Impact of Toceranib Phosphate and Carprofen on Survival and Quality of Life in Dogs with Inflammatory Mammary Carcinomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Initial Evaluation
2.3. Inclusion and Exclusion Criteria
- Presence of macroscopic lesions measurable according to response evaluation criteria in solid tumors RECIST criteria [24].
- Hematological and biochemical parameters less than grade 2 following VCOG-CTCAE [25].
- Previous use of NSAIDs and prednisolone was allowed with a washout period of 1 week before being included in the study.
- The use of other analgesic treatments was accepted as long as the patient was already receiving them for at least 5 days before starting the study.
- Dogs with regional or distant metastases were allowed to enter the study.
- An owner’s written consent was obtained in all cases, along with a document providing study information, QOL questionnaire document to be filled out every 2 weeks, and a document outlining the protocol to be followed.
- Prior treatment with chemotherapy or tyrosine kinase inhibitors.
- Diagnosis of other forms of mammary gland tumors.
2.4. Treatment Protocol
2.5. Data Collection
2.6. Treatment Response and Toxicity Assessment
2.7. Statistical Analysis
3. Results
3.1. Dogs and Tumor Characteristics
3.2. Treatment and Adverse Events
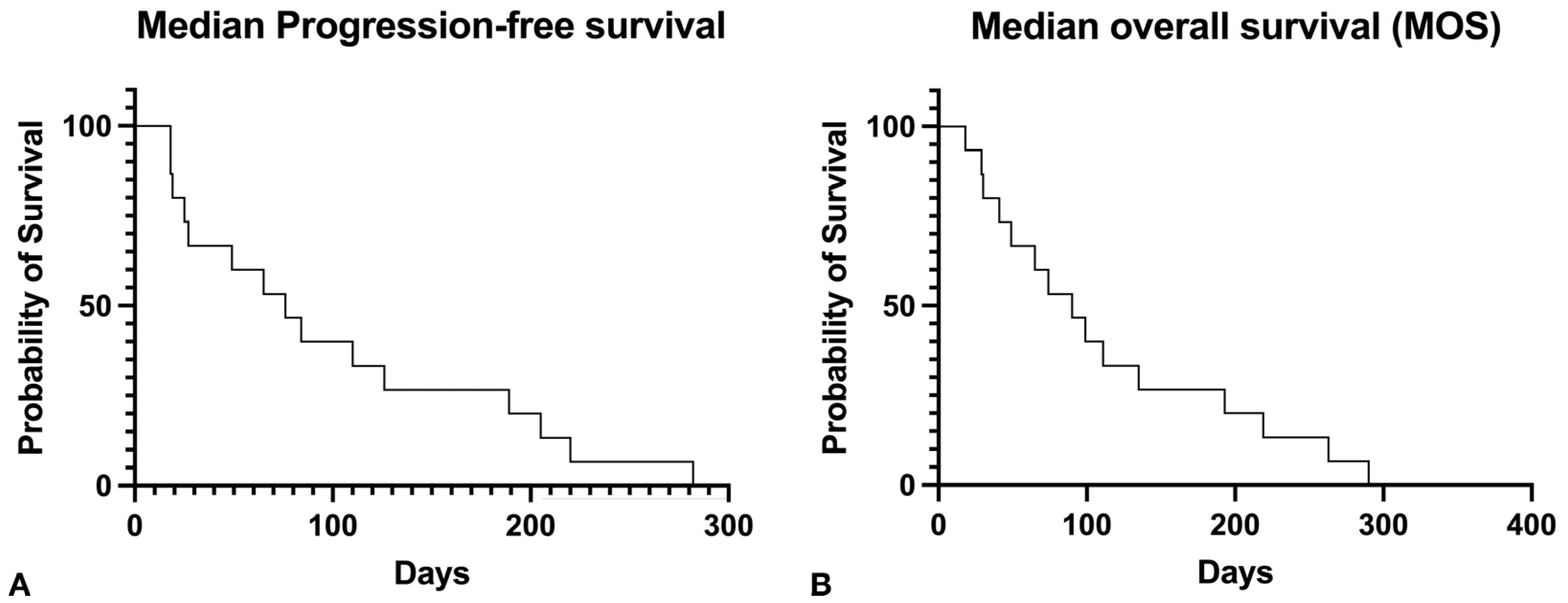
3.3. Treatment Response
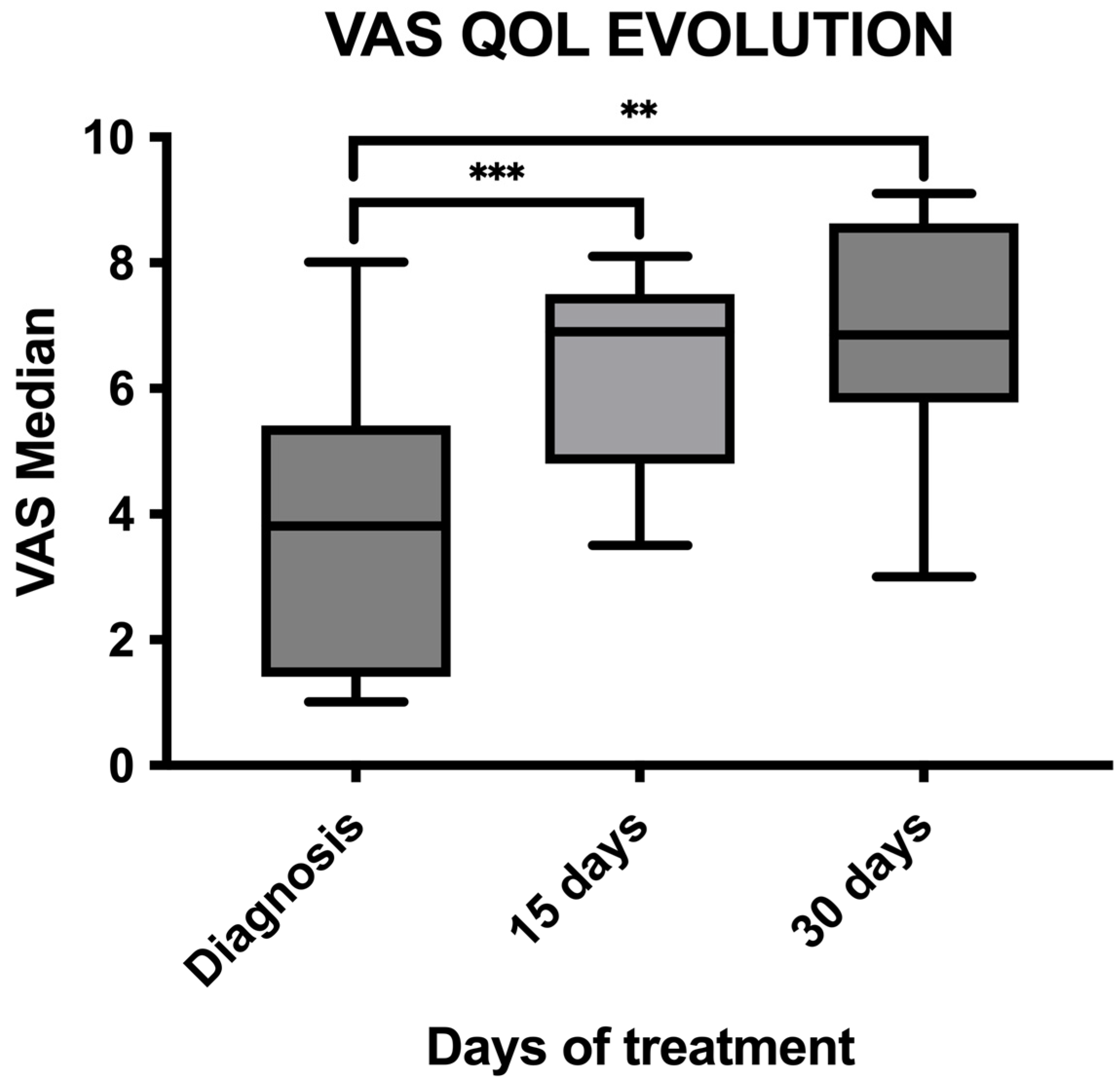
3.4. Quality of Life Assessment
3.5. Follow-Up and Rescue Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peña, L.; Pérez-Alenza, M.D.; Rodriguez-Bertos, A.; Nieto, A. Canine inflammatory mammary carcinoma: Histopathology, immunohistochemistry and clinical implications of 21 cases. Breast Cancer Res. Treat. 