Application of Principal Component Analysis as a Prediction Model for Feline Sporotrichosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, A.M.; Gonçalves, S.S.; De Carvalho, J.A.; Borba-Santos, L.P.; Rozental, S.; Camargo, Z.P.D. Current Progress on Epidemiology, Diagnosis, and Treatment of Sporotrichosis and Their Future Trends. J. Fungi 2022, 8, 776. [Google Scholar] [CrossRef] [PubMed]
- Orofino-Costa, R.; Macedo, P.M.D.; Rodrigues, A.M.; Bernardes-Engemann, A.R. Sporotrichosis: An Update on Epidemiology, Etiopathogenesis, Laboratory and Clinical Therapeutics. An. Bras. Dermatol. 2017, 92, 606–620. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.T.; Nascimento, L.F.D.J.; Barbosa, A.A.T.; Martins, M.P.; Tunon, G.I.L.; Santos, P.O.M.; Dantas-Torres, F.; Dolabella, S.S. The Rising Incidence of Feline and Cat-transmitted Sporotrichosis in Latin America. Zoonoses Public Health 2024, 71, 609–619. [Google Scholar] [CrossRef]
- Gremião, I.D.F.; Menezes, R.C.; Schubach, T.M.P.; Figueiredo, A.B.F.; Cavalcanti, M.C.H.; Pereira, S.A. Feline Sporotrichosis: Epidemiological and Clinical Aspects. Med. Mycol. 2015, 53, 15–21. [Google Scholar] [CrossRef]
- Gremião, I.D.F.; Oliveira, M.M.E.; Monteiro De Miranda, L.H.; Saraiva Freitas, D.F.; Pereira, S.A. Geographic Expansion of Sporotrichosis, Brazil. Emerg. Infect. Dis. 2020, 26, 621–624. [Google Scholar] [CrossRef]
- Thomson, P.; González, C.; Blank, O.; Ramírez, V.; Río, C.D.; Santibáñez, S.; Pena, P. Sporotrichosis Outbreak Due to Sporothrix brasiliensis in Domestic Cats in Magallanes, Chile: A One-Health-Approach Study. J. Fungi 2023, 9, 226. [Google Scholar] [CrossRef]
