Effects of Synbiotic Administration on Gut Microbiome and Fecal Bile Acids in Dogs with Chronic Hepatobiliary Disease: A Randomized Case–Control Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
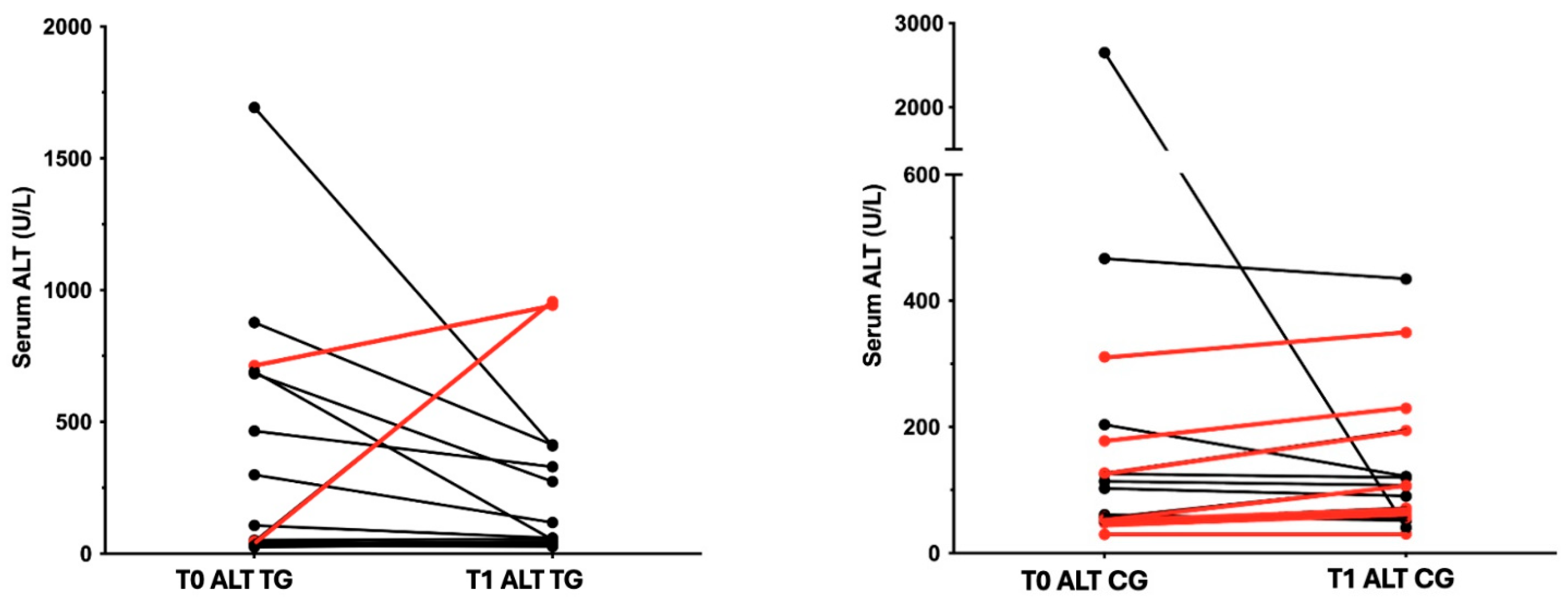
3.1. Animals
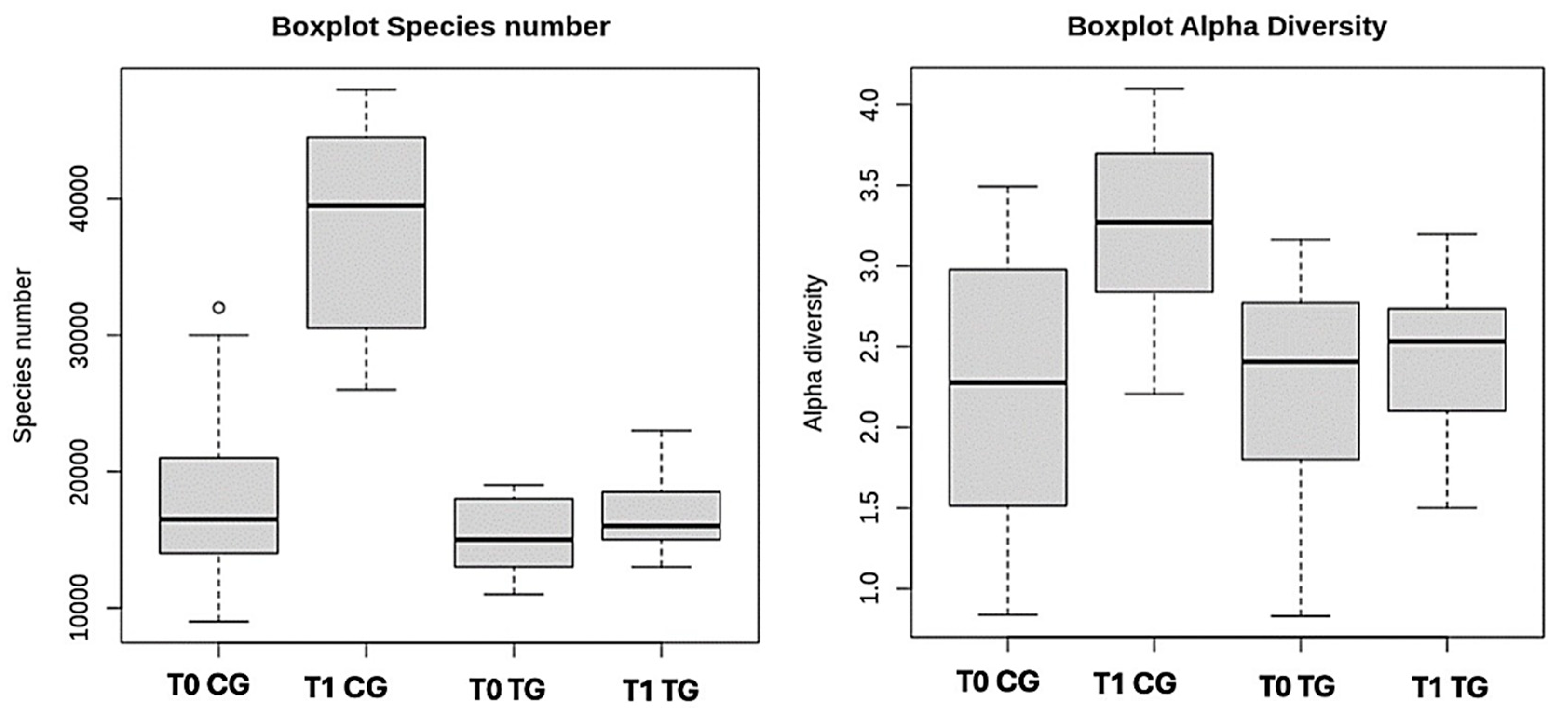
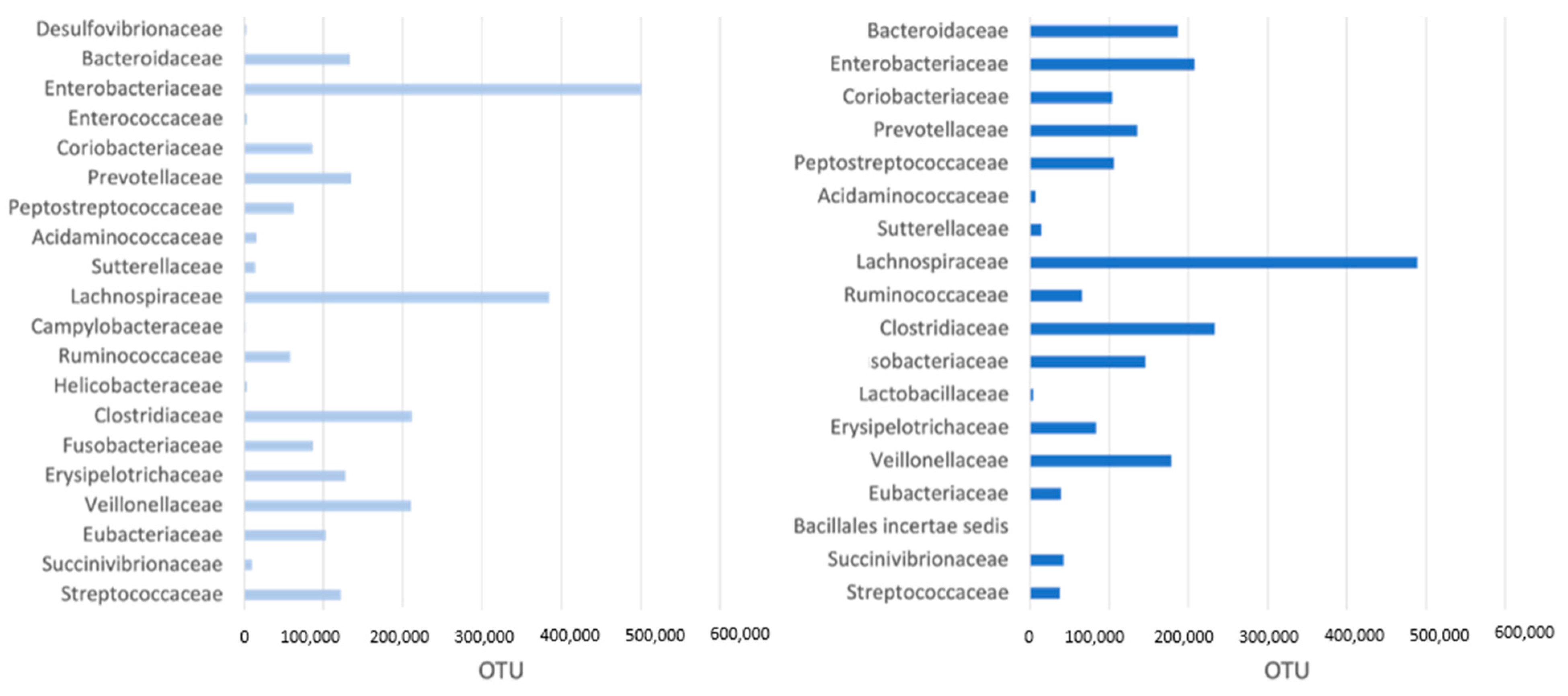
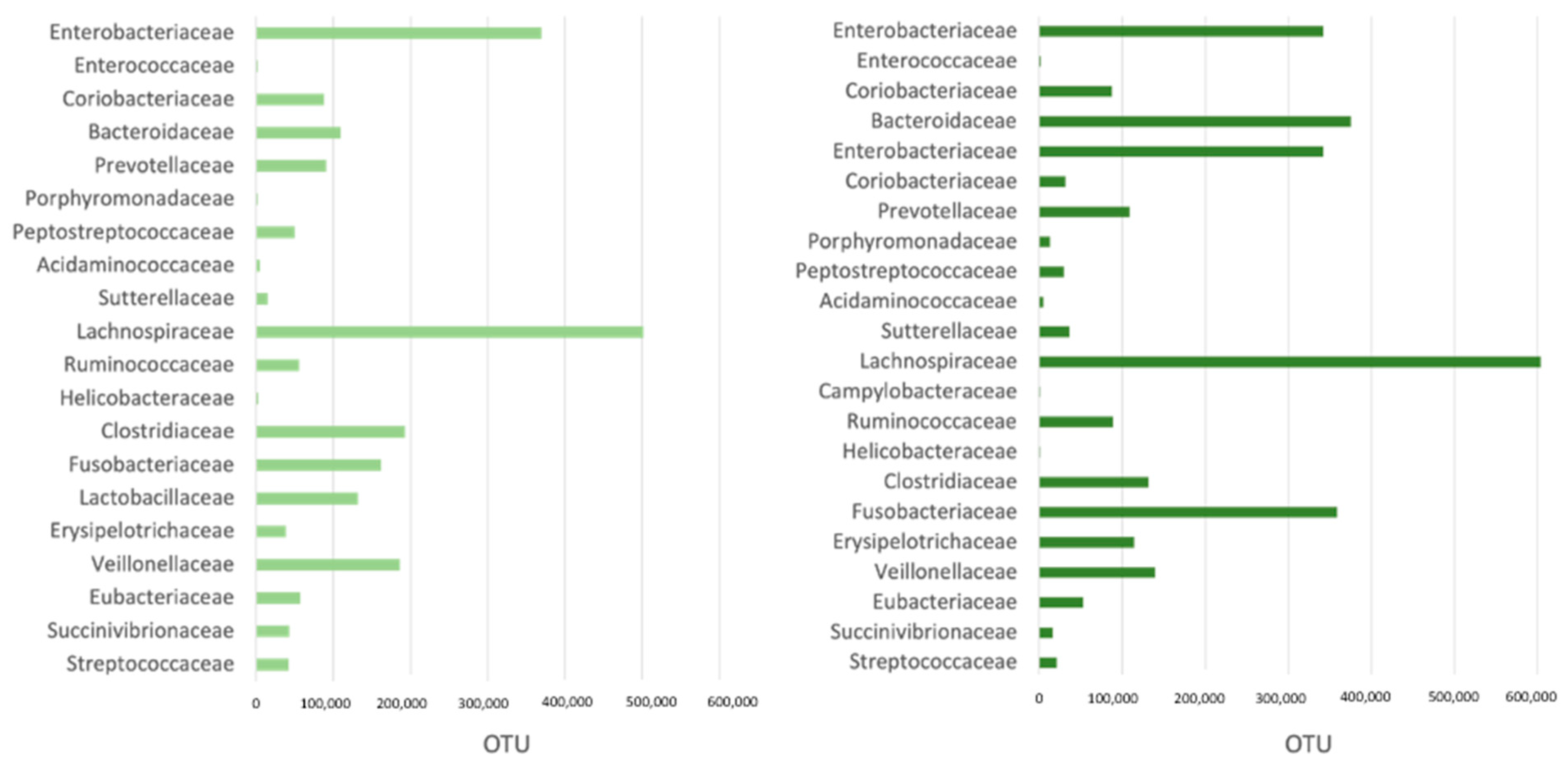
3.2. Microbiome
3.3. Fecal Bile Acids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Little, R.; Wine, E.; Kamath, B.M.; Griffiths, A.M.; Ricciuto, A. Gut microbiome in primary sclerosing cholangitis: A review. World J. Gastroenterol. 2020, 26, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Yuan, X.; Zeng, Y.; Wang, J.; Zhang, Y.; Xue, C.; Li, L. Crosstalk between Gut Microbiota and Bile Acids in Cholestatic Liver Disease. Nutrients 2023, 15, 2411. [Google Scholar] [CrossRef]
- Di Gregorio, M.C.; Cautela, J.; Galantini, L. Physiology and Physical Chemistry of Bile Acids. Int. J. Mol. Sci. 2021, 22, 1780. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhe, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Kobyliak, N.; Abenavoli, L.; Mykhalchyshyn, G.; Kononenko, L.; Boccuto, L.; Kyriienko, D.; Dynnyk, O. A Multi-strain Probiotic Reduces the Fatty Liver Index. Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J. Gastrointestin Liver Dis. 2018, 27, 41–49. [Google Scholar] [CrossRef]
- Bakhshimoghaddam, F.; Shateri, K.; Sina, M.; Hashemian, M.; Alizadeh, M. Daily Consumption of Synbiotic Yogurt Decreases Liver Steatosis in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. J. Nutr. 2018, 148, 1276–1284. [Google Scholar] [CrossRef]
