Pharmacokinetic Comparison of Tylvalosin Tartrate Nanocrystal Suspension and Soluble Powder in Broiler Chickens After Oral and Intravenous Administration
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Animals
2.3. Experimental Design and Sample Collection
2.4. Sample Preparation
2.5. UPLC-MS/MS Conditions
2.6. Standards Preparation and Method Validation
2.7. Data Analysis
3. Results
3.1. Validation of Analytical Methods
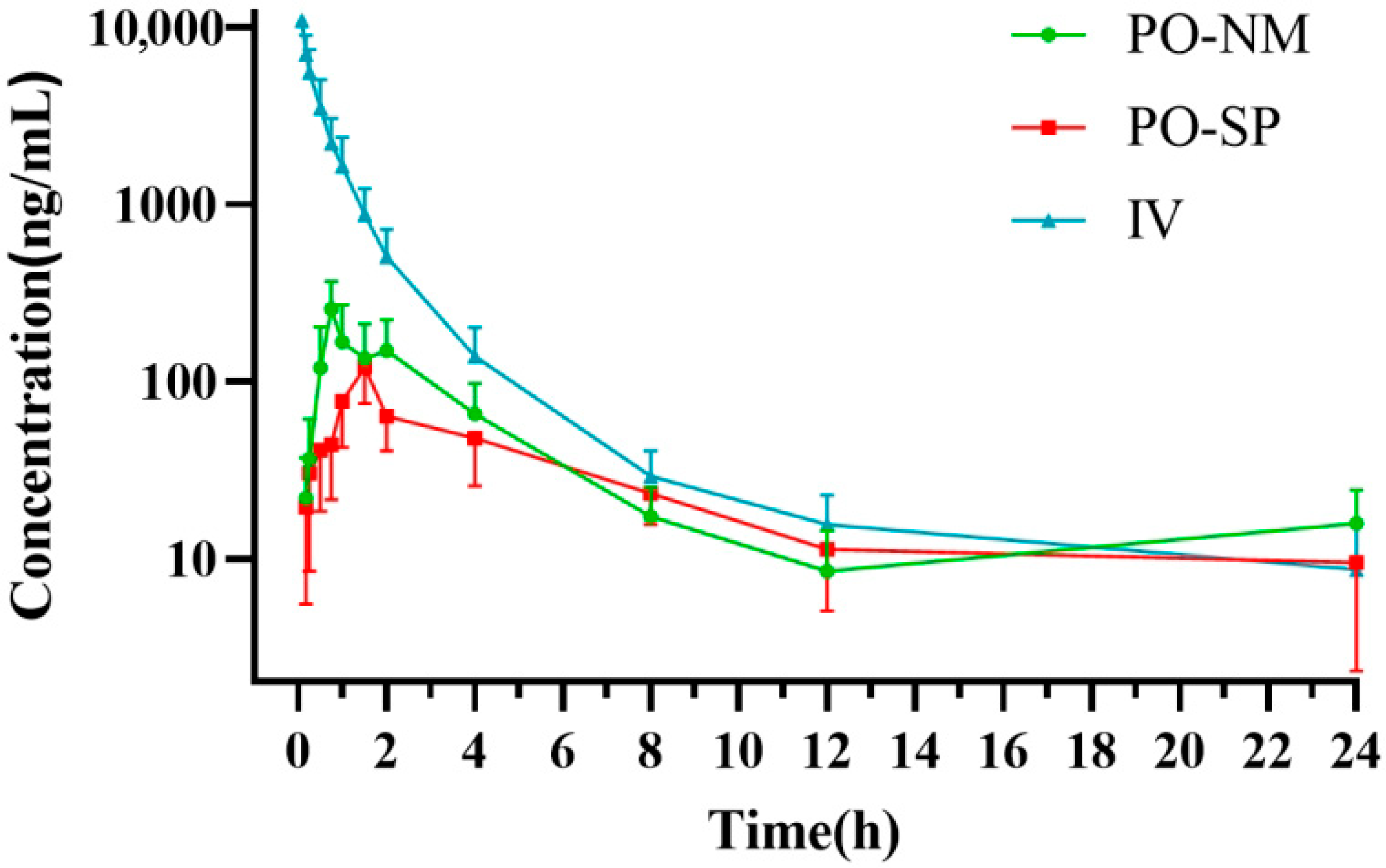
3.2. Pharmacokinetics
| Parameter | Units | PO-NM | PO-SP | IV |
|---|---|---|---|---|
| λz | 1/h | 0.11 ± 0.03 | 0.09 ± 0.03 | 0.16 ± 0.09 |
| t1/2λz | h | 6.54 ± 2.07 | 6.68 ± 1.49 | 4.26 ± 1.34 |
| Tmax | h | 0.71 ± 0.09 | 1.42 ± 0.18 | - |
| Cmax | ng/ml | 255.52 ± 111.88 | 120.45 ± 45.82 | - |
| AUClast | h·ng/ml | 918.90 ± 354.99 | 731.95 ± 374.28 | 6737.17 ± 2375.36 |
| AUC0–∞ | h·µg/mL | 1040.31 ± 377.33 | 839.85 ± 426.58 | 6833.51 ± 2454.11 |
| AUMC | h2·µg/mL | 10.48 ± 6.27 | 10.33 ± 9.43 | 13.86 ± 12.25 |
| MRT | h | 5.80 ± 1.92 | 6.64 ± 1.43 | 1.44 ± 0.23 |
| F | % | 15.73 ± 4.29 | 11.45 ± 4.66 | - |
| CL | L/h/Kg | - | - | 4.44 ± 1.77 |
| Vz | L/Kg | - | - | 35.99 ± 23.56 |
| Vss | L/kg | - | - | 5.55 ± 1.58 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feberwee, A.; de Wit, S.; Dijkman, R. Clinical expression, epidemiology, and monitoring of Mycoplasma gallisepticum and Mycoplasma synoviae: An update. Avian Pathol. 2022, 51, 2–18. [Google Scholar] [CrossRef]
- Yadav, J.P.; Singh, Y.; Jindal, N.; Mahajan, N.K. Rapid and specific detection of Mycoplasma gallisepticum and Mycoplasma synoviae infection in poultry using single and duplex PCR assays. J. Microbiol. Methods 2022, 192, 106365. [Google Scholar] [CrossRef] [PubMed]
- Mugunthan, S.P.; Kannan, G.; Chandra, H.M.; Paital, B. Infection, Transmission, Pathogenesis and Vaccine Development against Mycoplasma gallisepticum. Vaccines 2023, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Limpavithayakul, K.; Sasipreeyajan, J.; Pakpinyo, S. Molecular characterization and antimicrobial susceptibility profiles of Thai Mycoplasma synoviae isolates. Sci. Rep. 2023, 13, 2002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ye, X.; Wu, Y.; Huang, Z.; Gu, X.; Cai, Q.; Shen, X.; Jiang, H.; Ding, H. Determination of the Mutant Selection Window and Evaluation of the Killing of Mycoplasma gallisepticum by Danofloxacin, Doxycycline, Tilmicosin, Tylvalosin and Valnemulin. PLoS ONE 2017, 12, e0169134. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; Awad, N.F.S.; Hashem, Y.M.; Abdel-Rahman, M.A.; Abdelaziz, A.M.; Mohammed, I.A.A.; Abo-Shama, U.H. In vitro evaluation of various antimicrobials against field mycoplasma gallisepticum and mycoplasma synoviae isolates in Egypt. Poult. Sci. 2019, 98, 6281–6288. [Google Scholar] [CrossRef]