- Etchecopaz, A.; Toscanini, M.A.; Gisbert, A.; Mas, J.; Scarpa, M.; Iovannitti, C.A.; Bendezú, K.; Nusblat, A.D.; Iachini, R.; Cuestas, M.L. Sporothrix brasiliensis: A Review of an Emerging South American Fungal Pathogen, Its Related Disease, Presentation and Spread in Argentina. J. Fungi 2021, 7, 170. [Google Scholar] [CrossRef]
- De Oliveira, P.R.F.; De Carvalho, J.A.; Costa, T.R.; Silva, B.P.E.; Da Silva, G.G.; Rodrigues, A.M.; Mota, R.A. Emerging Cases of Cat-Transmitted Sporotrichosis Driven by Sporothrix brasiliensis in Northeast Brazil. Mycopathologia 2024, 189, 66. [Google Scholar] [CrossRef]
- Rabello, V.B.S.; Almeida, M.A.; Bernardes-Engemann, A.R.; Almeida-Paes, R.; De Macedo, P.M.; Zancopé-Oliveira, R.M. The Historical Burden of Sporotrichosis in Brazil: A Systematic Review of Cases Reported from 1907 to 2020. Braz. J. Microbiol. 2022, 53, 231–244. [Google Scholar] [CrossRef]
- Miranda, L.H.M.; Santiago, M.D.A.; Schubach, T.M.P.; Morgado, F.N.; Pereira, S.A.; Oliveira, R.D.V.C.D.; Conceição-Silva, F. Severe Feline Sporotrichosis Associated with an Increased Population of CD8low Cells and a Decrease in CD4+ Cells. Med. Mycol. 2015, 54, 29–39. [Google Scholar] [CrossRef]
- Gremião, I.D.F.; Miranda, L.H.M.; Reis, E.G.; Rodrigues, A.M.; Pereira, S.A. Zoonotic Epidemic of Sporotrichosis: Cat to Human Transmission. PLoS Pathog. 2017, 13, e1006077. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, L.H.M.; Silva, J.N.; Gremião, I.D.F.; Menezes, R.C.; Almeida-Paes, R.; Dos Reis, É.G.; De Oliveira, R.D.V.C.; De Araujo, D.S.D.A.; Ferreiro, L.; Pereira, S.A. Monitoring Fungal Burden and Viability of Sporothrix spp. in Skin Lesions of Cats for Predicting Antifungal Treatment Response. J. Fungi 2018, 4, 92. [Google Scholar] [CrossRef] [PubMed]