2003, 78, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Millanta, F.; Verin, R.; Asproni, P.; Giannetti, G.; Poli, A. A case of feline primary inflammatory mammary carcinoma: Clinicopathological and immunohistochemical findings. J. Feline Med. Surg. 2012, 14, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alenza, M.D.; Jiménez, A.; Nieto, A.I.; Peña, L. First description of feline inflammatory mammary carcinoma: Clinicopathological and immunohistochemical characteristics of three cases. Breast Cancer Res. 2004, 6, 4. [Google Scholar] [CrossRef]
- Marconato, L.; Romanelli, G.; Stefanello, D. Prognostic factors for dogs with mammary inflammatory carcinoma: 43 cases (2003–2008). J. Am. Vet. Med. Assoc. 2009, 235, 967–972. [Google Scholar] [CrossRef]
- Clemente, M.; Pérez-Alenza, M.D.; Illera, J.C.; Peña, L. Histological, immunohistological, and ultrastructural description of vasculogenic mimicry in canine mammary cancer. Vet. Pathol. 2010, 47, 265–274. [Google Scholar] [CrossRef]
- Clemente, M.; Pérez-Alenza, M.D.; Peña, L. Metastasis of canine inflammatory versus non-inflammatory mammary tumours. J. Comp. Pathol. 2010, 143, 157–163. [Google Scholar] [CrossRef]
- Chainitikun, S.; Saleem, S.; Lim, B.; Valero, V.; Ueno, N.T. Update on systemic treatment for newly diagnosed inflammatory breast cancer. J. Adv. Res. 2020, 29, 1–12. [Google Scholar] [CrossRef]
- Raposo, T.P.; Arias-Pulido, H.; Chaher, N.; Fiering, S.N.; Argyle, D.J.; Prada, J.; Pires, I.; Queiroga, F.L. Comparative aspects of canine an human inflammatory breast cancer. Semin. Oncol. 2017, 44, 288–300. [Google Scholar] [CrossRef]
- Clemente, M.; De Andrés, P.J.; Peña, L.; Pérez-Alenza, M.D. Survival time of dogs with inflammatory mammary cancer treated with palliative therapy alone or palliative therapy plus chemotherapy. Vet. Rec. 2009, 165, 78–81. [Google Scholar] [CrossRef]
- Souza, C.H.; Toledo-Piza, E.; Amorin, R.; Barboza, A.; Tobias, K.M. Inflammatory mammary carcinoma in 12 dogs: Clinical features, cyclooxygenase-2 expression, and response to piroxicam treatment. Can. Vet. J. 2009, 50, 506–510. [Google Scholar]
