Observed Trace Mineral Deficiencies in a Group of Locally Harvested Sheep in Hawai’i
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm Profile
2.2. Sample Collection
2.3. Mineral Analysis
2.4. Statistical Analysis
3. Result
3.1. Descriptive Analysis of Plasma and Liver Concentrations
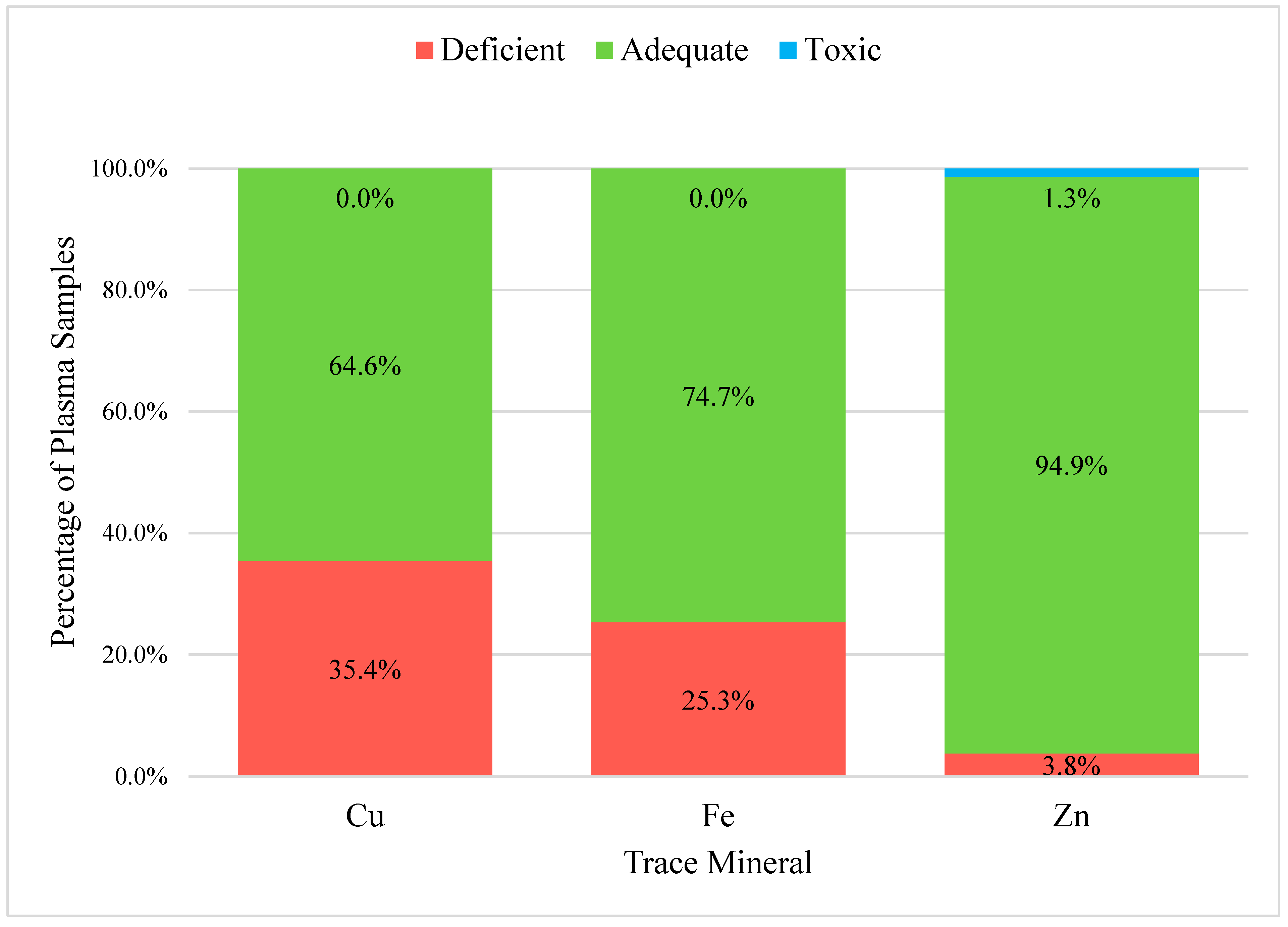
3.2. Adequate, Deficient, and Toxic Concentration Ranges
3.3. Liver and Plasma Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| As | Arsenic |
| Ba | Barium |
| Ca | Calcium |
| Cd | Cadmium |
| Co | Cobalt |
| Cr | Chromium |
| Cu | Copper |
| Fe | Iron |
| Mg | Magnesium |
| Mn | Manganese |
| Mo | Molybdenum |
| P | Phosphorus |
| SD | Standard Deviation |
| Se | Selenium |
| TM | Trace Mineral |
| Zn | Zinc |
References
- Whaley, J.R.; Froehlich, K.A.; Carroll, H.K. Surveying Production and Management Needs of US Sheep and Goat Producers. Sheep Goat Res. J. 2022, 37, 8–15. [Google Scholar]
- Farm Production Expenses Statistics State of Hawai‘i, 2007–2022; United States Department of Agriculture, National Agricultural Statistics Service: Honolulu, HI, USA, 2024.