- Eslamparast, T.; Poustchi, H.; Zamani, F.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in nonalcoholic fatty liver disease: A randomized. double-blind. placebo-controlled pilot study. Am. J. Clin. Nutr. 2014, 99, 535–542. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Analysis of the gut microbiome in dogs and cats. Vet. Clin. Pathol. 2022, 50 (Suppl. 1), 6–17. [Google Scholar] [CrossRef] [PubMed]
- Squire, N.; Lux, C.; Tolbert, K.; Lidbury, J.; Sun, X.; Suchodolski, J.S. Characterization of the Fecal Microbiome in Dogs Receiving Medical Management for Congenital Portosystemic Shunts. Front. Vet. Sci. 2022, 28, 897760. [Google Scholar] [CrossRef] [PubMed]
- Habermaass, V.; Olivero, D.; Gori, E.; Mariti, C.; Longhi, E.; Marchetti, V. Intestinal Microbiome in Dogs with Chronic Hepatobiliary Disease: Can We Talk about the Gut-Liver Axis? Animals 2023, 13, 3174. [Google Scholar] [CrossRef] [PubMed]
- Kakiyama, G.; Muto, A.; Takei, H.; Nittono, H.; Murai, T.; Kurosawa, T.; Hofmann, A.F.; Pandak, W.M.; Bajaj, J.S. A simple and accurate HPLC method for fecal bile acid profile in healthy and cirrhotic subjects: Validation by GC-MS and LC-MS. J. Lipid Res. 2014, 55, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.R.L.; Center, S.A.; Cullen, J.M.; Penninck, D.G.; Richter, K.P.; Twedt, D.C.; Watson, P.J. ACVIM consensus statement on the diagnosis and treatment of chronic hepatitis in dogs. J. Vet. Intern. Med. 2019, 33, 1173–1200. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.C.J.; Walsh, K.A.; Harris, J.A.; Moffett, B.F. Using ecological diversity measures with bacterial communities. FEMS Microbiol. Ecol. 2003, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marciňáková, M.; Simonova, M.; Strompfova, V.; Laukova, A. Oral application of Enterococcus faecium strain EE3 in healthy dogs. Folia Microbiol. 