- Rossi, F.; Sabattini, S.; Vascellari, M.; Marconato, L. The impact of toceranib, piroxicam and thalidomide with or without hypofractionated radiation therapy on clinical outcome in dogs with inflammatory mammary carcinoma. Vet. Comp. Oncol. 2018, 16, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.J.; McEntee, M.F.; Legendre, A.M. Review paper: Cancer chemopreventive compounds and canine cancer. Vet. Pathol. 2009, 46, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Poradowski, D.; Obmińska-Mrukowicz, B. Effect of selected nonsteroidal anti-inflammatory drugs on the viability of canine osteosarcoma cells of the D-17 line: In vitro studies. J. Vet. Res. 2019, 63, 399–403. [Google Scholar] [CrossRef]
- Stelio, P.L.; Luna, A.C.; Basílio, P.V.M.; Steagall, L.P.; Machado, F.Q.; Moutinho, R.K.; Takahira, C.V.S.B. Evaluation of adverse effects of long-term oral administration of carprofen, etodolac, flunixin meglumine, ketoprofen, and meloxicam in dogs. Am. J. Vet. Res. 2007, 68, 258–264. [Google Scholar] [CrossRef]
- London, C.A.; Hannah, A.L.; Zadovoskaya, R.; Chien, M.B.; Kollias-Baker, C.; Rosenberg, M.; Downing, S.; Post, G.; Boucher, J.; Shenoy, N.; et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin. Cancer Res. 2003, 9, 2755–2768. [Google Scholar]
- London, C.A.; Malpas, P.B.; Wood-Follis, S.L.; Boucher, J.F.; Rusk, A.W.; Rosenberg, M.P.; Henry, C.J.; Mitchener, K.L.; Klein, M.K.; Hintermeister, J.G.; et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin. Cancer Res. 2009, 15, 3856–3865. [Google Scholar] [CrossRef] [PubMed]
- Frezoulis, P.; Harper, A. The role of toceranib phosphate in dogs with non-mast cell neoplasia: A systematic review. Vet. Comp. Oncol. 2022, 20, 362–371. [Google Scholar] [CrossRef]
- Raposo, T.P.; Pires, I.; Prada, J.; Queiroga, F.L.; Argyle, D.J. Exploring new biomarkers in the tumour microenvironment of canine inflammatory mammary tumours. Vet. Comp. Oncol. 2017, 15, 655–666. [Google Scholar] [CrossRef]
