Diet Supplementation Influences Ghrelin System Expression in the Skin Appendages of the Sheep
Simple Summary
Abstract
1. Introduction
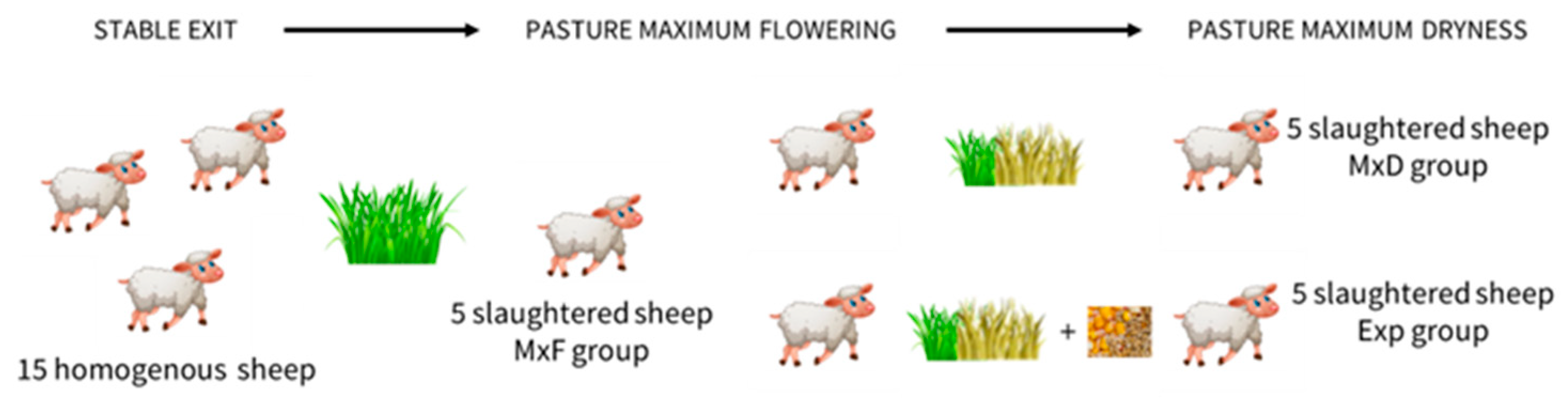
2. Materials and Methods
2.1. Immunohistochemistry
2.2. RNA Extraction and Real-Time PCR
2.3. Statistical Analysis
3. Results
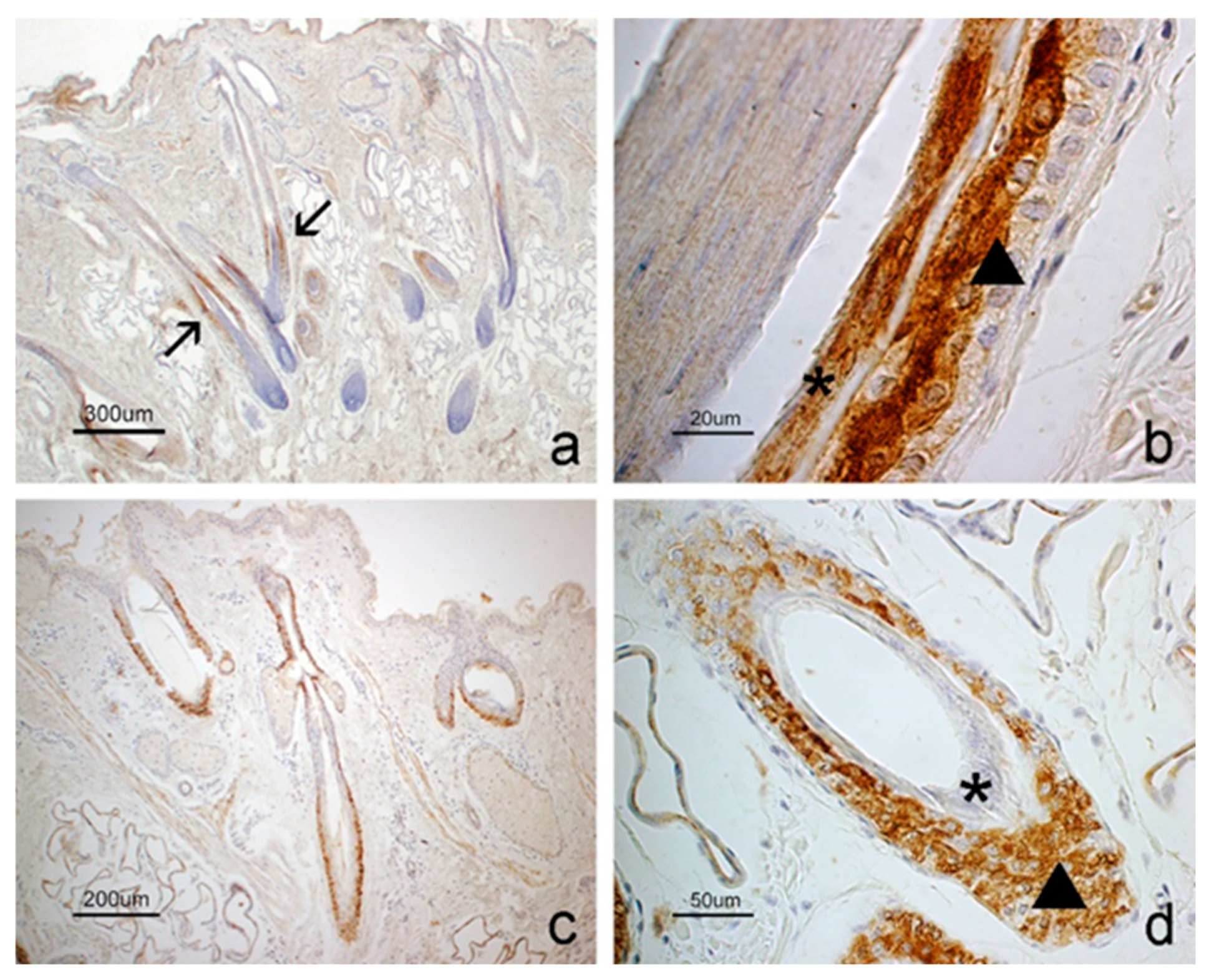
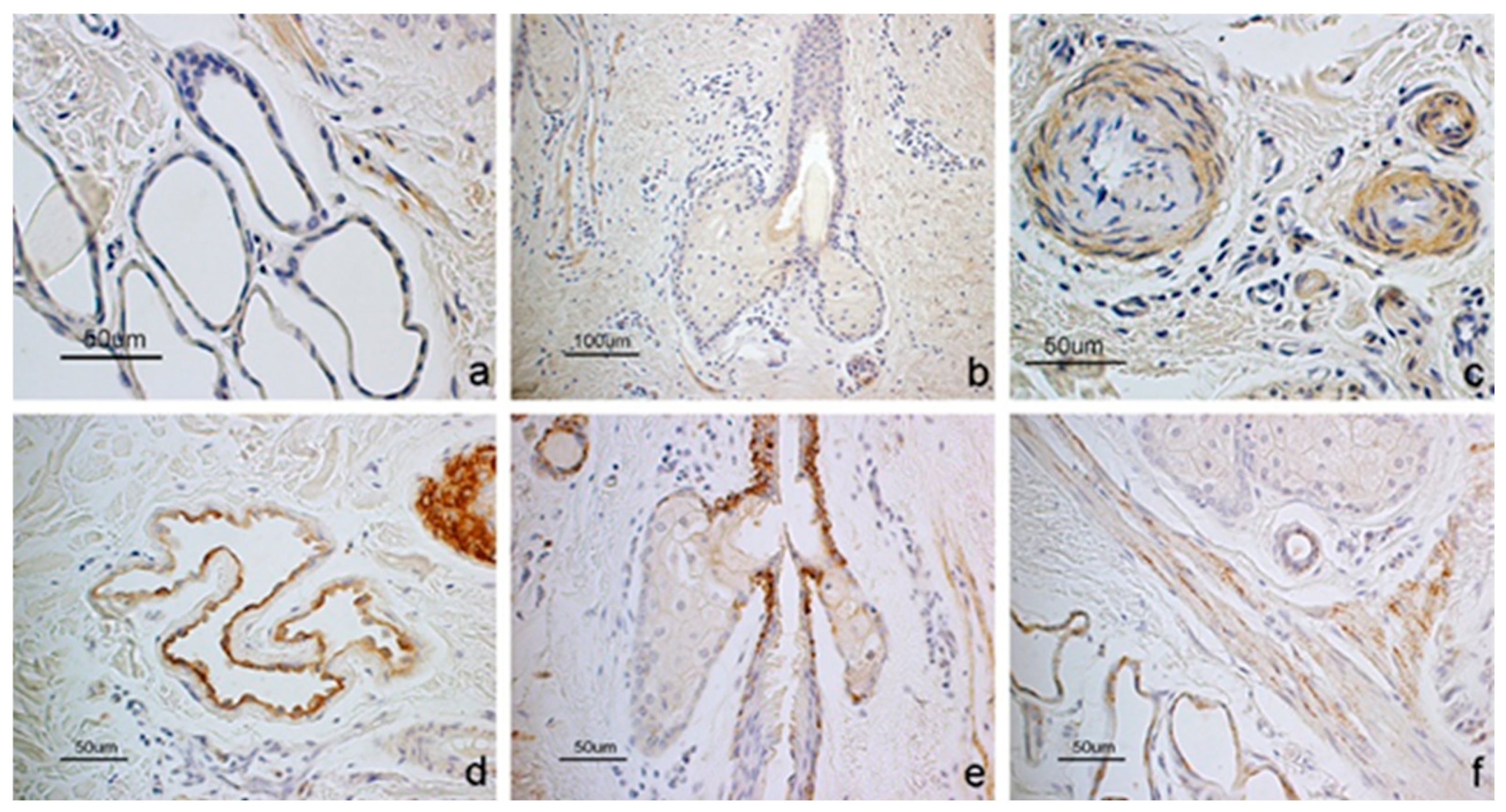
3.1. Immunohistochemistry
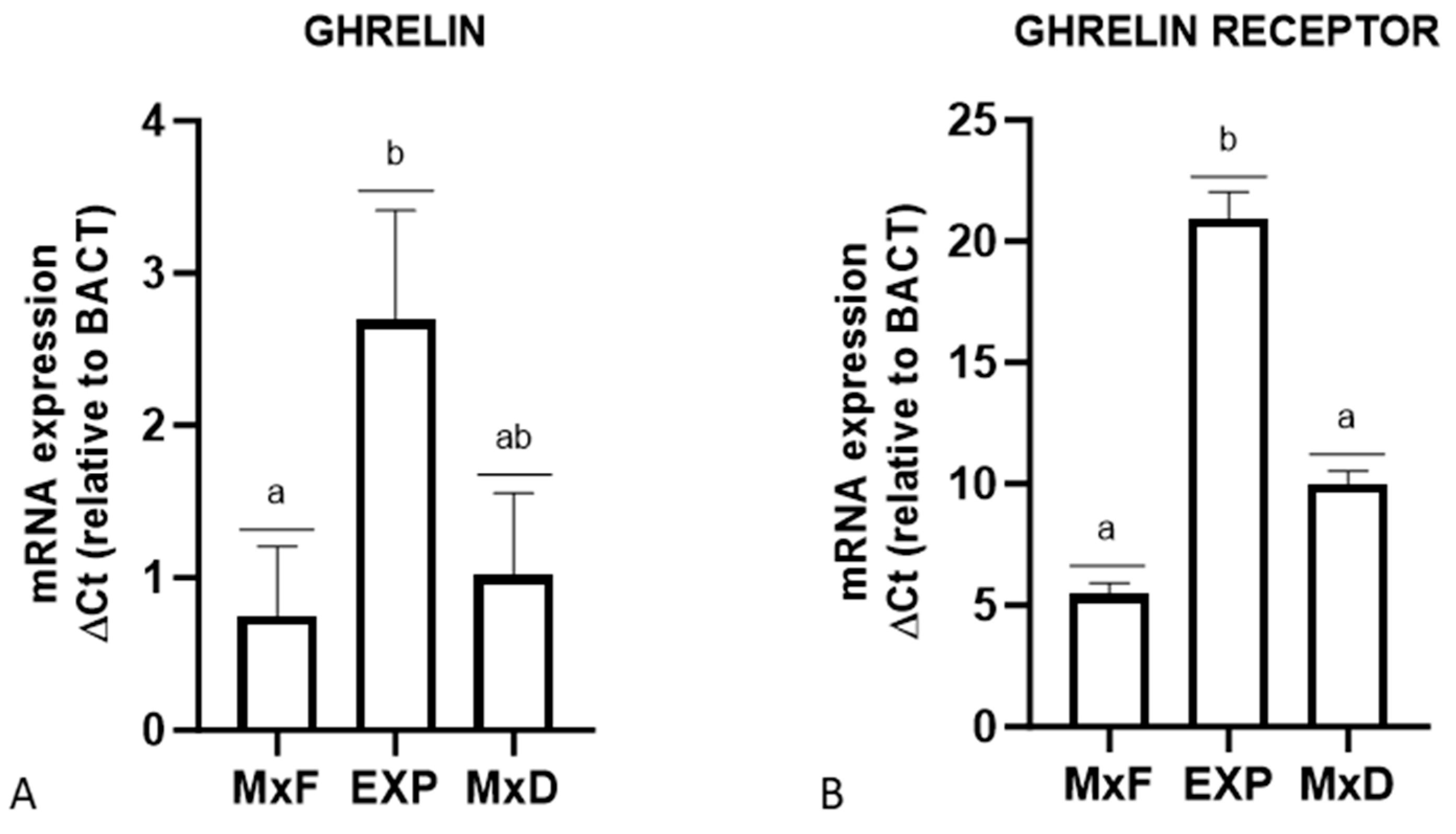
3.2. Real-Time PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Kojima, M.; Kangawa, K. Ghrelin: Structure and function. Physiol. Rev. 2005, 85, 495–522. [Google Scholar] [CrossRef]
- Date, Y.; Kojima, M.; Hosoda, H.; Sawaguchi, A.; Mondal, M.S.; Suganuma, T.; Matsukura, S.; Kangawa, K.; Nakazato, M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000, 141, 4255–4261. [Google Scholar] [CrossRef] [PubMed]
- Sakata, I.; Nakamura, K.; Yamazaki, M.; Matsubara, M.; Hayashi, Y.; Kangawa, K.; Sakai, T. Ghrelin-producing cells exist as two types of cells, closed- and opened-type cells, in the rat gastrointestinal tract. Peptides 2002, 23, 531–536. [Google Scholar] [CrossRef]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschöp, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Korbonits, M.; Bustin, S.A.; Kojima, M.; Jordan, S.; Adams, E.F.; Lowe, D.G.; Kangawa, K.; Grossman, A.B. The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J. Clin. Endocrinol. Metab. 2001, 86, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Korbonits, M.; Goldstone, A.P.; Gueorguiev, M.; Grossman, A.B. Ghrelin—A hormone with multiple functions. Front. Neuroendocrinol. 2004, 25, 27–68. [Google Scholar] [CrossRef]
- Schmid, D.A.; Held, K.; Ising, M.; Uhr, M.; Weikel, J.C.; Steiger, A. Ghrelin stimulates appetite, imagination of food, GH, ACTH, and cortisol, but does not affect leptin in normal controls. Neuropsychopharmacology 2005, 30, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Sugino, T.; Hasegawa, Y.; Kikkawa, Y.; Yamaura, J.; Yamagishi, M.; Kurose, Y.; Kojima, M.; Kangawa, K.; Terashima, Y. A transient ghrelin surge occurs just before feeding in a scheduled meal-fed sheep. Biochem. Biophys. Res. Commun. 