1. Introduction
Many countries around the world, especially in the Middle East, are working to promote the importance of camel milk and consider it a valuable alternative, primarily produced by Bedouins. As desert animals, camels have developed several adaptations to survive in harsh environments characterized by limited water, poor-quality feed, and high temperatures. They are large, strong, durable, and extremely resilient [
1]. Although camels are not bred specifically for milk production, they can continue producing milk for 18 months, even under extreme environmental conditions. Therefore, they are vital to the inhabitants of dry areas, serving as both a means of transportation and a source of food. Despite their lower milk production compared to cows, camels rank as the fifth-largest milk producers in the world [
2].
Camel milk has been reported to possess antidiabetic properties, potentially due to its high levels of insulin and insulin-like proteins as well as bioactive peptides that may regulate blood glucose levels [
3]. Studies have shown that patients with type 2 diabetes can reduce their fasting blood glucose levels and increase their insulin sensitivity by regularly consuming camel milk [
4,
5]. Additionally, camel milk has anti-inflammatory and antioxidant properties that may help prevent complications from diabetes [
6]. However, research specifically examining the influence of dietary salt on fatty acid profile and insulin levels in the blood and milk of ruminants remains limited [
7], and to our knowledge, there are no studies that have examined this relationship in camels.
Several factors, including diet composition, breed, and management system, may affect the levels of insulin and insulin-like peptides in camel milk. Among dietary factors, salt intake may influence blood insulin levels and, consequently, the insulin content in milk. Evidence from human and animal models suggests a link between salt consumption and insulin dynamics, where increased Na
+ intake can lead to hyperinsulinemia, likely due to Na
+ effects on cellular ion channels and pancreatic function [
8,
9]. High salt consumption may also increase tight junctions’ permeability in the mammary gland, potentially allowing more insulin to pass from blood to milk. Although direct studies on the mammary gland are scarce, a recent study in rats demonstrated that high Na
+ intake can disrupt gut tight junctions’ integrity and increase permeability [
10].
While mineral salts have been studied extensively in dairy cows [
11] and sheep [
12], and the effect of salt on fatty acids (FA) has been reported, several studies have found that the addition of mineral salts, particularly sodium bicarbonate, increases rumen pH. This increase in pH can improve rumen fermentation by favoring the production of acetate over propionate [
13]. This shift in hydrogenated fatty acid production could help alleviate the problems of low-fat milk in dairy cows [
14]. In addition, sodium bicarbonate and other mineral salts increase the rate of dilution of rumen fluid. This increased dilution rate may also contribute to an increase in the acetate-propionate ratio, particularly in cattle fed high-concentration feed [
13]. However, there is a significant research gap regarding their effects on camel milk composition, particularly in relation to FA profiles, since the FA profile of camel milk plays a significant role in its nutritional value and potential health benefits for humans. The greater content of unsaturated FA (UFA) and the larger casein micelles in camel milk result in a softer clot in the stomach, allowing for faster and more efficient digestion compared to cow’s milk. Additionally, the smaller fat globules in camel milk further support digestion [
15]. These characteristics make camel milk more suitable for individuals with bovine milk intolerance [
16]. Due to its nutritional value and numerous health benefits, camel milk is often referred to as the “white gold of the desert” [
17]. While marketing in Saudi Arabia is primarily done locally, camel milk is gradually gaining recognition in the international market.
To address the above-mentioned research gaps, we hypothesized that a moderate increase in dietary salt levels in dairy camels would alter systemic insulin dynamics, which might increase milk insulin content and modify the FA profile. This study is the first to comprehensively analyze the effects of increased dietary salt levels on both blood and milk insulin levels in dairy camels, as well as key FA related to human health, particularly those influencing cardiovascular disease risk. The objectives of the current study were to evaluate the impacts of two levels of dietary salt on milk yield, milk composition, and blood metabolites in dairy camels, with a specific focus on blood and milk insulin levels and the FA profile of milk.
2. Materials and Methods
2.1. Animals, Treatments, and Diets
Twelve multiparous female camels (578 ± 24 kg average body weight; 3.1 ± 0.3 parities; 105 ± 22 days in milk) were used in a crossover design with two periods, each lasting 3 weeks. In the first period, 6 camels were fed a control concentrate (CON; 1.4% salt; 99.7% purity), while the remaining 6 were fed a high-salt concentrate (SAL; 4.3% salt; 99.7% purity). In the second period, the groups were switched, with the CON camels from the 1st period receiving SAL, and the SAL camels receiving CON. The ingredients of CON and SAL concentrates are shown in
Table 1.
Animals were individually fed 6.5 kg of experimental concentrates (CON or SAL) and 3.8 kg of alfalfa hay daily. All animals consumed the full amounts of concentrates and alfalfa hay offered. Fresh water was provided separately to each treatment group early in the morning. Daily water consumption was recorded using a 200-L water trough, which was filled each morning. After the evening milking, the remaining water was measured, and the trough was emptied.