2006, 51, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Sengaut, J.; Januskevicius, A.; Januskeviciene, G.; Gabinaitis, P. The influence of probiotic bilavet on morphological blood parameters. digestibility and chemical composition of faeces in German shepherd dogs. Vet. Zootech. 2011, 55, 72–78. [Google Scholar]
- Strompfová, V.; Simonová, M.P.; Gancarčíková, S.; Mudroňová, D.; Farbáková, J.; Mad’ari, A.; Lauková, A. Effect of Bifidobacterium animalis B/12 administration in healthy dogs. Anaerobe 2014, 28, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, S.S. Value of Probiotics in Canine and Feline Gastroenterology. Vet. Clin. North. Am. Small Anim. Pract. 2021, 51, 171–217. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. J. Gastroenterol. 2012, 143, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Nor, M.H.; Ayob, N.; Mokhtar, N.M.; Raja Ali, R.A.; Tan, G.C.; Wong, Z.; Shafiee, N.H.; Wong, Y.P.; Mustangin, M.; Nawawi, K.N.M. The Effect of Probiotics (MCP® BCMC® Strains) on Hepatic Steatosis. Small Intestinal Mucosal Immune Function. and Intestinal Barrier in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 3192. [Google Scholar] [CrossRef] [PubMed]
- Escouto, G.S.; Port, G.Z.; Tovo, C.V.; Fernandes, S.A.; Peres, A.; Dorneles, G.P.; Houde, V.P.; Varin, T.V.; Pilon, G.; Marette, A.; et al. Probiotic Supplementation. Hepatic Fibrosis. and the Microbiota Profile in Patients with Nonalcoholic Steatohepatitis: A Randomized Controlled Trial. J. Nutr. 2023, 153, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Haga, S.; Aoyama, Y.; Kiriyama, S. Shortchain fatty acids suppress cholesterol synthesis in rat liver and intestine. J. Nutr. 1999, 129, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Carpi, R.Z.; Barbalho, S.M.; Sloan, K.P.; Laurindo, L.F.; Gonzaga, H.F.; Grippa, P.C.; Zutin, T.L.M.; Girio, R.J.S.; Repetti, C.S.F.; Detregiachi, C.R.P.; et al. The Effects of Probiotics, Prebiotics and Synbiotics in Non-Alcoholic Fat Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8805. [Google Scholar] [CrossRef] [PubMed]