- Gremião, I.D.F.; Rocha, E.M.d.S.d.; Montenegro, H.; Carneiro, A.J.B.; Xavier, M.O.; de Farias, M.R.; Monti, F.; Mansho, W.; Pereira, R.H.d.M.A.; Pereira, S.A.; et al. Guideline for the Management of Feline Sporotrichosis Caused by Sporothrix brasiliensis and Literature Revision. Braz. J. Microbiol. 2021, 52, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Azevedo, M.I.; Amaral, C.I.; Grom, N.A.; Marinho, F.; de Oliveira, C.S.F.; Soares, D.F.d.M.; Morais, M.H.F.; Brandão, S.T.; Menezes, R.C.; et al. Feline Sporotrichosis: Characterization of Cutaneous and Extracutaneous Lesions Using Different Diagnostic Methods. Vet. Pathol. 2024, 61, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Schubach, T.M.P.; Schubach, A.; Okamoto, T.; Barros, M.B.L.; Figueiredo, F.B.; Cuzzi, T.; Fialho-Monteiro, P.C.; Reis, R.S.; Perez, M.A.; Wanke, B. Evaluation of an Epidemic of Sporotrichosis in Cats: 347 Cases (1998–2001). J. Am. Vet. Med. Assoc. 2004, 224, 1623–1629. [Google Scholar] [CrossRef]
- Maschio-Lima, T.; Marques, M.D.R.; Lemes, T.H.; Brizzotti-Mazuchi, N.S.; Caetano, M.H.; De Almeida, B.G.; Bianco, L.M.; Monteiro, R.C.; Rodrigues, A.M.; De Camargo, Z.P.; et al. Clinical and Epidemiological Aspects of Feline Sporotrichosis Caused by Sporothrix brasiliensis and in vitro Antifungal Susceptibility. Vet. Res. Commun. 2021, 45, 171–179. [Google Scholar] [CrossRef]
- Reis, B.D.; Cobucci, F.O.; Zacaron, L.H.; D’Acri, A.M.; Lima, R.B.; Martins, C.J. Sporotrichosis in an Unusual Location—Case Report. An. Bras. Dermatol. 2015, 90 (Suppl. S1), 84–87. [Google Scholar] [CrossRef]
- Lopes-Bezerra, L.M.; Schubach, A.; Costa, R.O. Sporothrix schenckii and Sporotrichosis. An. Acad. Bras. Ciências 2006, 78, 293–308. [Google Scholar] [CrossRef]
- de Groot, T.; Puts, Y.; Berrio, I.; Chowdhary, A.; Meis, J.F. Development of Candida auris short tandem repeat typing and its application to a global collection of isolates. mBio 2020, 11, e02971-19. [Google Scholar] [CrossRef]