- Camacho, L.; Peña, L.; Gil, A.G.; Martín-Ruiz, A.; Dunner, S.; Illera, J.C. Immunohistochemical Vascular Factor Expression in Canine Inflammatory Mammary Carcinoma. Vet. Pathol. 2014, 51, 737–748. [Google Scholar] [CrossRef]
- Millanta, F.; Caneschi, V.; Ressel, L.; Citi, S.; Poli, A. Expression of Vascular Endothelial Growth Factor in Canine Inflammatory and Non-inflammatory Mammary Carcinoma. J. Comp. Pathol. 2010, 142, 36–42. [Google Scholar] [CrossRef]
- Alonso-Miguel, D.; Valdivia, G.; García-San José, P.; Alonso-Diez, Á.; Clares, I.; Portero, M.; Peña, L.; Pérez-Alenza, M.D. Clinical outcome of dogs diagnosed with canine inflammatory mammary cancer treated with metronomic cyclophosphamide, a cyclooxygenase-2 inhibitor and toceranib phosphate. Vet. Comp. Oncol. 2021, 20, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, M.A.; Kerrigan, S.M. Quality of Life Measurement in Prospective Studies of Cancer Treatments in Dogs and Cats. J. Vet. Intern. Med. 2014, 28, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.; Savary-Bataille, K.; Leeuw, B.; Argyle, D.J. Development of a questionnaire assessing health-related quality-of-life in dogs and cats with cancer. Vet. Comp. Oncol. 2011, 9, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.M.; Thamm, D.H.; Vail, D.M.; London, C. Response evaluation criteria for solid tumours in dogs (v1.0): A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 2015, 13, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Veterinary cooperative oncology group-common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet. Comp. Oncol. 2016, 14, 417–446. [CrossRef]
