Application of Minimally Invasive Oral Swab Samples for qPCR-Based Sexing in Neognathae Birds
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction and qPCR
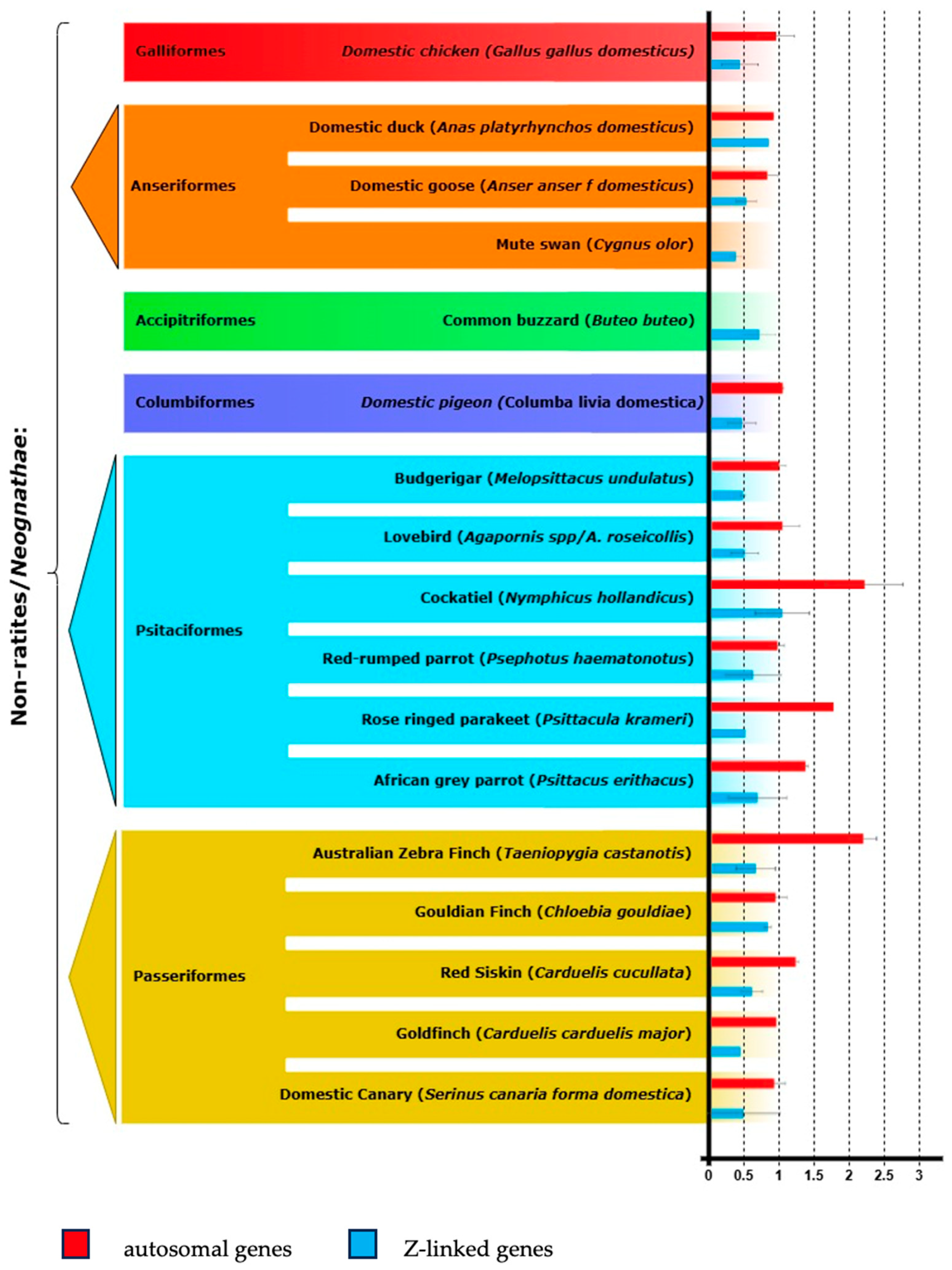
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brusatte, S.L.; O’Connor, J.K.; Jarvis, E.D. The origin and diversification of Birds. Curr. Biol. 2015, 25, R888–R898. [Google Scholar] [CrossRef]
- Kidd, A.H.; Kidd, R.M. Problems and benefits of bird ownership. Psychol. Rep. 1998, 83, 131–138. [Google Scholar] [CrossRef]
- Meyers, N.M. Perspectives on pet bird welfare from the pet industry. J. Am. Vet. Med. Assoc. 1998, 212, 1238–1242. [Google Scholar] [PubMed]
- Davis, C. Appreciating avian intelligence: The importance of a proper domestic environment. J. Am. Vet. Med. Assoc. 1998, 212, 1220–1222. [Google Scholar] [PubMed]
- Graham, D.L. Pet birds: Historical and modern perspectives on the keeper and the kept. J. Am. Vet. Med. Assoc. 1998, 212, 1216–1219. [Google Scholar] [PubMed]
- Wyndham, E. Diurnal cycle, behaviour and social organization of the budgerigar Melopsittacus undulatus. EMU—Austral Ornithol. 1980, 80, 25–33. [Google Scholar] [CrossRef]
- Peng, S.; Broom, D.M. The sustainability of keeping birds as pets: Should any be kept? Animals 2021, 11, 582. [Google Scholar] [CrossRef] [PubMed]
- Morinha, F.; Cabral, J.A.; Bastos, E. Molecular sexing of birds: A comparative review of polymerase chain reaction (PCR)-based methods. Theriogenology 2012, 78, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Morinha, F.; Travassos, P.; Seixas, F.; Santos, N.; Sargo, R.; Sousa, L.; Bastos, E. High-resolution melting analysis for bird sexing: A successful approach to molecular sex identification using different biological samples. Mol. Ecol. Resour. 2013, 13, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.; Double, M.C.; Orr, K.; Dawson, R.J. A DNA test to sex most birds. Mol. Ecol. 1998, 7, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R. Sex identification in birds. In Seminars in Avian and Exotic Pet Medicine; WB Saunders: Philadelphia, PA, USA, 2000; pp. 14–26. [Google Scholar]
- Nanda, I.; Schlegelmilch, K.; Haaf, T.; Schartl, M.; Schmid, M. Synteny conservation of the Z chromosome in 14 avian species (11 families) supports a role for Z dosage in avian sex determination. Cytogenet. Genome Res. 2008, 122, 150–156. [Google Scholar] [CrossRef]
- Rutkowska, J.; Lagisz, M.; Nakagawa, S. The long and the short of avian W chromosomes: No evidence for gradual W shortening. Biol. Lett. 2012, 8, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Sigeman, H.; Ponnikas, S.; Hansson, B. Whole-genome analysis across 10 songbird families within Sylvioidea reveals a novel autosome–sex chromosome fusion. Biol. Lett. 2020, 16, 20200082. [Google Scholar] [CrossRef] [PubMed]