- Hong, S.B.; Cho, H.S.; Shin, H.D.; Frisvad, J.C.; Samson, R.A. Novel Neosartorya species isolated from soil in Korea. Int. J. Syst. Evol. Microbiol. 2006, 56, 477–486. [Google Scholar] [CrossRef]
- Zhang, Y.; Hagen, F.; Stielow, B.; Rodrigues, A.M.; Samerpitak, K.; Zhou, X.; Feng, P.; Yang, L.; Chen, M.; Deng, S.; et al. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14 000 human and animal case reports. Persoonia 2015, 35, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with burrows-Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- Skinner, M.E.; Uzilov, A.V.; Stein, L.D.; Mungall, C.J.; Holmes, I.H. JBrowse: A next-generation genome browser. Genome Res. 2009, 19, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.E.; Camus, M.S.; Freeman, K.P.; Giori, L.; Hooijberg, E.H.; Jeffery, U.; Korchia, J.; Meindel, M.J.; Moore, A.R.; Sisson, S.C.; et al. ASVCP Guidelines: Principles of Quality Assurance and Standards for Veterinary Clinical Pathology (Version 3.0): Developed by the American Society for Veterinary Clinical Pathology’s (ASVCP) Quality Assurance and Laboratory Standards (QALS) Committee. Vet. Clin. Pathol. 2019, 48, 542–618. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.C. Schalm’s Veterinary Hematology, 4th ed.; Lea and Febiger: Philadelphia, PA, USA, 1986; pp. 126–139. [Google Scholar]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M. Clinical Biochemistry of Domestic Animals, 6th ed.; Academic Press: Amsterdam, The Netherlands; Elsevier: Boston, MA, USA, 2008. [Google Scholar]
- Boechat, J.S.; Oliveira, M.M.E.; Gremião, I.D.F.; Almeida-Paes, R.; Machado, A.C.D.S.; Zancopé-Oliveira, R.M.; Oliveira, R.D.V.C.; Morgado, D.S.; Corrêa, M.L.; Figueiredo, A.B.F.; et al. Sporothrix brasiliensis and Feline Sporotrichosis in the Metropolitan Region of Rio de Janeiro, Brazil (1998–2018). J. Fungi 2022, 8, 749. [Google Scholar] [CrossRef]