- Lees, P.; Giraudel, J.; Landoni, M.F.; Toutain, P.L. PK-PD integration and PK-PD modelling of nonsteroidal anti-inflammatory drugs: Principles and applications in veterinary pharmacology. J. Vet. Pharmacol. Ther. 2004, 27, 491–502. [Google Scholar] [CrossRef]
- Huang, G.; Okabe, M.; Kahar, P.; Tsunekawa, H.; Park, Y. Optimization of tylosin feeding rate profile in production of acetyl-isovaleryl tylosin (AIV) from tylosin by Streptomyces thermotolerans YN554. J. Biosci. Bioeng. 2001, 91, 504–508. [Google Scholar] [CrossRef]
- Yuan, W.; Jia, H.; Tang, X.; Xin, T.; Liu, X.; Wang, Z.; Li, X.; Zhao, Z.; Liu, L.; Liang, L.; et al. Tylvalosin demonstrates anti-parasitic activity and protects mice from acute toxoplasmosis. Life Sci. 2022, 294, 120373. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, X.; Zhao, X.; Zhang, M.; Zhang, W.; Hou, S.; Yuan, W.; Zhang, H.; Shi, L.; Jia, H.; et al. Tylvalosin exhibits anti-inflammatory property and attenuates acute lung injury in different models possibly through suppression of NF-κB activation. Biochem. Pharmacol. 2014, 90, 73–87. [Google Scholar] [CrossRef]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar] [CrossRef]
- Nowroozi, F.; Almasi, A.; Javidi, J.; Haeri, A.; Dadashzadeh, S. Effect of Surfactant Type, Cholesterol Content and Various Downsizing Methods on the Particle Size of Niosomes. Iran. J. Pharm. Res. 2018, 17 (Suppl. S2), 1–11. [Google Scholar]
- Abu-Basha, E.A.; Ismail, Z.B.; Idkaidek, N.M.; Hamzeh, E. Comparison of pharmacokinetics of two tylvalosin oral formulations in broiler chickens. J. Vet. Pharmacol. Ther. 2023, 46, 165–169. [Google Scholar] [CrossRef]
- Wen, Z.; Chen, S.; Meng, J.; Wu, Q.; Yu, R.; Xu, N.; Kong, J.; Zhang, L.; Cao, X. Pharmacokinetics of Tylvalosin Following Intravenous or Oral Administration at Different Doses in Broiler Chickens. Vet. Sci. 2025, 12, 118. [Google Scholar] [CrossRef]
- Lin, Y.S.; Thummel, K.E.; Thompson, B.D.; Totah, R.A.; Cho, C.W. Sources of Interindividual Variability. Methods Mol. Biol. 2021, 2342, 481–550. [Google Scholar]