2002, 295, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Shiiya, T.; Nakazato, M.; Mizuta, M.; Date, Y.; Mondal, M.S.; Tanaka, M.; Nozoe, S.; Hosoda, H.; Kangawa, K.; Matsukura, S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J. Clin. Endocrinol. Metab. 2002, 87, 240–244. [Google Scholar] [CrossRef]
- Szentirmai, E.; Hajdu, I.; Obal, F., Jr.; Krueger, J.M. Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Res. 2006, 1088, 131–140. [Google Scholar] [CrossRef]
- Lutter, M.; Sakata, I.; Osborne-Lawrence, S.; Rovinsky, S.A.; Anderson, J.G.; Jung, S.; Birnbaum, S.; Yanagisawa, M.; Elmquist, J.K.; Nestler, E.J.; et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat. Neurosci. 2008, 11, 752–753. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Saha, P.K.; Ma, X.; Henshaw, I.O.; Shao, L.; Chang, B.H.; Buras, E.D.; Tong, Q.; Chan, L.; McGuinness, O.P.; et al. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell. 2011, 10, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, N.; Moriya, J.; Yasumura, Y.; Uematsu, M.; Ono, F.; Shimizu, W.; Ueno, K.; Kitakaze, M.; Miyatake, K.; Kangawa, K. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation 2004, 110, 3674–3679. [Google Scholar] [CrossRef]
- Mathur, N.; Mehdi, S.F.; Anipindi, M.; Aziz, M.; Khan, S.A.; Kondakindi, H.; Lowell, B.; Wang, P.; Roth, J. Ghrelin as an Anti-Sepsis Peptide: Review. Front. Immunol. 2021, 11, 610363. [Google Scholar] [CrossRef]
- Howard, A.D.; Feighner, S.D.; Cully, D.F.; Arena, J.P.; Liberator, P.A.; Rosenblum, C.I.; Hamelin, M.; Hreniuk, D.L.; Palyha, O.C.; Anderson, J.; et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 1996, 273, 974–977. [Google Scholar] [CrossRef]
- Camiña, J.P. Cell biology of the ghrelin receptor. J. Neuroendocrinol. 2006, 18, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Koca, S.S.; Ozgen, M.; Sarikaya, M.; Dagli, F.; Ustundag, B.; Isik, A. Ghrelin prevents the development of dermal fibrosis in bleomycin-induced scleroderma. Clin. Exp. Dermatol. 2014, 39, 176–181. [Google Scholar] [CrossRef]