- Bernabe, L.F.; Portela, R.; Nguyen, S.; Kisseberth, W.C.; Pennell, M.Y.M.F.; London, C.A. Evaluation of the adverse event profile and pharmacodynamics of toceranib phosphate administered to dogs with solid tumors at doses below the maximum tolerated dose. BMC Vet. Res. 2013, 9, 190. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Prado, M.C.M.; Macedo, S.A.L.; Guiraldelli, G.G.; de Faria Lainetti, P.; Leis-Filho, A.F.; Kobayashi, P.E.; Laufer-Amorim, R.; Fonseca-Alves, C.E. Investigation of the Prognostic Significance of Vasculogenic Mimicry and Its Inhibition by Sorafenib in Canine Mammary Gland Tumors. Front Oncol. 2019, 19, 1445. [Google Scholar] [CrossRef]
- Vasudev, N.S.; Reynolds, A. Anti-angiogenic therapy for cancer: Current progress, unresolved questions and future directions. Angiogenesis 2014, 17, 471–494. [Google Scholar] [CrossRef]
- Pérez-Alenza, M.D.; Tabanera, E.; Peña, L. Inflammatory mammary carcinoma in dogs: 33 cases (1995–1999). J. Am. Vet. Med. Assoc. 2001, 219, 1110–1114. [Google Scholar] [CrossRef]
- Robertson, F.M.; Bondy, M.; Yang, W.; Yamauchi, H.; Wiggins, S.K.S.; Krishnamurthy, S.; Le-Petross, H.; Bidaut, L.; Player, A.N.; Barsky, S.H.; et al. Inflammatory breast cancer: The disease, the biology, the treatment. CA Cancer J. Clin. 2010, 60, 351–375. [Google Scholar] [CrossRef]
- Marconato, L.; Buracco, P.; Aresu, L. Perspectives on the design of clinical trials for targeted therapies and immunotherapy in veterinary oncology. Vet. J. 2015, 205, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.M. Cancer clinical trials: Development and implementation. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 1033–1057. [Google Scholar] [CrossRef]
- Yang, W.T.; Le-Petross, H.T.; Macapinlac, H.; Carkaci, S.; Gonzalo-Angulo, A.M.; Dawood, S.; Resetkova, E.; Hortobagyi, G.N.; Cristofanilli, M. Inflammatory breast cancer: PET/CT, MRI, mammography, and sonography findings. Breast Cancer Res. 2008, 109, 416–421. [Google Scholar] [CrossRef]
- Stockhaus, C.; Kohn, B.; Rudolph, R.; Brunnberg, L.; Giger, U. Correlation of haemostatic abnormalities with tumour stage and characteristics in dogs with mammary carcinoma. J. Small Anim. Pract. 1999, 40, 326–331. [Google Scholar] [CrossRef]