- Lucena, R.; Novales, M.; Blanco, B.; Hernández, E.; Ginel, P.J. Effect of probiotic Enterococcus faecium SF68 on liver function in healthy dogs. J. Vet. Intern. Med. 2019, 33, 2628–2634. [Google Scholar] [CrossRef] [PubMed]
- Rodenes-Gavidia, A.; Lamelas, A.; Bloor, S.; Hobson, A.; Treadway, S.; Haworth, J.; Vijayakumar, V.; Naghibi, M.; Day, R.; Chenoll, E. An insight into the functional alterations in the gut microbiome of healthy adults in response to a multi-strain probiotic intake: A single arm open label trial. Front. Cell Infect. Microbiol. 2023, 13, 1240267. [Google Scholar] [CrossRef]
- Lin, D.; Peters, B.A.; Friedlander, C.; Freiman, H.J.; Goedert, J.J.; Sinha, R.; Miller, G.; Bernstein, M.A.; Hayes, R.B.; Ahn, J. Association of dietary fibre intake and gut microbiota in adults. Br. J. Nutr. 2018, 120, 1014–1022. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Lu, M.; Cai, J.; Lu, B.; Luo, C.; Dai, M. Habitual Diet Pattern Associations with Gut Microbiome Diversity and Composition: Results from a Chinese Adult Cohort. Nutrients 2022, 14, 2639. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wu, Y.; Pham, Q.; Li, R.W.; Yu, L.; Chen, M.; Boue, S.M.; Yokoyama, W.; Li, B.; Wang, T.T.Y. Effects of differences in resistant starch content of rice on intestinal microbial composition. J. Agric. Food Chem. 2021, 69, 8017–8027. [Google Scholar] [CrossRef] [PubMed]