- Qu, R.; Chen, X.; Hu, J.; Fu, Y.; Peng, J.; Li, Y.; Chen, J.; Li, P.; Liu, L.; Cao, J.; et al. Ghrelin protects against contact dermatitis and psoriasiform skin inflammation by antagonizing TNF-α/NF-κB signaling pathways. Sci. Rep. 2019, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Zouboulis, C.C. Hormones and the pilosebaceous unit. Dermato-endocrinology 2009, 1, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Eurell, J.A.; Frappier, B.L. Dellmann’s Textbook of Veterinary Histology, 6th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013. [Google Scholar]
- Catorci, A.; Ottaviani, G.; Kosić, I.V.; Cesaretti, S. Effect of spatial and temporal patterns of stress and disturbance intensities in a sub-Mediterranean grassland. Plant Biosyst. 2011, 146, 352–367. [Google Scholar] [CrossRef]
- Mercati, F.; Maranesi, M.; Dall’Aglio, C.; Petrucci, L.; Pasquariello, R.; Tardella, F.M.; De Felice, E.; Scocco, P. Apelin System in Mammary Gland of Sheep Reared in Semi-Natural Pastures of the Central Apennines. Animals 2018, 8, E223. [Google Scholar] [CrossRef]
- Palmioli, E.; Dall’Aglio, C.; Fagotti, A.; Simoncelli, F.; Dobrzyn, K.; Di Rosa, I.; Maranesi, M.; De Felice, E.; Scocco, P.; Mercati, F. Leptin system is not affected by different diets in the abomasum of the sheep reared in semi-natural pastures of the Central Apennines. Ann. Anat. 2023, 247, 152069. [Google Scholar] [CrossRef]
- Palmioli, E.; Dall’Aglio, C.; Bellesi, M.; Tardella, F.M.; Moscatelli, S.; Scocco, P.; Mercati, F. The Apelinergic System Immuno-Detection in the Abomasum and Duodenum of Sheep Grazing on Semi-Natural Pasture. Animals 2021, 11, 3173. [Google Scholar] [CrossRef] [PubMed]
- Maranesi, M.; Di Loria, A.; Dall’Aglio, C.; Piantedosi, D.; Lepri, E.; Ciaramella, P.; Mercati, F. Leptin System in Obese Dog Skin: A Pilot Study. Animals 2020, 10, 2338. [Google Scholar] [CrossRef] [PubMed]