- CerdÁ, R.O.; Petruccelli, M.; Piscopo, M.; Origlia, J.; Landoni, M. Impact of the type of catheter on the absorption of tylvalosin (acetylvaleryltylosin) administered orally to broiler chickens. J. Vet. Pharmacol. Ther. 2010, 33, 202–203. [Google Scholar] [CrossRef]
- Abo El-Ela, F.; El Banna, H.; El-Deen, M.; El-Gendy, A.A.M.; Tohamy, M. Pharmacokinetics of Tylvalosin Alone or in Combination with Vitamin E in Broiler Chickens. Asian J. Anim. Vet. Adv. 2015, 10, 556–566. [Google Scholar] [CrossRef][Green Version]
- Elbadawy, M.; Aboubakr, M.; Abugomaa, A. Pharmacokinetics of Tylvalosin in Broiler Turkeys (Meleagris Gallopavo) After Single Intravenous and Oral Administration. Front. Vet. Sci. 2019, 6, 355. [Google Scholar] [CrossRef]
- El-Tareef, F.S.; Abo-El-Sooud, K.; Karmi, M.; Hafez, A. Effect of theophylline on serum and milk pharmacokinetics of tylosin following intramuscular administration in lactating goats. BMC Vet. Res. 2024, 20, 251. [Google Scholar] [CrossRef]
- Elazab, S.T.; Elshater, N.S.; Hashem, Y.H.; Park, S.-C.; Hsu, W.H. Pharmacokinetics, tissue residues, and ex vivo pharmacodynamics of tylosin against Mycoplasma anatis in ducks. J. Vet. Pharmacol. Ther. 2020, 43, 57–66. [Google Scholar] [CrossRef]
- Ji, L.W.; Dong, L.L.; Ji, H.; Feng, X.W.; Li, D.; Ding, R.L.; Jiang, S.X. Comparative pharmacokinetics and bioavailability of tylosin tartrate and tylosin phosphate after a single oral and i.v. administration in chickens. J. Vet. Pharmacol. Ther. 2014, 37, 312–315. [Google Scholar] [CrossRef]
- Poźniak, B.; Tikhomirov, M.; Motykiewicz-Pers, K.; Bobrek, K.; Świtała, M. Allometric analysis of tylosin tartrate pharmacokinetics in growing male turkeys. J. Vet. Sci. 2020, 21, e35. [Google Scholar] [CrossRef]
- Mechesso, A.F.; Lee, S.J.; Park, N.H.; Park, S.C. Pharmacokinetic parameters and optimal dosage of a florfenicol and tylosin mixture in beagle dogs. Vet. Med. 2018, 63, 329–334. [Google Scholar] [CrossRef]
- Shafiq, S.; Shakeel, F.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K.; Ali, M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur. J. Pharm. Biopharm. 2007, 66, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J. Clin. Pharmacol. 2018, 58 (Suppl. S10), S10–S25. [Google Scholar] [CrossRef] [PubMed]
- Kuan, I.H.S.; Wright, D.F.B.; Duffull, S.B. The influence of flip-flop in population pharmacokinetic analyses. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 285–287. [Google Scholar] [CrossRef]