- Rau, M.; Rehman, A.; Dittrich, M.; Groen, A.K.; Hermanns, H.M.; Seyfried, F.; Beyersdorf, N.; Dandekar, T.; Rosenstiel, P.; Geier, A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United Eur. Gastroenterol. J. 2018, 6, 1496–1507. [Google Scholar] [CrossRef]
- Blake, A.B.; Guard, B.C.; Honneffer, J.B.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Altered microbiota. fecal lactate. and fecal bile acids in dogs with gastrointestinal disease. PLoS ONE 2019, 14, e0224454. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut Microbiota-Derived Short Chain Fatty Acids Are Potential Mediators in Gut Inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Schumann, R.R.; Leong, S.R.; Flaggs, G.W.; Gray, P.W.; Wright, S.D.; Mathison, J.C.; Tobias, P.S.; Ulevitch, R.J. Structure and function of lipopolysaccha ride binding protein. Science 1990, 249, 1429–1431. [Google Scholar] [CrossRef] [PubMed]
- Vatanen, T.; Kostic, A.D.; d’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hämäläinen, A.M.; et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 2016, 165, 842–853. [Google Scholar] [CrossRef]
- Fei, N.; Bruneau, A.; Zhang, X.; Wang, R.; Wang, J.; Rabot, S.; Gérard, P.; Zhao, L. Endotoxin producers overgrowing in human gut microbiota as the causative agents for nonalcoholic fatty liver disease. mBio 2020, 11, e03263-19. [Google Scholar] [CrossRef] [PubMed]
- Arrese, M.; Cabrera, D.; Kalergis, A.M.; Feldstein, A.E. Innate immunity and inflammation in NAFLD/NASH. Dig. Dis. Sci. 2016, 61, 1294–1303. [Google Scholar] [CrossRef]
- Abu-Shanab, A.; Quigley, E.M. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 691–701. [Google Scholar] [CrossRef]