All animals were kept under the same management conditions in the spring, from February to April 2023. Camels were hand-milked twice daily (9 a.m. and 5 p.m.). Calves stayed with their mothers from after the evening milking until 5 a.m.
2.2. Samples, Measurements, and Chemical Analysis
Daily records of milk production were maintained for the last seven days of each period. Milk samples (120 mL) for composition were taken during days 6 and 7 of each period. These samples were immediately placed in an ice chest and transported to the biotechnology laboratory of the Animal Production Department. For the FA profile, milk samples from both days were combined, and a representative sample was taken. Feed samples were also collected and stored at 4 °C until the chemical analysis.
The dry matter, protein, ether extract, NDF, ADF, and ash contents of feeds (
Table 2) were determined according to the official methods of the AOAC [
18]. Fat was extracted from feed samples using Soxhlet extraction (Soxtec 8000 extraction device-FOSS-Nils Foss Allé 1-DK-3400 Hilleroed-Denmark). The extracted fat was analyzed in triplicate to determine the FA profile of the feeds (
Table 2).
Infrared technology was used with the Milkoscan™FT+ device (Foss Electric-Allé 1-DK-3400 Hilleroed, Denmark) to measure the concentrations of fat, lactose, protein, total solids (TS), and solids non-fat (SNF) in milk. Insulin was determined in skimmed milk using a bovine ELISA kit (Mercodia Diagnostics, Uppsala, Sweden) according to the manufacturer’s protocol. Reacted ELISA plates were read using an automatic reader (RX Monza, Randox, UK) at 450 nm.
Milk samples were centrifuged for 15 min at 4 °C and 3500 rpm to separate the fat. The top fat layer was then collected and stored in glass tubes at −20 °C until further analysis [
19]. For the methylation of FA, 1 mg of milk fat was placed in glass tubes, followed by the addition of 500 µL of hexane (99%) and 250 µL of sodium methoxide (0.5 M in methanol) to prevent the isomerization of UFA. The mixture was vortexed for 10 s. The tubes were then placed in a beaker of distilled water at 50 °C for 20 s, removed, and cooled to 24 °C for 2 min. Then, 250 µL of 5% methanolic hydrochloric acid was added to the cooled samples to extract all fat, and the samples were vortexed again for 10 s. The tubes were returned to the beaker at 50 °C for an additional 20 s. After cooling, 1000 µL of hexane was added to dissolve and extract pure fat. Finally, the hexane layer was carefully removed from the top of the tube and transferred into vials, which were cooled at 4 °C for subsequent chromatographic analysis.
The FA methyl esters were analyzed using a gas chromatography-mass spectrometer (GC-MS). Helium (1.8 mL/min) was used as the carrier gas, with injector and detector temperatures set at 245 °C. The oven temperature was initially set at 60 °C (1 min) and then increased to 120 °C at a rate of 20 °C/min, maintaining this final temperature for 15 min [
20]. Samples were injected at a ratio of 1:20 into a BPX-70 capillary column (60 m × 0.25 mm × 0.25 mm; SGE, Melbourne, Australia). The FAs were identified by comparing the retention time of the peaks with those obtained by certified standards (FAME MIX C6-C24, CLA FAME, Sigma Aldrich, St. Louis, MO, USA). Peaks were integrated using data acquisition software (Agilent ChemStation, version B.04.01), and FA quantification was performed using an external standard calibration. The results were expressed in grams per 100 g of fat.
At the end of each period, blood samples were collected, and plasma was separated and frozen for the analysis of insulin and other metabolites. Insulin concentrations in blood plasma were determined using a bovine ELISA kit (Mercodia Diagnostics) and reacted ELISA plates were read by an automatic reader at 450 nm. Quimica Clinica Aplicada kits (QCA, Tarragona, Spain) were used to determine the concentrations of glucose, total protein, albumin, globulin, triglycerides, and urea. Analyses were performed using colorimetric methods with the RX Monza chemical analyzer spectrophotometer (Randox Laboratories Ltd., Crumlin, UK).
2.3. Lipid Quality Indices
The values of saturated FA (SFA), MUFA, and polyunsaturated FA (PUFA) were used to calculate lipid quality indices as follows:
Thrombogenic index (TI) =
[
21].
Atherogenic index (AI):
Hypocholesterolemic fatty acids (hFA) ;
Hypercholesterolemic fatty acids (HFA) = C12:0 + C14:0 + C16:0;
hFA:HFA ratio (h/H)
according to Santos-Silva et al. [
22].
The transfer rates of OA, LA, and ALA were calculated using their intake and milk output values according to Stergiadis et al. [
23] as follows:
FA content in milk (g)/FA intake (g), where:
2.4. Statistical Analyses
The MIXED procedure of SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) was used to analyze the repeatedly measured data (i.e., water intake, milk yield, and milk composition). The statistical mixed model considered the effects of treatment (CON vs. SAL) and period as fixed effects, the interaction of treatment × period, the random effect of the animal, and the residual error. Since the experimental design was a crossover with two experimental periods, the interaction treatment × period accounted for the possible residual treatment effect from the previous period.