- Pagno, K.C.; Barbosa, J.D.; Salvarani, F.M.; Bomjardim, H.A.; Faial, K.C.; Sousa, R.S.; Gava, A.; Perotta, J.H.; Barros Filho, I.R. Micromineral concentrations (copper, cobalt, iron, molybdenum and zinc) in the liver of dairy cows from Campos Gerais Region, Paraná state, Brazil. Pesqui. Veterinária Bras. 2023, 43, e07137. [Google Scholar] [CrossRef]
- Sadri, H.; Khordadmehr, M.; Akbari, H.; Shirazi, J.; Jafari-Khataylou, Y.; Eskandari, S.; Mirarabshahi, B.S.; Abdolmaleki, A. Nutritional deficiencies and abortions in sheep and goats: An in-depth study from East Azerbaijan Province, Northwest Iran. PLoS ONE 2025, 20, e0327768. [Google Scholar] [CrossRef]
- Swarup, D.; Patra, R.C.; Naresh, R.; Kumar, P.; Shekhar, P.; Balagangatharathilagar, M. Lowered blood copper and cobalt contents in goats reared around lead–zinc smelter. Small Rumin. Res. 2006, 63, 309–313. [Google Scholar] [CrossRef]
- Thorne, M.S.; Hewlett, J.P.; Fukumoto, G.K.; Oshiro, M.A. Development of an individual free-choice mineral supplementation program for improved grazing management in Hawaii’s rangelands. In Proceedings of the 71st Annual Meeting of the Society for Range Management, Sparks, NV, USA, 27 January–3 February 2018. [Google Scholar]
- Zervas, G.; Rissaki, M.; Deligeorgis, S. Free-choice consumption of mineral lick blocks by fattening lambs fed ad libitum alfalfa hay and concentrates with different trace mineral content. Livest. Prod. Sci. 2001, 68, 251–258. [Google Scholar] [CrossRef]
- Espinoza, J.; McDowell, L.; Wilkinson, N.; Conrad, J.; Martin, F. Forage and soil mineral concentrations over a three-year period in a warm climate region of central Florida. II. Trace minerals. Livest. Res. Rural Dev. 1991, 3, 20–27. [Google Scholar]
- Chelliah, G.; Myer, B.; Carter, J.; McDowell, L.; Wilkinson, N.; Blount, A. Mineral Concentrations of Annual Cool Season Pasture Forages in North Florida during the Winter-Spring Grazing Season: II. Trace Minerals. J. Plant Nutr. 2006, 31, 1774–1788. [Google Scholar] [CrossRef]
- Spears, J.; Brandao, V.; Heldt, J. Invited Review: Assessing trace mineral status in ruminants, and factors that affect measurements of trace mineral status. Appl. Anim. Sci. 2022, 38, 252–267. [Google Scholar] [CrossRef]
- Herdt, T.H.; Hoff, B. The use of blood analysis to evaluate trace mineral status in ruminant livestock. Vet. Clin: Food Anim. Pract. 2011, 27, 255–283. [Google Scholar] [CrossRef] [PubMed]
- Pacific Region Livestock Slaughter; National Agricultural Statistics Service: Washington, DC, USA, 2024.