- Maranesi, M.; Palmioli, E.; Dall’Aglio, C.; Marini, D.; Anipchenko, P.; De Felice, E.; Scocco, P.; Mercati, F. Resistin in endocrine pancreas of sheep: Presence and expression related to different diets. Gen. Comp. Endocrinol. 2024, 348, 114452. [Google Scholar] [CrossRef]
- Zerani, M.; Catone, G.; Maranesi, M.; Gobbetti, A.; Boiti, C.; Parillo, F. Gonadotropin-releasing hormone 1 directly affects corpora lutea lifespan in Mediterranean buffalo (Bubalus bubalis) during diestrus: Presence and in vitro effects on enzymatic and hormonal activities. Biol. Reprod. 2012, 87, 45. [Google Scholar] [CrossRef]
- Maranesi, M.; Petrucci, L.; Leonardi, L.; Bufalari, A.; Parillo, F.; Boiti, C.; Zerani, M. Kisspeptin/kisspeptin receptor system in pseudopregnant rabbit corpora lutea: Presence and function. Sci. Rep. 2019, 9, 5044. [Google Scholar] [CrossRef] [PubMed]
- Maranesi, M.; Dall’Aglio, C.; Acuti, G.; Cappelli, K.; Trabalza Marinucci, M.; Galarini, R.; Suvieri, C.; Zerani, M. Effects of Dietary Polyphenols from Olive Mill Waste Waters on Inflammatory and Apoptotic Effectors in Rabbit Ovary. Animals 2021, 11, 1727. [Google Scholar] [CrossRef] [PubMed]
- Ng’ambi, J.W.; Tyasi, T.L. A Review of Studies on Improvement of Sheep Resilience to Climate Change Stresses. In Sheep Farming—Sustainability From Traditional to Precision Production; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Grandini, M.; Medici, M.; Canavari, M.; Palmioli, E.; Mercati, F.; Catorci, A.; Scocco, P. Consumer Liking and Value Perception of Mountain Cheese from Different Pasture Periods: Evidence for Mountain Systems Supporting Policies. Mt. Res. Dev. 2022, 42, R1–R7. [Google Scholar] [CrossRef]
- Scocco, P.; Piermarteri, K.; Malfatti, A.; Tardella, F.M.; Catorci, A. Effects of summer rainfall variations on sheep body state and farming sustainability in sub-mediterranean pastoral system. Span. J. Agric. Res. 2016, 14, 8. [Google Scholar] [CrossRef]