- Clemente, M.; Sánchez-Archidona, A.R.; Sardón, D.; Díez, L.; Martín-Ruiz, A.; Caceres, S.; Sassi, F.; Pérez-Alenza, M.D.; Illera, J.C.; Dunner, S.; et al. Different role of COX-2 and angiogenesis in canine inflammatory and non-inflammatory mammary cancer. Vet. J. 2013, 197, 427–432. [Google Scholar] [CrossRef]
- Queiroga, F.L.; Perez-Alenza, M.D.; Silvan, G.; Peña, L.; Lopes, C.; Illera, J.C. Cox-2 levels in canine mammary tumors, including inflammatory mammary carcinoma: Clinicopathological features and prognostic significance. Anticancer Res. 2005, 25, 4269–4275. [Google Scholar]
- Mansa, S.; Palmer, E.; Grøndahl, C.; Lønaas, L.N.G. Long-term treatment with carprofen of 805 dogs with osteoarthritis. Vet. Rec. 2007, 160, 427–430. [Google Scholar] [CrossRef]
- Holtermann, N.; Kiupel, M.; Kessler, M.; Teske, E.; Betz, D.; Hirschberger, J. Masitinib monotherapy in canine epitheliotropic lymphoma. Vet. Comp. Oncol. 2016, 1, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Peña, L.; Alonso-Díez, A.; Brunetti, B.; Muscatello, L.V.; Benazzi, C.; Pérez-Alenza, M.D.; Sarli, G. P-Glycoprotein and Breast Cancer Resistance Protein in Canine Inflammatory and Noninflammatory Grade III Mammary Carcinomas. Vet. Pathol. 2019, 56, 840–847. [Google Scholar] [CrossRef] [PubMed]
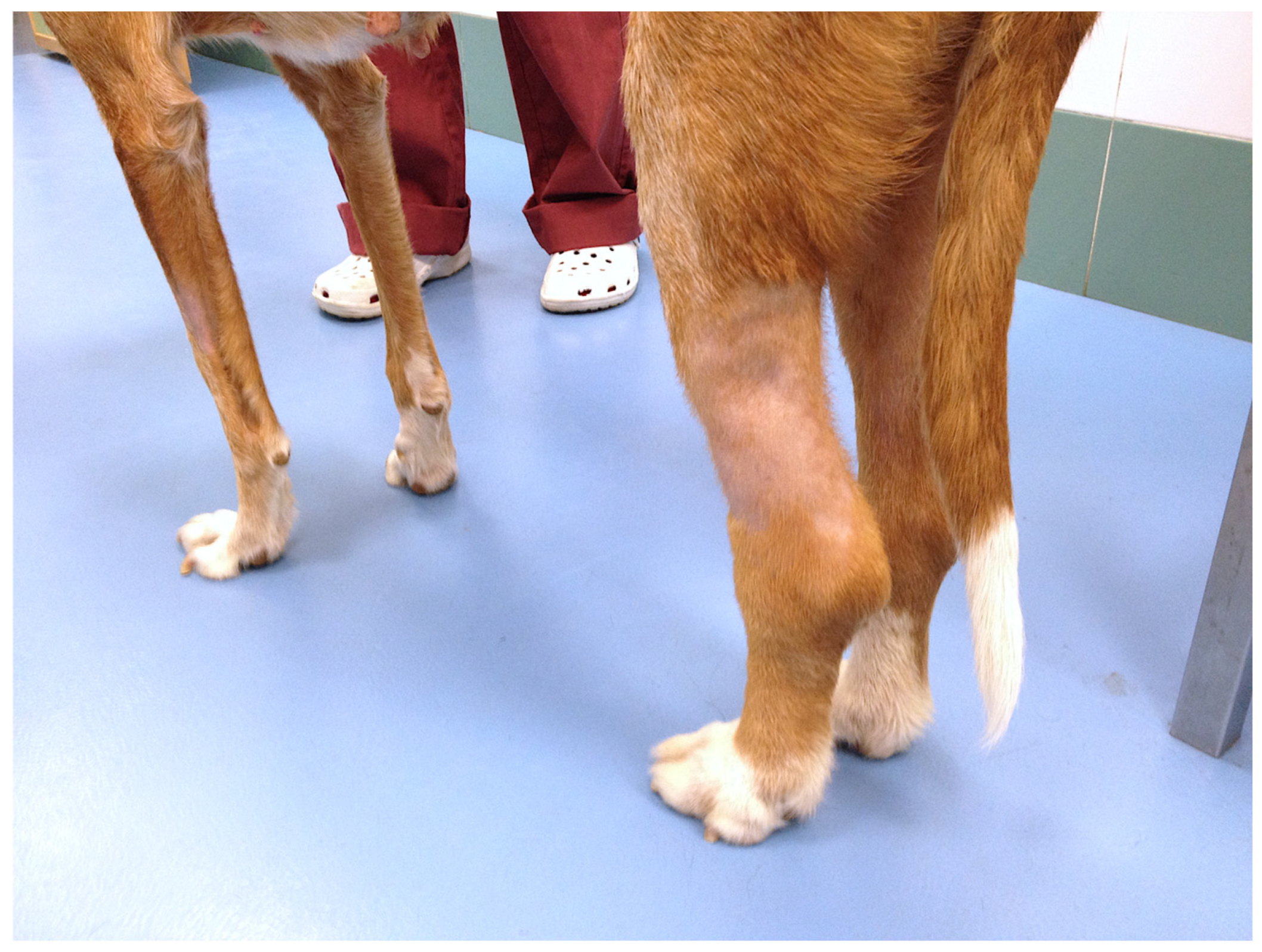
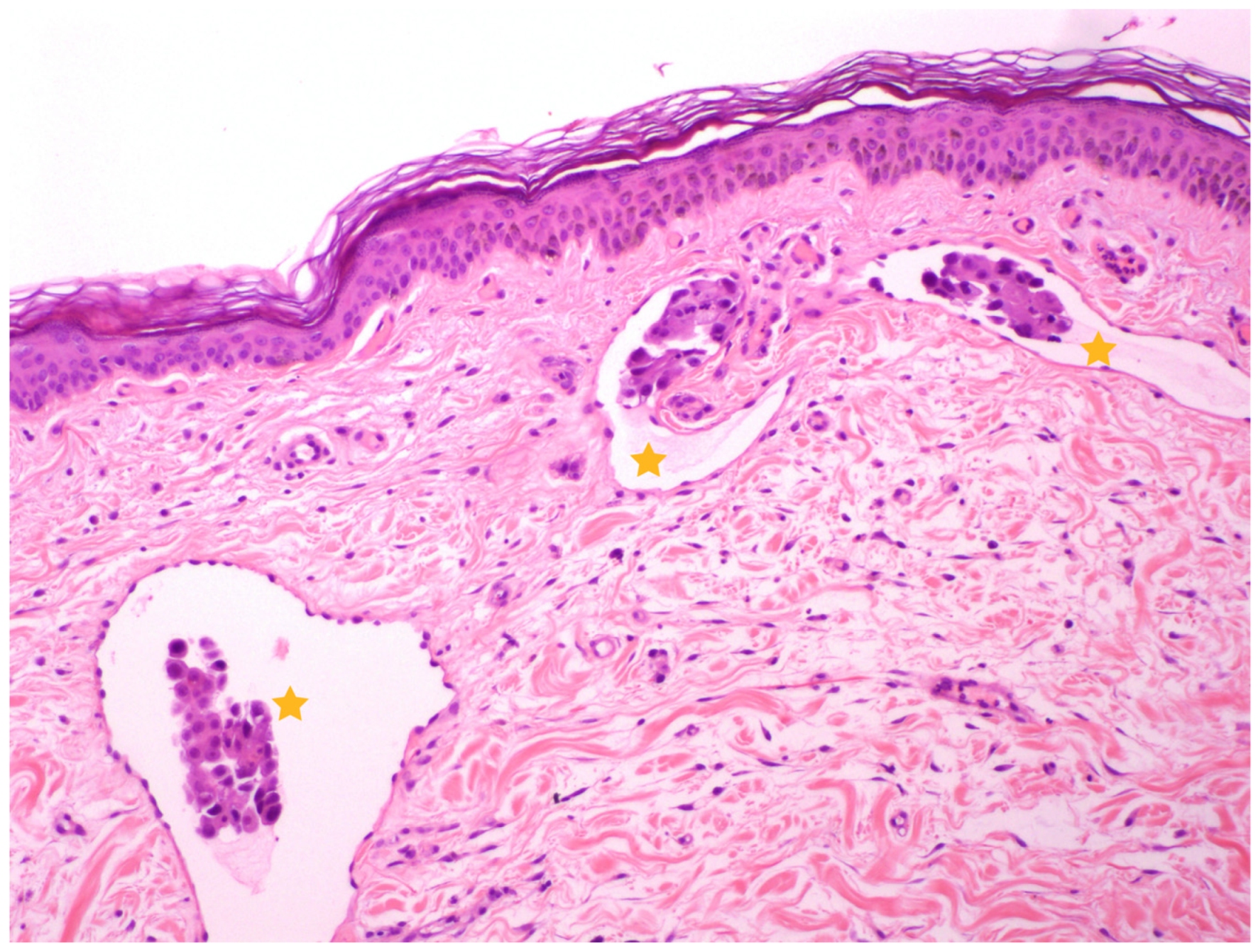
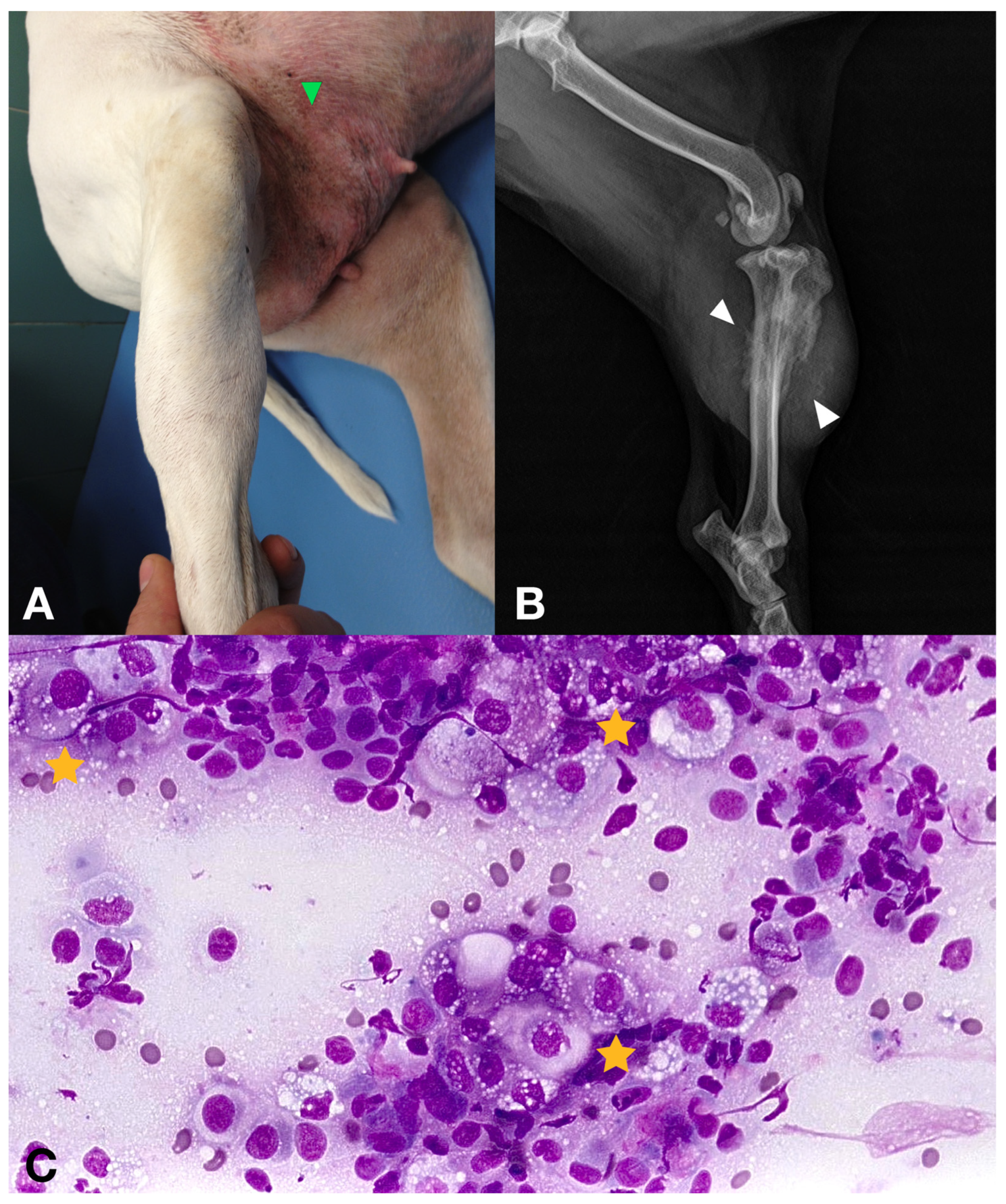
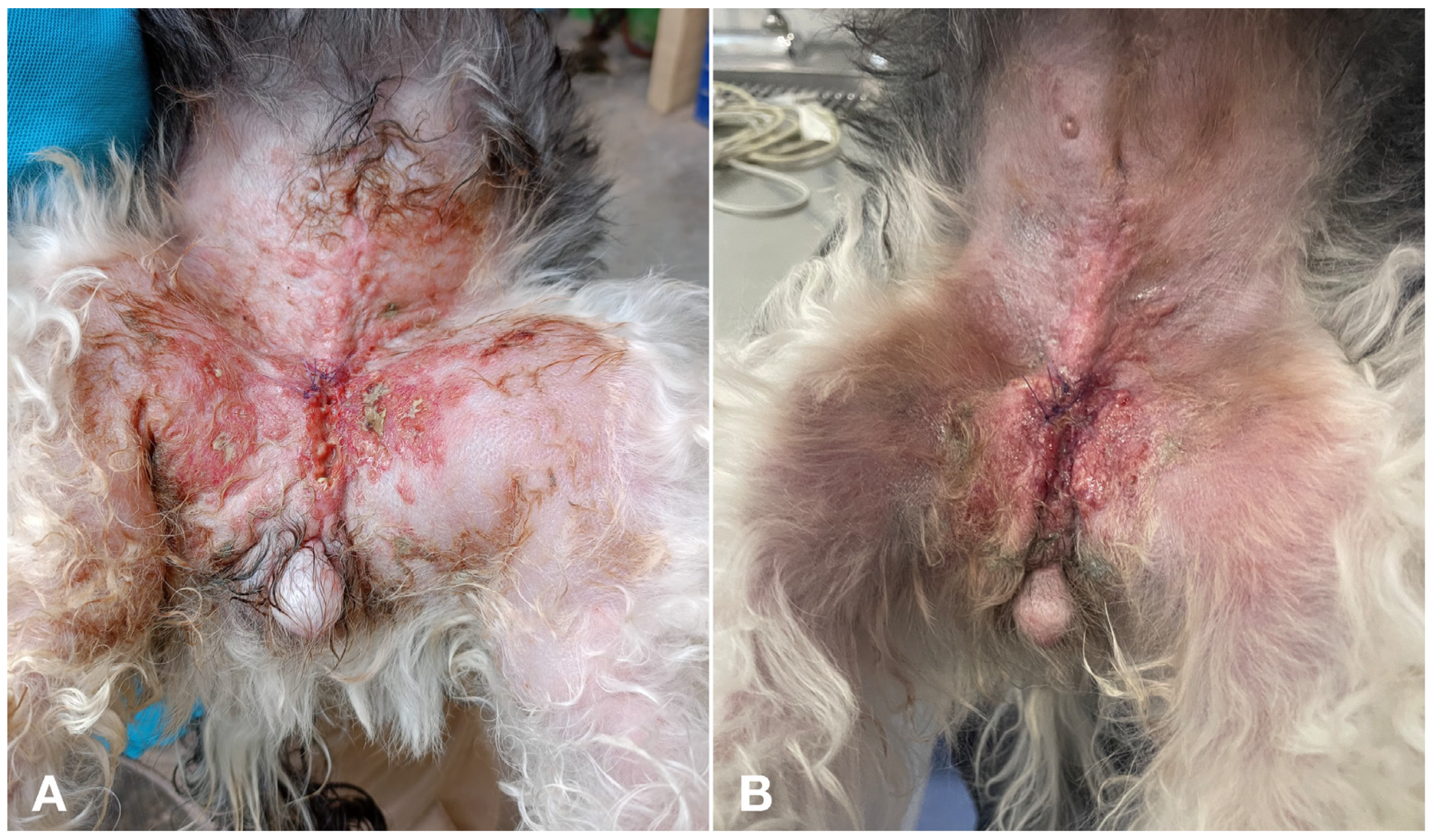
| Case | Age | Weight (kg) | Breed | Spayed | Years since OHE | Location | Other Characteristics | Size (cm) | Local or Distant Metastasis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | 15.5 | Mixed | No | ND | L3–L5 | Lymphedema Skin nodules | 5.3 | NDM |
| 2 | 13 | 23.3 | Mixed | No | ND | L3 | Skin nodules | 5.4 | NDM |
| 3 | 14 | 22.4 | Mixed | Yes | 10 | R2–R3 | Skin nodules | 6.6 | NDM |