- Gunski, R.J.; Cañedo, A.D.; Garnero, A.D.V.; Ledesma, M.A.; Coria, N.; Montalti, D.; Degrandi, T.M. Multiple sex chromosome system in penguins (Pygoscelis, Spheniscidae). Comp. Cytogenet. 2017, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, J.; Bachtrog, D.; An, N.; Huang, Q.; Jarvis, E.D.; Zhang, G. Complex evolutionary trajectories of sex chromosomes across bird taxa. Science 2014, 346, 1246338. [Google Scholar] [CrossRef]
- Petrou, E.L.; Scott, L.C.; McKeeman, C.M.; Ramey, A.M. Molecular sexing of birds using quantitative PCR (qPCR) of sex-linked genes and logistic regression models. Mol. Ecol. Resour. 2024, 24, e13946. [Google Scholar] [CrossRef]
- Fridolfsson, A.K.; Ellegren, H. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 1999, 30, 116–121. [Google Scholar] [CrossRef]
- Lee, J.C.; Tsai, L.C.; Hwa, P.Y.; Chan, C.L.; Huang, A.; Chin, S.C.; Wang, L.C.; Lin, J.T.; Linacre, A.; Hsieh, H.M. A novel strategy for avian species and gender identification using the CHD gene. Mol. Cell Probes 2010, 24, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Sudo-Yamaji, A.; Abe, M.; Murase, T.; Tsubota, T. Sex identification by alternative polymerase chain reaction methods in Falconiformes. Zool. Sci. 2003, 20, 339–344. [Google Scholar] [CrossRef]
- Bermudez-Humaran, L.G.; Chávez-Zamarripa, P.; Guzmán-Velasco, A.; Leal-Garza, C.H.; Montes de Oca-Luna, R. Loss of restriction site DdeI, used for avian molecular sexing, in Oreophasis derbianus. Reprod. Domest. Anim. 2002, 37, 321–323. [Google Scholar] [CrossRef]
- Chang, H.W.; Cheng, C.A.; Gu, D.L.; Chang, C.C.; Su, S.H.; Wen, C.H.; Cheng, C.C. High-throughput avian molecular sexing by SYBR green-based real-time PCR combined with melting curve analysis. BMC Biotechnol. 2008, 8, 12. [Google Scholar] [CrossRef]
- Fridolfsson, A.K.; Cheng, H.; Copeland, N.G.; Jenkins, N.A.; Liu, H.C.; Raudsepp, T.; Woodage, T.; Chowdhary, B.; Halverson, J.; Ellegren, H. Evolution of the avian sex chromosomes from an ances- tral pair of autosomes. Proc. Natl. Acad. Sci. USA 1998, 95, 8147–8152. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.; Binder, M.; Smith, C.; Andrews, J.; Reed, K.; Smith, M.; Millar, C.; Lambert, D.; Sinclair, A. ASW: A gene with conserved avian W-linkage and female specific expression in chick embry- onic gonad. Dev. Genes Evol. 2000, 210, 243–249. [Google Scholar] [CrossRef]
- Suh, A.; Kriegs, J.O.; Brosius, J.; Schmitz, J. Retroposon insertions and the chronology of avian sex chromosome evolution. Mol. Biol. Evol. 2011, 28, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, R.S.; de Kloet, S.R. Evolution of the spindlin gene in birds: Independent cessation of the recombination of sex chromosomes at the spindlin locus in neognathous birds and tinamous, a palaeognathous avian family. Genetica 2003, 119, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xue, F.; Li, L.; Li, X.; Yue, B.; Li, J. A triple-primer PCR approach for the sex identification of endangered Phasianidae birds. Eur. J. Wildl. Res. 2012, 58, 289–294. [Google Scholar] [CrossRef]