- Alves, R.C.; Soares, Y.G.S.; Costa, D.F.L.; Firmino, M.O.; Brito Junior, J.R.C.; Souza, A.P.; Galiza, G.J.N.; Dantas, A.F.M. Fungal Diseases in Dogs and Cats in Northeastern Brazil. Pesqui. Veterinária Bras. 2023, 43, e07169. [Google Scholar] [CrossRef]
- De Miranda, L.H.M.; Santiago, M.D.A.; Frankenfeld, J.; Reis, E.G.D.; Menezes, R.C.; Pereira, S.A.; Gremião, I.D.F.; Hofmann-Lehmann, R.; Conceição-Silva, F. Neutrophil Oxidative Burst Profile Is Related to a Satisfactory Response to Itraconazole and Clinical Cure in Feline Sporotrichosis. J. Fungi 2024, 10, 422. [Google Scholar] [CrossRef]
- Romani, L. Immunity to Fungal Infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef]
- Carlos, I.Z.; Sassá, M.F.; Da Graça Sgarbi, D.B.; Placeres, M.C.P.; Maia, D.C.G. Current Research on the Immune Response to Experimental Sporotrichosis. Mycopathologia 2009, 168, 1–10. [Google Scholar] [CrossRef]
- Maia, D.C.G.; Sassá, M.F.; Placeres, M.C.P.; Carlos, I.Z. Influence of Th1/Th2 Cytokines and Nitric Oxide in Murine Systemic Infection Induced by Sporothrix schenckii. Mycopathologia 2006, 161, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Uenotsuchi, T.; Takeuchi, S.; Matsuda, T.; Urabe, K.; Koga, T.; Uchi, H.; Nakahara, T.; Fukagawa, S.; Kawasaki, M.; Kajiwara, H.; et al. Differential Induction of Th1-Prone Immunity by Human Dendritic Cells Activated with Sporothrix schenckii of Cutaneous and Visceral Origins to Determine Their Different Virulence. Int. Immunol. 2006, 18, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Horwath, M.C.; Fecher, R.A.; Deepe, G.S. Histoplasma capsulatum, Lung Infection and Immunity. Future Microbiol. 2015, 10, 967–975. [Google Scholar] [CrossRef] [PubMed]