- McDowell, L.R.; Arthington, J.D. Minerals for Grazing Ruminants in Tropical Regions; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2005. [Google Scholar]
- Vellema, P.; Rutten, V.; Hoek, A.; Moll, L.; Wentink, G. The effect of cobalt supplementation on the immune response in vitamin B12 deficient Texel lambs. Vet. Immunol. Immunopathol. 1996, 55, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.; Stoate, C.; Kendall, N. Willow leaves as a cobalt supplement for weaned lambs. Livest. Sci. 2022, 264, 105047. [Google Scholar] [CrossRef]
- Hession, D.V.; Kendall, N.R.; Hanrahan, J.P.; Keady, T.W.J. The effects of supplementation with cobalt, and method of administration, on ewe reproduction and offspring performance to weaning. Livest. Sci. 2021, 251, 104661. [Google Scholar] [CrossRef]
- Keady, T.; Hanrahan, J.; Fagan, S. Cobalt supplementation, alone or in combination with vitamin B12 and selenium: Effects on lamb performance and mineral status. J. Anim. Sci. 2017, 95, 379–386. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L. Minerals in Animal and Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Suttle, N. Mineral Nutrition of Livestock; Cabi GB: Wallingford, UK, 2022. [Google Scholar]
- Adogwa, A.; Mutani, A.; Ramnanan, A.; Ezeokoli, C. The effect of gastrointestinal parasitism on blood copper and hemoglobin levels in sheep. Can. Vet. J. 2005, 46, 1017. [Google Scholar] [PubMed]
- Stevenson, M.H.; Stokes, A.; McNeal, L. An Introduction to Sheep and Goat Parasite Management in Hawai‘i. CTAHR Ext. 2012, LM-24. Available online: https://www.ctahr.hawaii.edu/oc/freepubs/pdf/LM-24.pdf (accessed on 14 October 2025).
- Casanova, V.P.; Aires, A.R.; Collet, S.G.; Krause, A.; Moresco, R.N.; Bochi, G.V.; Silva, A.S.; Leal, M.L. Iron supplementation for lambs experimentally infected by Haemonchus contortus: Response to anemia and iron store in the bone marrow. Pesqui. Veterinária Bras. 2018, 38, 1543–1548. [Google Scholar] [CrossRef]
- Burke, J.; Miller, J.; Olcott, D.; Olcott, B.; Terrill, T. Effect of copper oxide wire particles dosage and feed supplement level on Haemonchus contortus infection in lambs. Vet. Parasitol. 2004, 123, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.; Glenn, J.; Farver, T. Copper oxide wire particles for the treatment of copper deficiency in sheep. Small Rumin. Res. 1999, 35, 7–12. [Google Scholar] [CrossRef]
- Meng, L.; Jin, X.; Qi, Z.; Mi, L. Effects of dietary minerals deficiency and supplementation on different parts of muscle minerals content in grazing Mongolian sheep. Front. Vet. Sci. 2024, 11, 1301852. [Google Scholar] [CrossRef]
- Meng, L.; Jin, X.; Qi, Z.; Mi, L. Dietary copper levels affect mineral absorbability, rumen microbial composition and metabolites of the grazing Mongolian sheep. Anim. Feed Sci. Technol. 2024, 312, 115970. [Google Scholar] [CrossRef]
- Akalın, P.P.; Bülbül, B.; Coyan, K.; Başpınar, N.; Kırbaş, M.; Bucak, M.N.; Güngör, Ş.; Öztürk, C. Relationship of blood and seminal plasma ceruloplasmin, copper, iron and cadmium concentrations with sperm quality in Merino rams. Small Rumin. Res. 2015, 133, 135–139. [Google Scholar] [CrossRef]
- Quillian, E.; Miller, W.; Gentry, R.; Heinmiller, S.; Neathery, M. Maximum safe dietary magnesium and effects of high dietary magnesium on zinc metabolism in Holstein calves. J. Dairy Sci. 1980, 63, 457–463. [Google Scholar] [CrossRef]