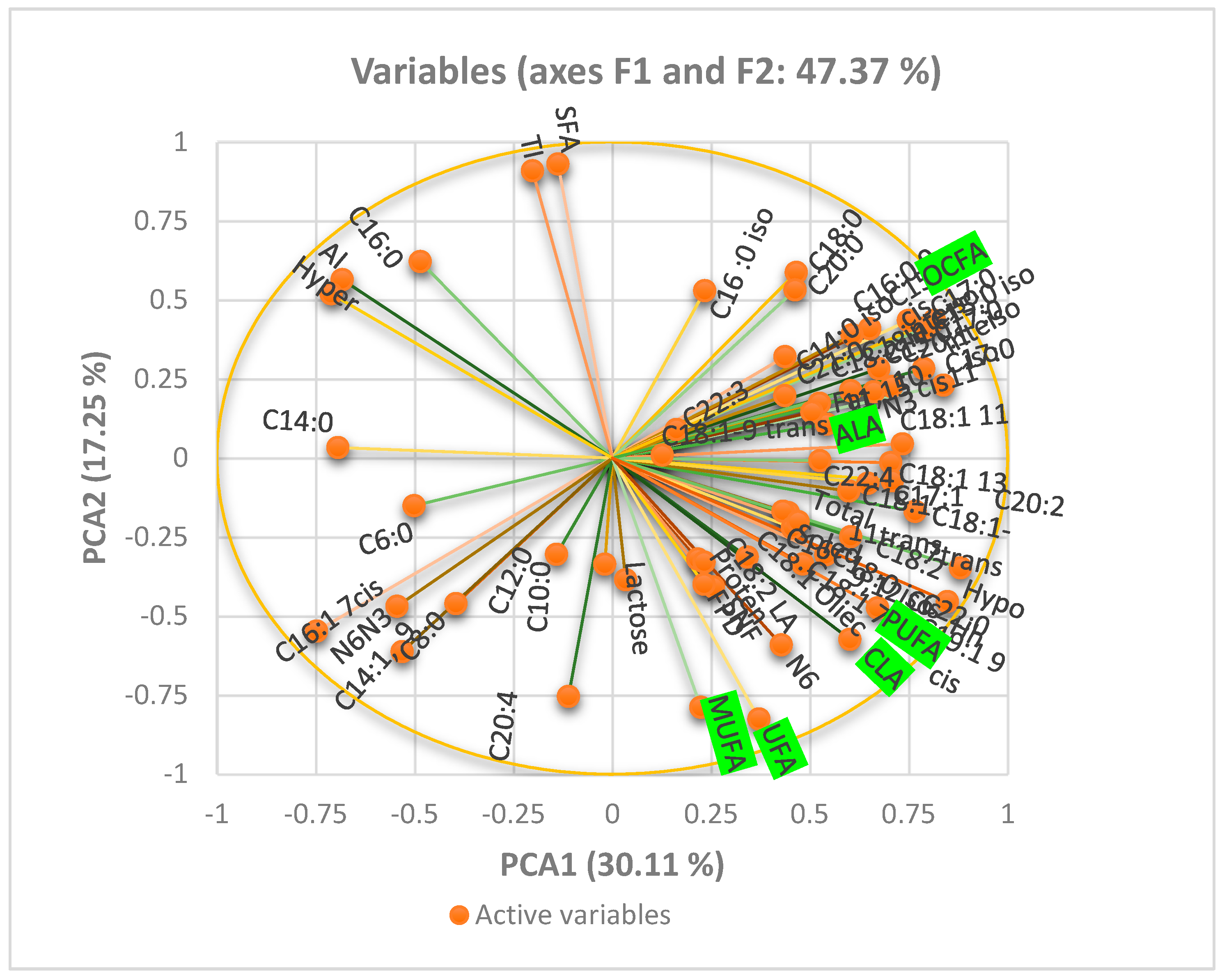
For the data measured only once at the end of each period (i.e., milk FA and blood metabolites), a GLM procedure was used. The model included the effects of treatment, period, and their interaction. The normality of the data distribution was checked, and transformations were applied to blood insulin values. The Tukey test was used to identify differences in least square means. Significance was declared at p < 0.05 and tendencies at p < 0.10. The XLstat-2021 was used to calculate the principal component analysis (PCA).
5. Conclusions
This study provides valuable insights into the effects of dietary salt supplementation on dairy camels. While moderate salt inclusion did not negatively affect milk yield or major milk components, it altered the FA profile, reducing beneficial FA and increasing the atherogenic index. Although milk insulin levels remained unchanged, salt supplementation increased blood insulin levels without affecting blood glucose, suggesting a potential impact on the animal’s insulin sensitivity. Additionally, salt supplementation decreased total protein, albumin, and globulin levels, indicating potential effects on protein metabolism and fluid balance. These findings suggest that careful consideration should be given to dietary salt levels in camel feeding regimes, balancing the need to meet mineral requirements with potential impacts on milk quality and human health. Further research is warranted to fully elucidate the complex interplay between dietary salt and camel milk composition, particularly concerning insulin dynamics and FA metabolism.