| 4 | 12 | 42.6 | Mastín | Yes | 11 | R4–R5 | Lymphedema Skin nodules | 4.7 | Iliac and inguinal lymph node |
| 5 | 16 | 13.5 | Mixed | No | ND | L4–L5 | Skin | 5.5 | Diaphyseal tibial aggressive bone lesion |
| 6 | 11 | 6.1 | Yorkshire Terrier | No | ND | R3 | Skin ulcers | 3.4 | NDM |
| 7 | 8 | 3.3 | Yorkshire Terrier | Yes | 7 | R1 | Skin nodules | 2.3 | NDM |
| 8 | 11 | 15.5 | Mixed | No | ND | L5–R5 | Lymphedema | 6.7 | Popliteal lymph node |
| 9 | 13 | 42.7 | Mixed | Yes | 1 | R1 | Skin ulcers | 5.6 | NDM |
| 10 | 12 | 9.1 | French Bulldog | No | ND | L3–L4 | Skin ulcers | 4.6 | NDM |
| 11 | 8 | 27.8 | Labrador | No | ND | L2–L3 | Skin nodules | 8.4 | NDM |
| 12 | 14 | 33.9 | Rottweiller | Yes | 5 | R4 | Skin nodules | 9.6 | Iliac lymph node |
| 13 | 8 | 14.2 | English Cocker spaniel | Yes | 7 | L2–L3 | Skin nodules | 3.4 | NDM |
| 14 | 15 | 28.6 | German Shepherd | Yes | 5 | L4–L5 | Lymphedema Skin nodules | 8.7 | Iliac and inguinal lymph node |
| 15 | 10 | 29.9 | Boxer | No | ND | R4 | Skin nodules | 6.7 | Iliac and inguinal lymph node |
| Topic | Question | Before Treatment | 15 Days after | 30 Days after |
|---|---|---|---|---|
| Happiness | My dog wants to play | 3 | 3.867 * (p = 0.0039) | 4.214 * (p = 0.049) |
| My dog interacts with me | 4.133 | 4.867 (p = 0.0078) | 4.286 (p = 0.9) | |
| My dog is happy | 3.571 | 4.467 * (p = 0.0039) | 4.286 (p = 0.1250) | |
| Mental status | My dog has more good days than bad days | 3.071 | 4.2 * (p = 0.0010) | 4.214 * (p = 0.0059) |
| My dog is awake less | 2.643 | 1.733 * (p = 0.0010) | 2 (p = 0.12) | |
| My dog is depressed | 2.5 | 1.667 * (p = 0.0010) | 2.071 (p = 0.273) | |
| Pain | My dog is in pain | 4 | 2.267 * (p = 0.0002) | 2.286 * (p = 0.0020) |
| Appetite | My dog eats all of their food portion | 2.786 | 3.867 (p = 0.1675) | 4.143 * (p = 0.0082) |
| My dog is sick | 1.071 | 1.4 (p = 0.1875) | 1.143 (p = 0.9) | |
| My dog wants treats/snacks | 3.286 | 4.133 (p = 0.12) | 4.429 * (p = 0.0115) | |