- Toghdory, A.; Asadi, M.; Ghoorchi, T.; Hatami, M. Impacts of organic manganese supplementation on blood mineral, biochemical, and hematology in Afshari Ewes and their newborn lambs in the transition period. J. Trace Elem. Med. Biol. 2023, 79, 127215. [Google Scholar] [CrossRef]
- Asadi, M.; Toghdory, A.; Ghoorchi, T.; Hatami, M. The effect of maternal organic manganese supplementation on performance, immunological status, blood biochemical and antioxidant status of Afshari ewes and their newborn lambs in transition period. J. Anim. Physiol. Anim. Nutr. 2024, 108, 493–499. [Google Scholar] [CrossRef] [PubMed]
- National Research Council; Division on Earth and Life Studies; Board on Agriculture and Natural Resources; Committee on Minerals and Toxic Substances in Diets and Water for Animals. Mineral Tolerance of Animals: 2005; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Ho, S.; Miller, W.; Gentry, R.; Neathery, M.; Blackmon, D. Effects of high but nontoxic dietary manganese and iron on their metabolism by calves. J. Dairy Sci. 1984, 67, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- National Research Council of the National Academics. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, And New World Camelids; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Moyano Tapia, J.C.; Leib, S.A.; Marini, P.R.; Fischman, M.L. Effect of mineral supplementation on the macromineral concentration in blood in pre-and postpartum blackbelly sheep. Animals 2020, 10, 1206. [Google Scholar] [CrossRef]
- Fukumoto, G.K.; Thorne, M.S.; Silva, J.H.; Deenik, J.L. Suitability map for forage-finished beef production using GIS technology: Hawai‘i island. Univ. Hawai‘i Manoa CTAHR 2015, PRM-7, 1–6. [Google Scholar]
- Deenik, J.; McClellan, A.T. Soils of Hawai‘i. Univ. Hawai‘i Manoa CTAHR 2007, SCM-20. [Google Scholar]

| Farms | Number of Samples | Provided Mineral Supplements |
|---|---|---|
| A | 3 | No Response |
| B | 9 | No Response |
| C | 18 | Yes |
| D | 2 | No Response |
| E | 14 | Yes |
| F | 1 | No Response |
| G | 9 | Yes |
| H | 6 | No Response |
| I | 10 | No Response |
| J | 8 | Yes |
| K | 3 | No Response |
| Liver Descriptive Statistics | |||||
|---|---|---|---|---|---|
| Mean | SD | Sum | Min | Max | |
| Ba (µg/g) | 0.042 | 0.021 | 3.345 | 0.010 | 0.130 |
| Cd (µg/g) | 0.049 | 0.027 | 3.822 | 0.010 | 0.110 |
| Cr (µg/g) | 0.040 | 0.020 | 3.266 | 0.018 | 0.150 |
| Co (µg/g) | 0.040 | 0.024 | 2.844 | 0.011 | 0.100 |
| Cu (µg/g) | 49.730 | 28.704 | 3929 | 2.900 | 120.000 |
| Fe (µg/g) | 66.346 | 28.868 | 5175 | 27.000 | 140.000 |
| Mn (µg/g) | 3.181 | 0.864 | 241.820 | 0.520 | 5.700 |
| Mo (µg/g) | 0.819 | 0.446 | 67.143 | 0.027 | 1.800 |
| Se (µg/g) | 0.465 | 0.159 | 36.740 | 0.210 | 0.760 |
| Zn (µg/g) | 38.718 | 6.750 | 3020 | 27.000 | 72.000 |
| Plasma Descriptive Statistics | |||||
|---|---|---|---|---|---|
| Mean | SD | Sum | Min | Max | |
| Ca (µg/g) | 86.764 | 9.082 | 6768 | 47.500 | 104.000 |
| Cu (µg/g) | 0.811 | 0.195 | 62.440 | 0.370 | 1.300 |
| Fe (µg/g) | 1.138 | 0.415 | 86.450 | 0.230 | 2.100 |
| Mg (µg/g) | 22.551 | 4.585 | 1714 | 15.000 | 53.900 |
| P (µg/g) | 64.623 | 16.014 | 4976 | 1.000 | 97.100 |
| Zn (µg/g) | 1.361 | 4.464 | 104.820 | 0.490 | 40.000 |
| Liver Trace Elements | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ba | Cd | Cr | Co | Cu | Fe | Mn | Mo | Se | Zn | ||
| Liver Trace Elements | Ba | 1.00 1 0 2 | 0.208 0.073 | −0.039 0.748 | −0.163 0.184 | 0.042 0.721 | 0.066 0.572 | 0.040 0.735 | −0.185 0.103 | −0.233 0.042 | 0.159 0.172 |
| Cd | - | 1.00 0 | −0.070 0.544 | −0.257 0.036 | 0.078 0.508 | −0.050 0.673 | 0.051 0.670 | −0.033 0.773 | −0.199 0.087 | 0.163 0.166 | |