- Scocco, P.; Mercati, F.; Tardella, F.M.; Catorci, A. Increase of forage dryness induces differentiated anatomical response in the sheep rumen compartments. Microsc. Res. Tech. 2016, 79, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Cicek, D.; Demir, B.; Erder, I.; Kuloglu, T.; Ucer, O.; Aydin, S.; Ucak, H.; Dertlioglu, S.; Kalayci, M. Ghrelin in the pilosebaceous unit: Alteration of ghrelin in patients with acne vulgaris. Eur. J. Dermatol. 2015, 25, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Gnanapavan, S.; Kola, B.; Bustin, S.A.; Morris, D.G.; McGee, P.; Fairclough, P.; Bhattacharya, S.; Carpenter, R.; Grossman, A.B.; Korbonits, M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 2002, 87, 2988. [Google Scholar] [CrossRef]
- Oshima, H.; Rochat, A.; Kedzia, C.; Kobayashi, K.; Barrandon, Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 2001, 104, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Sahin, I.; Ozkan, Y.; Dag, E.; Gunay, A.; Guzel, S.P.; Catak, Z.; Ozercan, M.R. Examination of the tissue ghrelin expression of rats with diet-induced obesity using radioimmunoassay and immunohistochemical methods. Mol. Cell Biochem. 2012, 365, 165–173. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.; Li, H.; Yang, Z.; Zeng, Y.; Liu, J.; Hao, Y.; Li, R. Ghrelin accelerates wound healing through GHS-R1a-mediated MAPK-NF-κB/GR signaling pathways in combined radiation and burn injury in rats. Sci. Rep. 2016, 6, 27499. [Google Scholar] [CrossRef]
- Delhanty, P.J.; van der Eerden, B.C.; van der Velde, M.; Gauna, C.; Pols, H.A.; Jahr, H.; Chiba, H.; van der Lely, A.J.; van Leeuwen, J.P. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J. Endocrinol. 2006, 188, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Andreis, P.G.; Malendowicz, L.K.; Trejter, M.; Neri, G.; Spinazzi, R.; Rossi, G.P.; Nussdorfer, G.G. Ghrelin and growth hormone secretagogue receptor are expressed in the rat adrenal cortex: Evidence that ghrelin stimulates the growth, but not the secretory activity of adrenal cells. FEBS Lett. 2003, 536, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, I.; Muccioli, G.; Granata, R.; Deghenghi, R.; Ghigo, E.; Ohlsson, C.; Isgaard, J. Natural (ghrelin) and synthetic (hexarelin) GH secretagogues stimulate H9c2 cardiomyocyte cell proliferation. J. Endocrinol. 