- Kroczak, A.; Wołoszyńska, M.; Wierzbicki, H.; Kurkowski, M.; Grabowski, K.A.; Piasecki, T.; Urantówka, A.D. New Bird sexing strategy developed in the order Psittaciformes involves multiple markers to avoid sex misidentification: Debunked myth of the Universal DNA marker. Genes 2021, 12, 878. [Google Scholar] [CrossRef]
- Kroczak, A.; Wierzbicki, H.; Urantówka, A.D. In Silico Analysis of Seven PCR Markers Developed from the CHD1, NIPBL and SPIN Genes Followed by Laboratory Testing Shows How to Reliably Determine the Sex of Musophagiformes Species. Genes 2022, 13, 932. [Google Scholar] [CrossRef]
- Medeiros, R.J.; King, R.A.; Symondson, W.O.; Cadiou, B.; Zonfrillo, B.; Bolton, M.; Thomas, R.J. Molecular evidence for gender differences in the migratory behaviour of a small seabird. PLoS ONE 2012, 7, e46330. [Google Scholar] [CrossRef]
- Çakmak, E.; Akın Pekşen, Ç.; Bilgin, C.C. Comparison of three different primer sets for sexing birds. J. Vet. Diagn. Investig. 2017, 29, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.E.; Jones, K.L.; Sandercock, B.K.; Wisely, S.M. Heteroduplex molecules cause sexing errors in a standard molecular protocol for avian sexing. Mol. Ecol. Resour. 2009, 9, 61–65. [Google Scholar] [CrossRef]
- Dawson, D.A.; Darby, S.; Hunter, F.M.; Krupa, A.P.; Jones, I.L.; Burke, T. A critique of avian CHD-based molecular sexing protocols illustrated by a Z-chromosome polymorphism detected in auklets. Mol. Ecol. Notes 2001, 1, 201–204. [Google Scholar] [CrossRef]
- Zhang, P.; Han, J.; Liu, Q.; Zhang, J.; Zhang, X. Sex Identification of Four Penguin Species Using Locus-Specific PCR. Zoo Biol. 2013, 32, 257–261. [Google Scholar] [CrossRef]
- Faux, C.E.; McInnes, J.C.; Jarman, S.N. High-throughput real-time PCR and melt curve analysis for sexing Southern Ocean seabirds using fecal samples. Theriogenology 2014, 81, 870–874. [Google Scholar] [CrossRef] [PubMed]
- He, P.J.; Yu, J.Q.; Fang, S.G. Sex identification of the black swan (Cygnus atratus) using the locus-specific PCR and implications for its reproduction. Reprod. Domest. Anim. 2005, 40, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Turcu, M.C.; Paștiu, A.I.; Bel, L.V.; Cocostîrc, V.; Lucaci, F.; Pusta, D.L. DNA Sex Identification Using Different Biological Samples from Four Companion Bird Species. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Hortic. 2022, 79, 44–53. [Google Scholar] [CrossRef]
- Turcu, M.C.; Paștiu, A.I.; Bel, L.V.; Pusta, D.L. A comparison of feathers and oral swab samples as DNA sources for molecular sexing in companion birds. Animals 2023, 13, 525. [Google Scholar] [CrossRef] [PubMed]