- De Souza, E.W.; Borba, C.D.M.; Pereira, S.A.; Gremião, I.D.F.; Langohr, I.M.; Oliveira, M.M.E.; De Oliveira, R.D.V.C.; Da Cunha, C.R.; Zancopé-Oliveira, R.M.; De Miranda, L.H.M.; et al. Clinical Features, Fungal Load, Coinfections, Histological Skin Changes, and Itraconazole Treatment Response of Cats with Sporotrichosis Caused by Sporothrix brasiliensis. Sci. Rep. 2018, 8, 9074. [Google Scholar] [CrossRef] [PubMed]
- Van De Veerdonk, F.L.; Netea, M.G. T-Cell Subsets and Antifungal Host Defenses. Curr. Fungal Infect. Rep. 2010, 4, 238–243. [Google Scholar] [CrossRef]
- Tizard, I.R. Veterinary Immunology, 9th ed.; Elsevier Health Sciences: London, UK, 2013. [Google Scholar]
- Cerón, J.J.; Eckersall, P.D.; Martínez-Subiela, S. Acute Phase Proteins in Dogs and Cats: Current Knowledge and Future Perspectives. Vet. Clin. Pathol. 2005, 34, 85–99. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Fernandes, G.F.; Araujo, L.M.; Della Terra, P.P.; Dos Santos, P.O.; Pereira, S.A.; Schubach, T.M.P.; Burger, E.; Lopes-Bezerra, L.M.; De Camargo, Z.P. Proteomics-Based Characterization of the Humoral Immune Response in Sporotrichosis: Toward Discovery of Potential Diagnostic and Vaccine Antigens. PLoS Negl. Trop. Dis. 2015, 9, e0004016. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Romero-Romero, L.; Govezensky, T.; Rosales, C. Neutrophil to Lymphocyte Ratio and Principal Component Analysis Offer Prognostic Advantage for Dogs with Mammary Tumors. Front. Vet. Sci. 2023, 10, 1187271. [Google Scholar] [CrossRef]
- Dunbar, D.; Babayan, S.A.; Krumrie, S.; Haining, H.; Hosie, M.J.; Weir, W. Assessing the Feasibility of Applying Machine Learning to Diagnosing Non-Effusive Feline Infectious Peritonitis. Sci. Rep. 2024, 14, 2517. [Google Scholar] [CrossRef]