2002, 175, 201–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jeffery, P.L.; Herington, A.C.; Chopin, L.K. Expression and action of the growth hormone releasing peptide ghrelin and its receptor in prostate cancer cell lines. J. Endocrinol. 2002, 172, R7–R11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murata, M.; Okimura, Y.; Iida, K.; Matsumoto, M.; Sowa, H.; Kaji, H.; Kojima, M.; Kangawa, K.; Chihara, K. Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. J. Biol. Chem. 2002, 277, 5667–5674. [Google Scholar] [CrossRef] [PubMed]
- Lien, G.S.; Lin, C.H.; Yang, Y.L.; Wu, M.S.; Chen, B.C. Ghrelin induces colon cancer cell proliferation through the GHS-R, Ras, PI3K, Akt, and mTOR signaling pathways. Eur. J. Pharmacol. 2016, 776, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Lago, F.; Dieguez, C.; Gómez-Reino, J.; Gualillo, O. Adipokines as emerging mediators of immune response and inflammation. Nat. Clin. Pract. Rheumatol. 2007, 3, 716–724. [Google Scholar] [CrossRef]
- Mercati, F.; Scocco, P.; Maranesi, M.; Acuti, G.; Petrucci, L.; Cocci, P.; Renzi, A.; De Felice, E.; Dall’Aglio, C. Apelin system detection in the reproductive apparatus of ewes grazing on semi-natural pasture. Theriogenology 2019, 139, 156–166. [Google Scholar] [CrossRef]
- Mercati, F.; Dall’Aglio, C.; Timperi, L.; Scocco, P.; De Felice, E.; Maranesi, M. Epithelial expression of the hormone leptin by bovine skin. Eur. J. Histochem. 2019, 63, 2993. [Google Scholar] [CrossRef]
- Rozza-de-Menezes, R.E.; Gaglionone, N.C.; Andrade-Losso, R.M.; Siqueira, O.H.K.; Almeida, L.M.; Peruzini, K.D.S.; Guimarães-Filho, M.A.C.; Brum, C.I.; Geller, M.; Cunha, K.S. Receptor of ghrelin is expressed in cutaneous neurofibromas of individuals with neurofibromatosis 1. Orphanet. J. Rare Dis. 2017, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Dall’Aglio, C.; Maranesi, M.; Di Loria, A.; Piantedosi, D.; Ciaramella, P.; Alterisio, M.C.; Lepri, E.; Mercati, F. Effects of Obesity on Adiponectin System Skin Expression in Dogs: A Comparative Study. Animals 2021, 11, 2308. [Google Scholar] [CrossRef]