- Turcu, M.C.; Paștiu, A.I.; Bel, L.V.; Pusta, D.L. Minimally invasive sampling methods for molecular sexing of wild and companion birds. Animals 2023, 13, 3417. [Google Scholar] [CrossRef] [PubMed]
- Mazzoleni, S.; Němec, P.; Albrecht, T.; Lymberakis, P.; Kratochvíl, L.; Rovatsos, M. Long-term stability of sex chromosome gene content allows accurate qPCR-based molecular sexing across birds. Mol. Ecol. Resour. 2021, 21, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Handel, C.M.; Pajot, L.M.; Talbot, S.L.; Sage, G.K. Use of buccal swabs for sampling DNA from nestling and adult birds. Wildl. Soc. Bull. 2006, 34, 1094–1100. [Google Scholar] [CrossRef]
- Rovatsos, M.; Vukić, J.; Lymberakis, P.; Kratochvíl, L. Evolutionary stability of sex chromosomes in snakes. Proc. R. Soc. B 2015, 282, 20151992. [Google Scholar] [CrossRef]
- Cankar, K.; Štebih, D.; Dreo, T.; Žel, J.; Gruden, K. Critical points of DNA quantification by real-time PCR–effects of DNA extraction method and sample matrix on quantification of genetically modified organisms. BMC Biotechnol. 2006, 6, 37. [Google Scholar] [CrossRef] [PubMed]
| Order | Species | Male | Female |
|---|---|---|---|
| Accipitriformes | Buteo buteo | 1 | 1 |
| Galliformes | Gallus gallus domesticus | 1 | 1 |
| Anseriformes | Cygnus cygnus | 1 | 1 |
| Anser anser f domesticus | 1 | 1 | |
| Anas platyrhynchos domesticus | 1 | 1 | |
| Passeriformes | Serinus canaria forma domestica | 1 | 1 |
| Taeniopygia castanotis | 1 | 1 | |
| Chloebia gouldiae | 1 | 1 | |
| Carduelis cucullata | 1 | 1 | |
| Carduelis carduelis major | 1 | 1 | |
| Columbiformes | Columba livia domestica | 1 | 1 |
| Psittaciformes | Psittacus erithacus | 1 | 1 |
| Psittacula krameri | 1 | 1 | |
| Psephotus haematonotus | 1 | 1 | |
| Nymphicus hollandicus | 1 | 1 | |
| Agapornis fischeri | 1 | 1 | |
| Melopsittacus undulatus | 1 | 1 | |
| TOTAL | 17 | 17 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turcu, M.-C.; Paștiu, A.I.; Bel, L.-V.; Doboși, A.-A.; Pusta, D.L. Application of Minimally Invasive Oral Swab Samples for qPCR-Based Sexing in Neognathae Birds. Vet. Sci. 2025, 12, 73. https://doi.org/10.3390/vetsci12010073
Turcu M-C, Paștiu AI, Bel L-V, Doboși A-A, Pusta DL. Application of Minimally Invasive Oral Swab Samples for qPCR-Based Sexing in Neognathae Birds. Veterinary Sciences. 2025; 12(1):73. https://doi.org/10.3390/vetsci12010073
Chicago/Turabian StyleTurcu, Maria-Carmen, Anamaria Ioana Paștiu, Lucia-Victoria Bel, Anca-Alexandra Doboși, and Dana Liana Pusta. 2025. "Application of Minimally Invasive Oral Swab Samples for qPCR-Based Sexing in Neognathae Birds" Veterinary Sciences 12, no. 1: 73. https://doi.org/10.3390/vetsci12010073
APA StyleTurcu, M.-C., Paștiu, A. I., Bel, L.-V., Doboși, A.-A., & Pusta, D. L. (2025). Application of Minimally Invasive Oral Swab Samples for qPCR-Based Sexing in Neognathae Birds. Veterinary Sciences, 12(1), 73. https://doi.org/10.3390/vetsci12010073