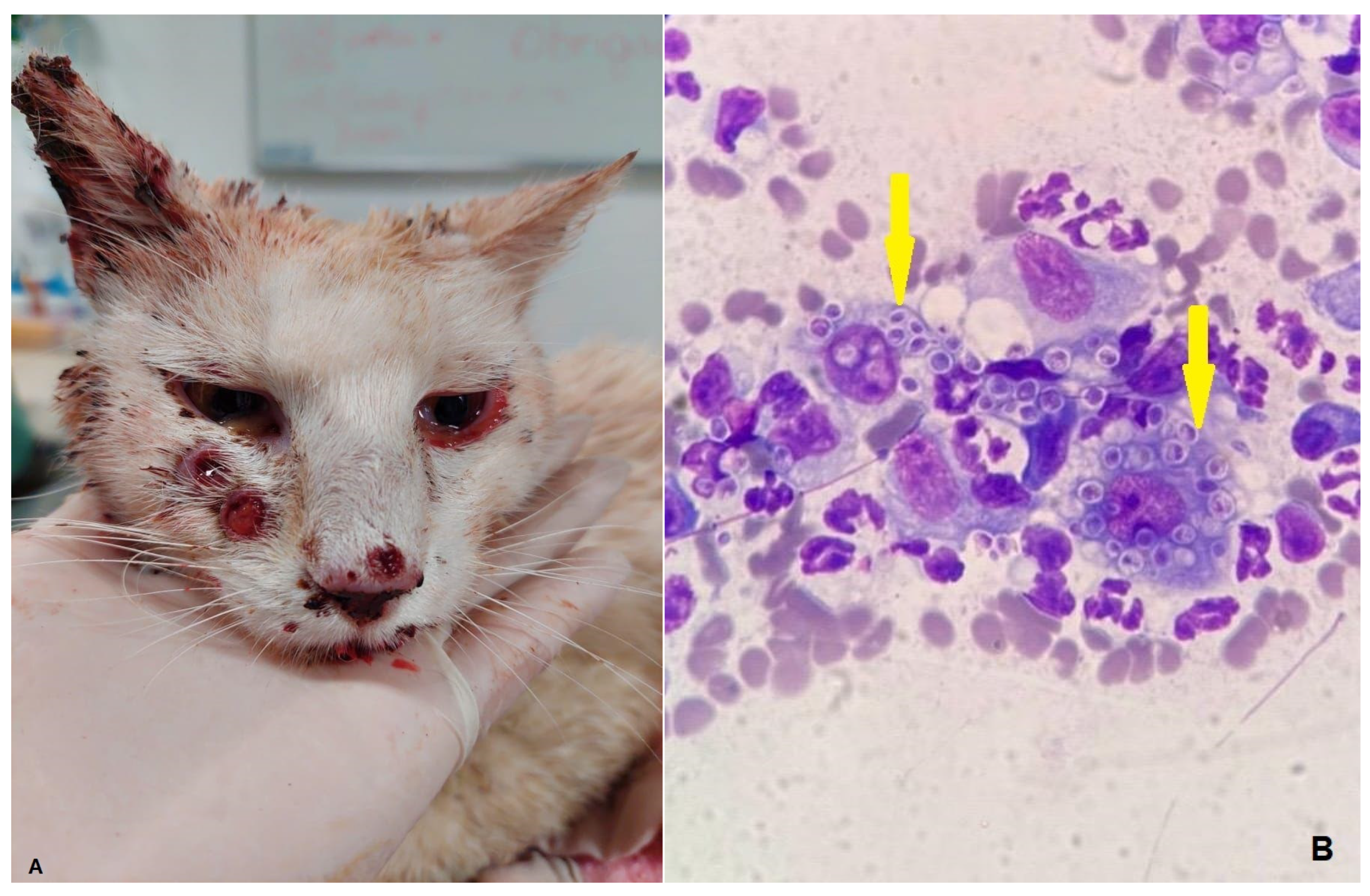
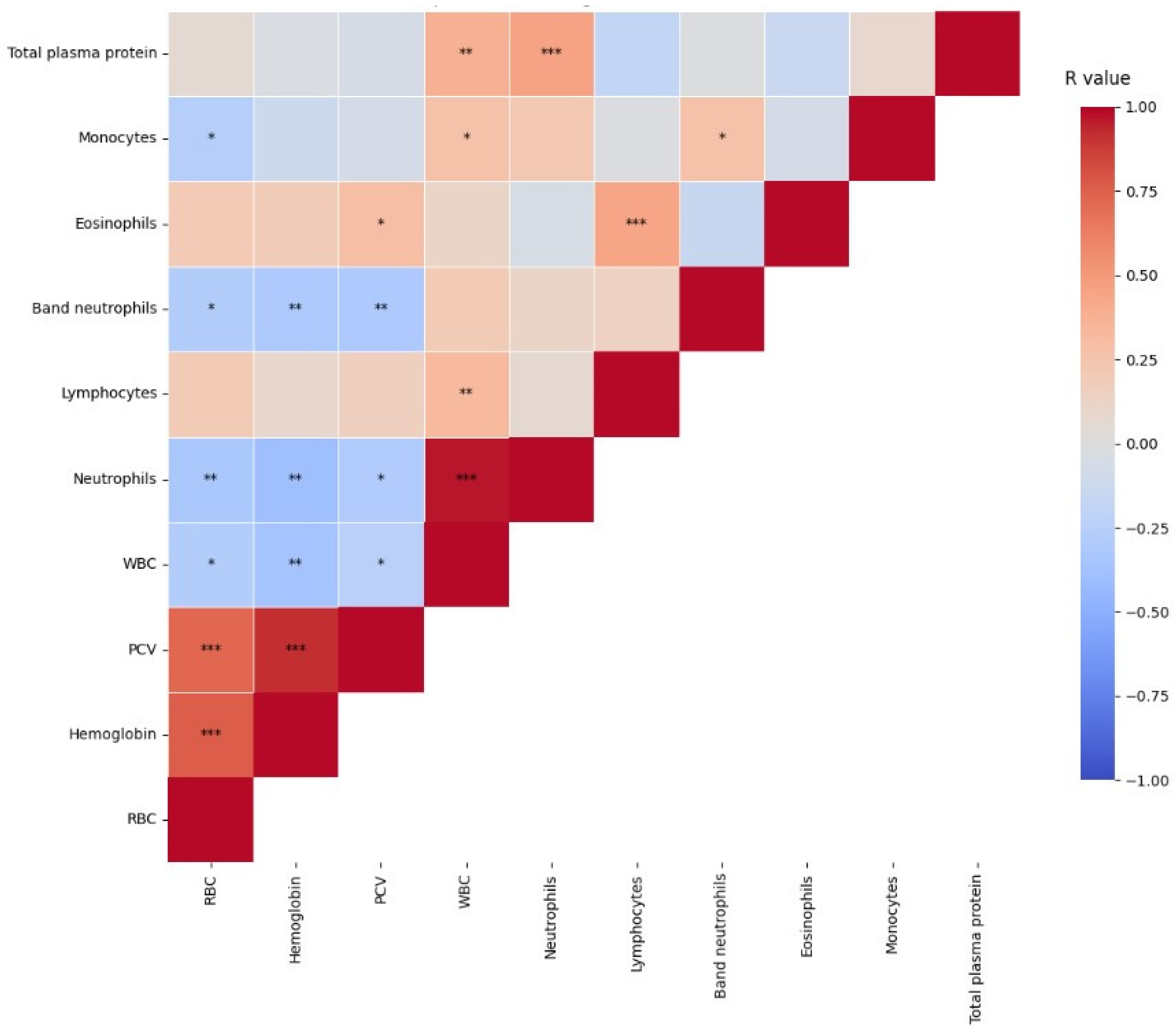
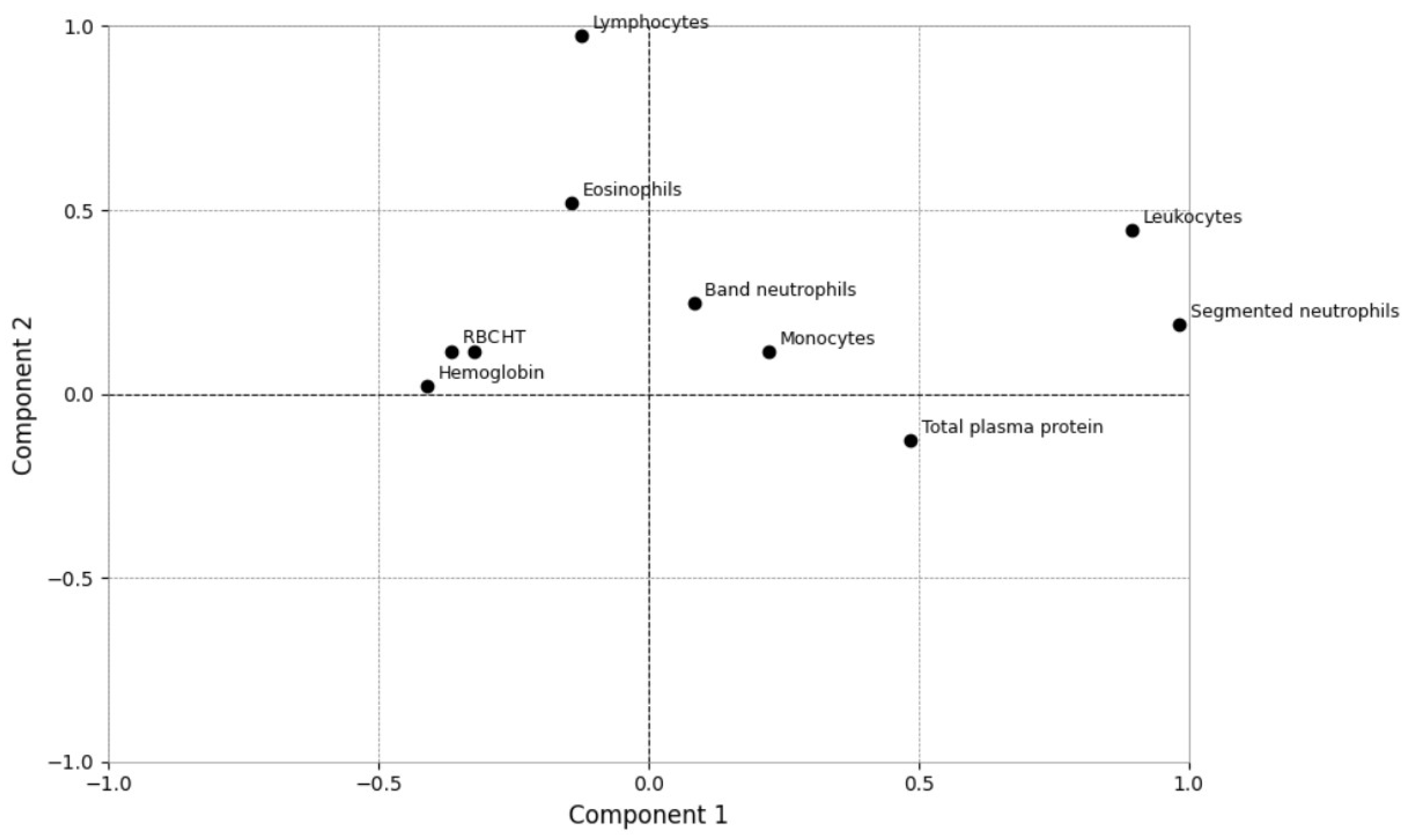
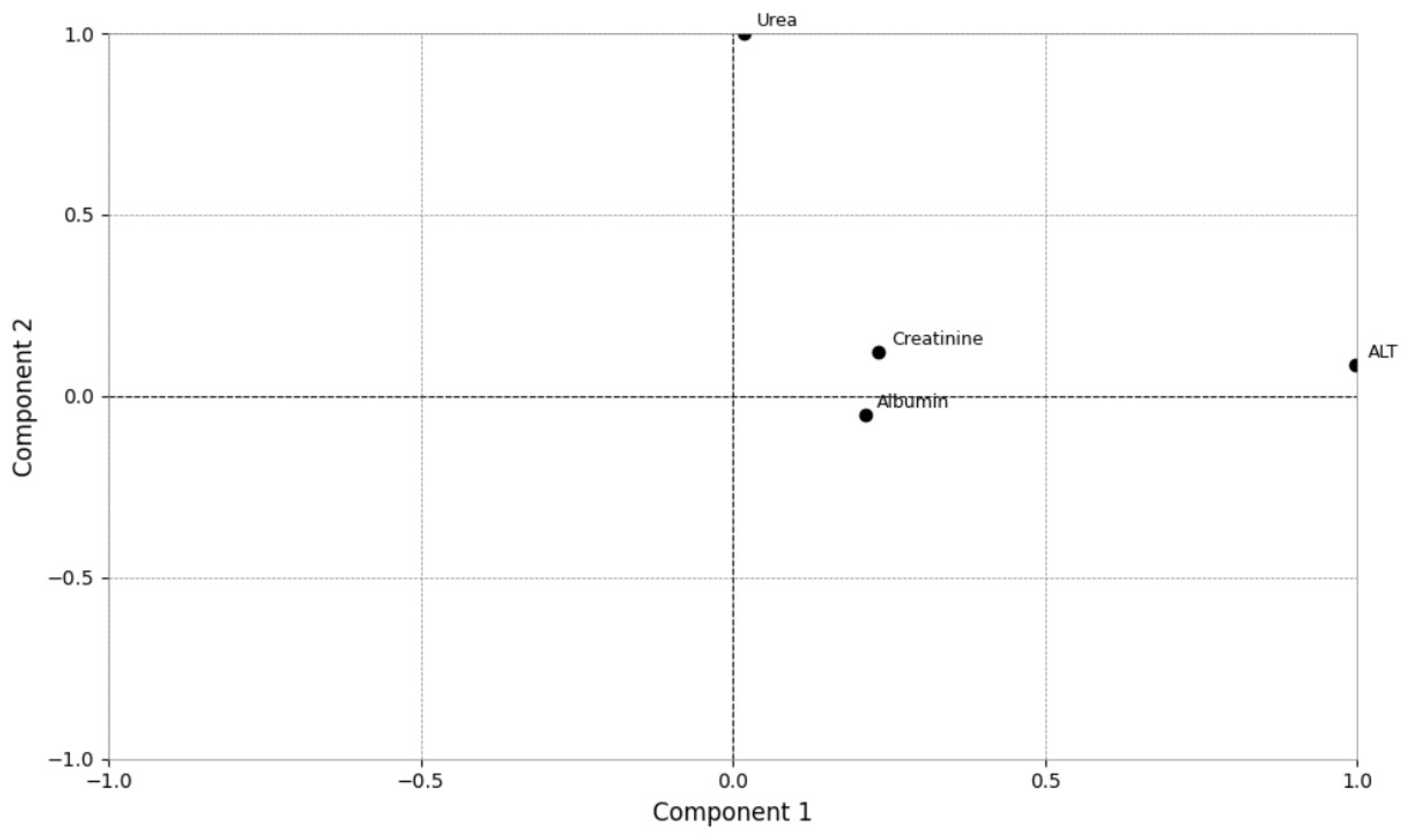
| Analytes | Group 1 (n = 13) Median (Min–Max) [25–75%] | Group 2 (n = 57) Median (Min–Max) [25–75%] | p Value | RI [20] |
|---|---|---|---|---|
| RBC (106/µL) | 7.87 (5.08–9.88) [6.68–9.45] | 7.42 (3.65–12.3) [6.10–8.86] | 0.370 | 5–10 |
| Hemoglobin (g/L) | 127 (100–166) [109–140] | 117 (64–175) [89–131] | 0.063 | 80–150 |
| Hematocrit (%) | 40 (28–45) [32.5–41.7] | 35.5 (19–48) [29.7–40] | 0.149 | 30–45 |
| WBC (/µL) | 11,150 (6100–22,600) [7288–15,638] | 14,675 (4250–46,200) [9825–20,975] | 0.128 | 5500–19,500 |
| Segmented Neutrophils (/µL) | 5931 (2869–13,728) [3616–9042] | 9923 (2805–44,352) [5319–12,360] | 0.027 | 2500–12,500 |
| Band neutrophils (/µL) | 30.5 (0–624) [0–146.3] | 65 (0–3048) [0–541] | 0.329 | 0–300 |
| Lymphocytes (/µL) | 3445 (1761–9270) [2372–4837] | 3,194 (408–9168) [1775–4842] | 0.347 | 1500–70,000 |
| Eosinophils (/µL) | 723 (176–1390) [543–1081] | 433 (0–3850) [216–733] | 0.032 | 100–1500 |
| Monocytes (/µL) | 228 (0–1408) [61.7–626.3] | 395.5 (0–1899) [133–875] | 0.247 | 100–1400 |
| Platelets (103/µL) | 300 (240–516) [249–401] | 337 (195–860) [283–459] | 0.24 | 300–800 |
| Total plasma protein (g/L) | 71 (64–82) [65–79] | 78 (62–120) [74–84] | 0.019 | 60–80 |
| Analytes | Group 1 (n = 13) Median (Min–Max) [25–75%] | Group 2 (n = 57) Median (Min–Max) [25–75%] | p Value | RI [21] |
|---|---|---|---|---|
| Total serum protein * (g/L) | 75 (63–76) [63–76] | 76 (62–86) [67–81] | 0.558 | 60–80 |
| Albumin (g/L) | 26.9 (15.2–31.5) [16.9–30.6] | 26 (9.1–33.9) [18.11–28.7] | 0.532 | 28–39 |
| Creatinine (µmol/L) | 116.6 (99–135.2) [107.8–121.1] | 102.5 (30.9–164.4) [86.6–121.1] | 0.053 | 70–177 |