- Rocha-Sousa, A.; Saraiva, J.; Henriques-Coelho, T.; Falcão-Reis, F.; Correia-Pinto, J.; Leite-Moreira, A.F. Ghrelin as a novel locally produced relaxing peptide of the iris sphincter and dilator muscles. Exp. Eye Res. 2006, 83, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Montégut, L.; Lopez-Otin, C.; Magnan, C.; Kroemer, G. Old Paradoxes and New Opportunities for Appetite Control in Obesity. Trends Endocrinol. Metab. 2021, 32, 264–294. [Google Scholar] [CrossRef]
- Allain, D.; Renieri, C. Genetics of fibre production and fleece characteristics in small ruminants, Angora rabbit and South American camelids. Animal 2010, 4, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.A.; Tucker, D.J. Effects of nutrition and origin on the amino acid, grease, and suint composition and color of cashmere and guard hairs. J. Appl. Polym. Sci. 2010, 117, 409–420. [Google Scholar] [CrossRef]
- Trüeb, R.M. The impact of oxidative stress on hair. Int. J. Cosmet. Sci. 2015, 37 (Suppl. S2), 25–30. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, H.; Mohri, Y.; Grachtchouk, M.; Asakawa, K.; Matsumura, H.; Oshima, M.; Takayama, N.; Kato, T.; Nishimori, Y.; Sorimachi, Y.; et al. Obesity accelerates hair thinning by stem cell-centric converging mechanisms. Nature 2021, 595, 266–271. [Google Scholar] [CrossRef]
| Gene | Primers | bp | |
|---|---|---|---|
| GhRL | F | GGAACCTAAGAAGCCGTCAGG | 105 |
| R | ATTTCCAGCTCGTCCTCTGC | ||
| GHS-R | F | CCATCTTCATGCTGGTCGGA | 131 |
| R | GAAGATGCTGGACACCCACA | ||
| ACTB | F | CCTTAGCAACCATGCTGTGA | 130 |
| R | AAGCTGGTGCAGGTAGAGGA |
| Skin Appendage | Tested Molecule | MxF | Exp | MxD | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Hair follicles | GhRL | 2.19 | 0.63 | 2.85 | 0.39 | 2.43 | 0.68 |
| GHS-R | 1.71 | 0.78 | 2.59 | 0.53 | 2.23 | 0.73 | |
| Sweat glands | GhRL | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| GHS-R | 1.54 | 0.75 | 2.49 | 0.6 | 2.19 | 0.78 | |