- Sarwar, A.R.; Iqbal, F.M.; Jamil, M.A.; Abbas, K. Nanocrystals of Mangiferin Using Design Expert: Preparation, Characterization, and Pharmacokinetic Evaluation. Molecules 2023, 28, 5918. [Google Scholar] [CrossRef]
- Abonashey, S.G.; Hassan, H.; Shalaby, M.A.; Fouad, A.G.; Mobarez, E.; El-Banna, H.A. Formulation, pharmacokinetics, and antibacterial activity of florfenicol-loaded niosome. Drug Deliv. Transl. Res. 2024, 14, 1077–1092. [Google Scholar] [CrossRef]
- Macedo, L.O.; Masiero, J.F.; Bou-Chacra, N.A. Drug Nanocrystals in Oral Absorption: Factors That Influence Pharmacokinetics. Pharmaceutics 2024, 16, 1141. [Google Scholar] [CrossRef]
- Deng, F.; Bae, Y.H. Bile acid transporter-mediated oral drug delivery. J. Control Release 2020, 327, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, W.; Xiong, S.; Luo, J.; Li, Y.; Zhao, Y.; Wang, Q.; Zhang, Z.; Chen, X.; Chen, T. Highly stabilized nanocrystals delivering Ginkgolide B in protecting against the Parkinson’s disease. Int. J. Pharm. 2020, 577, 119053. [Google Scholar] [CrossRef] [PubMed]
- Kala, S.G.; Chinni, S. Development and Characterization of Venetoclax Nanocrystals for Oral Bioavailability Enhancement. AAPS PharmSciTech 2021, 22, 92. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, A.; Qing, Y.; Gu, Y.; Huang, J.; Ma, X.; Li, J. Pharmacokinetic Comparison of Tylvalosin Tartrate Nanocrystal Suspension and Soluble Powder in Broiler Chickens After Oral and Intravenous Administration. Vet. Sci. 2025, 12, 1004. https://doi.org/10.3390/vetsci12101004
Lin A, Qing Y, Gu Y, Huang J, Ma X, Li J. Pharmacokinetic Comparison of Tylvalosin Tartrate Nanocrystal Suspension and Soluble Powder in Broiler Chickens After Oral and Intravenous Administration. Veterinary Sciences. 2025; 12(10):1004. https://doi.org/10.3390/vetsci12101004
Chicago/Turabian StyleLin, Ao, Yanzhe Qing, Yani Gu, Jingjie Huang, Xinxin Ma, and Jiancheng Li. 2025. "Pharmacokinetic Comparison of Tylvalosin Tartrate Nanocrystal Suspension and Soluble Powder in Broiler Chickens After Oral and Intravenous Administration" Veterinary Sciences 12, no. 10: 1004. https://doi.org/10.3390/vetsci12101004
APA StyleLin, A., Qing, Y., Gu, Y., Huang, J., Ma, X., & Li, J. (2025). Pharmacokinetic Comparison of Tylvalosin Tartrate Nanocrystal Suspension and Soluble Powder in Broiler Chickens After Oral and Intravenous Administration. Veterinary Sciences, 12(10), 1004. https://doi.org/10.3390/vetsci12101004