| Hydration | My dog drinks as normal | 4.571 | 4.8 (p = 0.06) | 4.643 (p = 0.9) |
| My dog has diarrhea | 1.286 | 1.533 (p = 0.5) | 1.571 (p = 0.75) | |
| My dog urinates the same quantity as always | 4.857 | 4.733 (p = 0.9) | 4.714 (p = 0.75) | |
| Mobility | My dog is moving normally | 3.286 | 4 * (p = 0.0078) | 4.143 * (p = 0.0215) |
| My dog is resting all day | 1.8 | 1.533 (p = 0.218) | 1.643 (p = 0.9) | |
| My dog has higher activity than normal | 2.143 | 3.133 * (p = 0.0020) | 3.143 * (p = 0.0039) | |
| General health | Health status compared to before the cancer diagnosis | 2.33 | 4.27 * (p = 0.0001) | 4.143 * (p = 0.0005) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-de la Virgen, M.; Del Portillo Miguel, I.; Maiques, E.; Pérez Roger, I.; Poch, E.; Borrego, J. Impact of Toceranib Phosphate and Carprofen on Survival and Quality of Life in Dogs with Inflammatory Mammary Carcinomas. Vet. Sci. 2024, 11, 430. https://doi.org/10.3390/vetsci11090430
Garcia-de la Virgen M, Del Portillo Miguel I, Maiques E, Pérez Roger I, Poch E, Borrego J. Impact of Toceranib Phosphate and Carprofen on Survival and Quality of Life in Dogs with Inflammatory Mammary Carcinomas. Veterinary Sciences. 2024; 11(9):430. https://doi.org/10.3390/vetsci11090430
Chicago/Turabian StyleGarcia-de la Virgen, Miguel, Isabel Del Portillo Miguel, Elisa Maiques, Ignacio Pérez Roger, Enric Poch, and Juan Borrego. 2024. "Impact of Toceranib Phosphate and Carprofen on Survival and Quality of Life in Dogs with Inflammatory Mammary Carcinomas" Veterinary Sciences 11, no. 9: 430. https://doi.org/10.3390/vetsci11090430
APA StyleGarcia-de la Virgen, M., Del Portillo Miguel, I., Maiques, E., Pérez Roger, I., Poch, E., & Borrego, J. (2024). Impact of Toceranib Phosphate and Carprofen on Survival and Quality of Life in Dogs with Inflammatory Mammary Carcinomas. Veterinary Sciences, 11(9), 430. https://doi.org/10.3390/vetsci11090430