| Comparison | Z | P. Unadjusted | P. Adjusted (Holm) |
|---|---|---|---|
| Exp-MxD | 4.13866414 | 3.49 × 10−5 | <0.001 *** |
| Exp-MxF | 6.789895389 | 1.12 × 10−11 | <0.001 *** |
| MxD-MxF | 2.651231249 | 0.008019891 | 0.0080 ** |
| Comparison | Difference | Lower | Upper | p-Value |
|---|---|---|---|---|
| MxD-Exp | −0.36 | −0.62615 | −0.09385 | 0.0045 ** |
| MxF-Exp | −0.87333 | −1.13948 | −0.60718 | <0.001 *** |
| MxF-MxD | −0.51333 | −0.77948 | −0.24718 | <0.001 *** |
| Comparison | Difference | Lower | Upper | p-Value |
|---|---|---|---|---|
| MxD-Exp | −0.30667 | −0.58158 | −0.03175 | 0.02455 * |
| MxF-Exp | −0.95333 | −1.22825 | −0.67842 | <0.001 *** |
| MxF-MxD | −0.64667 | −0.92158 | −0.37175 | <0.001 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maranesi, M.; Dall’Aglio, C.; Moscatelli, S.; Palmioli, E.; Coliolo, P.; Marini, D.; Guelfi, G.; Scocco, P.; Mercati, F. Diet Supplementation Influences Ghrelin System Expression in the Skin Appendages of the Sheep. Vet. Sci. 2025, 12, 41. https://doi.org/10.3390/vetsci12010041
Maranesi M, Dall’Aglio C, Moscatelli S, Palmioli E, Coliolo P, Marini D, Guelfi G, Scocco P, Mercati F. Diet Supplementation Influences Ghrelin System Expression in the Skin Appendages of the Sheep. Veterinary Sciences. 2025; 12(1):41. https://doi.org/10.3390/vetsci12010041
Chicago/Turabian StyleMaranesi, Margherita, Cecilia Dall’Aglio, Sara Moscatelli, Elisa Palmioli, Paola Coliolo, Daniele Marini, Gabriella Guelfi, Paola Scocco, and Francesca Mercati. 2025. "Diet Supplementation Influences Ghrelin System Expression in the Skin Appendages of the Sheep" Veterinary Sciences 12, no. 1: 41. https://doi.org/10.3390/vetsci12010041
APA StyleMaranesi, M., Dall’Aglio, C., Moscatelli, S., Palmioli, E., Coliolo, P., Marini, D., Guelfi, G., Scocco, P., & Mercati, F. (2025). Diet Supplementation Influences Ghrelin System Expression in the Skin Appendages of the Sheep. Veterinary Sciences, 12(1), 41. https://doi.org/10.3390/vetsci12010041