| Cr | - | - | 1.00 0 | 0.061 0.617 | −0.345 0.002 | 0.072 0.533 | 0.143 0.329 | −0.070 0.538 | −0.135 0.237 | 0.074 0.523 | |
| Co | - | - | - | 1.00 0 | −0.368 0.002 | 0.179 0.145 | −0.168 0.167 | 0.298 0.012 | 0.463 <0.0001 | 0.199 0.330 | |
| Cu | - | - | - | - | 1.00 0 | −0.214 0.065 | −0.119 0.314 | 0.170 0.135 | 0.086 0.463 | −0.141 0.226 | |
| Fe | - | - | - | - | - | 1.00 0 | −0.058 0.624 | −0.243 0.032 | −0.093 0.430 | 0.235 0.042 | |
| Mn | - | - | - | - | - | - | 1.00 0 | −0.362 0.001 | −0.370 0.001 | −0.180 0.125 | |
| Mo | - | - | - | - | - | - | - | 1.00 0 | 0.720 <0.0001 | −0.015 0.896 | |
| Se | - | - | - | - | - | - | - | - | 1.00 0 | 0.022 0.849 | |
| Zn | - | - | - | - | - | - | - | - | - | 1.00 0 | |
| Plasma Trace Elements | |||||||
|---|---|---|---|---|---|---|---|
| Ca | Cu | Fe | Mg | P | Zn | ||
| Liver Trace Elements | Ba | 0.041 1 0.726 2 | −0.091 0.440 | 0.175 0.135 | −0.014 0.901 | −0.017 0.887 | −0.0006 0.996 |
| Cd | −0.161 0.157 | 0.074 0.535 | −0.011 0.923 | −0.131 0.271 | 0.208 0.078 | −0.132 0.266 | |
| Cr | −0.121 0.294 | −0.070 0.554 | 0.023 0.842 | −0.150 0.197 | 0.104 0.372 | −0.064 0.582 | |
| Co | −0.004 0.974 | 0.082 0.509 | 0.139 0.264 | −0.071 0.573 | −0.118 0.340 | 0.056 0.654 | |
| Cu | 0.120 0.306 | 0.216 0.064 | −0.214 0.069 | 0.042 0.733 | 0.153 0.189 | −0.130 0.268 | |
| Fe | −0.078 0.507 | 0.057 0.629 | 0.427 0.0002 | 0.138 0.274 | −0.385 0.0008 | 0.252 0.031 | |
| Mn | 0.155 0.195 | −0.326 0.006 | 0.303 0.011 | −0.091 0.452 | 0.064 0.597 | −0.118 0.326 | |
| Mo | 0.122 0.289 | 0.294 0.009 | −0.171 0.140 | 0.092 0.430 | 0.152 0.319 | 0.125 0.280 | |
| Se | 0.296 0.009 | 0.036 0.002 | −0.168 0.152 | 0.0121 0.919 | 0.056 0.633 | 0.003 0.983 | |
| Zn | −0.180 0.125 | 0.359 0.001 | −0.225 0.057 | −0.221 0.062 | −0.156 0.188 | −0.023 0.844 | |
| Plasma Trace Elements | |||||||
|---|---|---|---|---|---|---|---|
| Ca | Cu | Fe | Mg | P | Zn | ||
| Plasma Trace Elements | Ca | 1.00 1 0 2 | 0.235 0.040 | 0.001 0.990 | 0.0009 0.994 | 0.317 0.005 | −0.007 0.949 |
| Cu | - | 1.00 0 | −0.318 0.005 | 0.087 0.457 | 0.030 0.794 | 0.161 0.164 | |
| Fe | - | - | 1.00 0 | −0.0008 0.995 | 0.004 0.973 | 0.047 0.686 | |
| Mg | - | - | - | 1.00 0 | −0.389 0.0005 | 0.814 <0.0001 | |
| P | - | - | - | - | 1.00 0 | −0.462 <0.0001 | |
| Zn | - | - | - | - | - | 1.00 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, S.N.R.; Bulosan, J.S.; Thorne, M.S.; Oshiro, M.A.; Odani, J.S.; Reichhardt, C.C. Observed Trace Mineral Deficiencies in a Group of Locally Harvested Sheep in Hawai’i. Vet. Sci. 2025, 12, 1002. https://doi.org/10.3390/vetsci12101002
Nishimura SNR, Bulosan JS, Thorne MS, Oshiro MA, Odani JS, Reichhardt CC. Observed Trace Mineral Deficiencies in a Group of Locally Harvested Sheep in Hawai’i. Veterinary Sciences. 2025; 12(10):1002. https://doi.org/10.3390/vetsci12101002
Chicago/Turabian StyleNishimura, Shaye N. R., Janae S. Bulosan, Mark S. Thorne, Melelani A. Oshiro, Jenee S. Odani, and Caleb C. Reichhardt. 2025. "Observed Trace Mineral Deficiencies in a Group of Locally Harvested Sheep in Hawai’i" Veterinary Sciences 12, no. 10: 1002. https://doi.org/10.3390/vetsci12101002
APA StyleNishimura, S. N. R., Bulosan, J. S., Thorne, M. S., Oshiro, M. A., Odani, J. S., & Reichhardt, C. C. (2025). Observed Trace Mineral Deficiencies in a Group of Locally Harvested Sheep in Hawai’i. Veterinary Sciences, 12(10), 1002. https://doi.org/10.3390/vetsci12101002