| Urea (mmol/L) | 8.79 (6.62–11.2) [7.72–10.4] | 7.70 (1.64–16.5) [6.82–9.27] | 0.171 | 3.6–10.7 |
| ALT (U/L) | 54.2 (5.0–79.2) [30.0–74.0] | 29.6 (1.3–216.0) [11.7–57.0] | 0.325 | 10–100 |
| GGT (U/L) | 2.0 (1.3–3.9) [1.5–2.8] | 2.0 (0.7–9.6) [2–3.2] | 0.267 | 0–6 |
| Analytes | B | S.E. | Wald | df | Sig. | Exp (B) | 95% C.I. for EXP (B) | |
|---|---|---|---|---|---|---|---|---|
| Inferior | Superior | |||||||
| Urea | 0.037 | 0.011 | 12.190 | 1.000 | 0.000 | 1.038 | 1.016 | 1.060 |
| Total plasma protein | 1.201 | 0.509 | 5.562 | 1.000 | 0.018 | 3.322 | 1.225 | 9.012 |
| Constant | 7.789 | 3.786 | 4.234 | 1.000 | 0.040 | 0.000 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pegoraro, F.B.; Mangrich-Rocha, R.M.V.; Weber, S.H.; de Farias, M.R.; Schmidt, E.M.d.S. Application of Principal Component Analysis as a Prediction Model for Feline Sporotrichosis. Vet. Sci. 2025, 12, 32. https://doi.org/10.3390/vetsci12010032
Pegoraro FB, Mangrich-Rocha RMV, Weber SH, de Farias MR, Schmidt EMdS. Application of Principal Component Analysis as a Prediction Model for Feline Sporotrichosis. Veterinary Sciences. 2025; 12(1):32. https://doi.org/10.3390/vetsci12010032
Chicago/Turabian StylePegoraro, Franco Bresolin, Rita Maria Venâncio Mangrich-Rocha, Saulo Henrique Weber, Marconi Rodrigues de Farias, and Elizabeth Moreira dos Santos Schmidt. 2025. "Application of Principal Component Analysis as a Prediction Model for Feline Sporotrichosis" Veterinary Sciences 12, no. 1: 32. https://doi.org/10.3390/vetsci12010032
APA StylePegoraro, F. B., Mangrich-Rocha, R. M. V., Weber, S. H., de Farias, M. R., & Schmidt, E. M. d. S. (2025). Application of Principal Component Analysis as a Prediction Model for Feline Sporotrichosis. Veterinary Sciences, 12(1), 32. https://doi.org/10.3390/vetsci12010032