- Machado, M.V.; Cortez-Pinto, H. Gut microbiota and nonalcoholic fatty liver disease. Ann. Hepatol. 2012, 11, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Devillard, E.; McIntosh, F.M.; Duncan, S.H.; Wallace, R.J. Metabolism of linoleic acid by human gut bacteria: Different routes for biosynthesis of conjugated linoleic acid. J. Bacteriol. 2007, 189, 2566–2570. [Google Scholar] [CrossRef]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of urease-and uricase-containing. indole-and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef]
- Sheridan, P.O.; Martin, J.C.; Lawley, T.D.; Browne, H.P.; Harris, H.M.; Bernalier-Donadille, A.; Duncan, S.H.; O’Toole, P.W.; Scott, K.P.; Flint, H.J. Polysaccharide utilization loci and nutritional specialization in a dominant group of butyrate-producing human colonic Firmicutes. Microb. Genom. 2016, 2, e000043. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Carbajal, A.; Nirmalkar, K.; Pérez-Lizaur, A.; Hernández-Quiroz, F.; Ramírez-del-Alto, S.; García-Mena, J.; Hernández-Guerrero, C. Gut Microbiota and Predicted Metabolic Pathways in a Sample of Mexican Women Affected by Obesity and Obesity Plus Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 438. [Google Scholar] [CrossRef] [PubMed]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Kedenko, I.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef. Microbes 2017, 8, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Salonen, A.; Lahti, L.; Salojärvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014, 8, 2218. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55. [Google Scholar] [CrossRef]
- Kameyama, K.; Itoh, K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Zheng, R.D.; Sun, X.Q.; Ding, W.J.; Wang, X.Y.; Fan, J.G. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatob. Pancreat. Dis. Int. 2017, 16, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, M.N.; Sergeant, M.; Kay, G.; Iqbal, T.; Chan, J.; Constantinidou, C.; Trivedi, P.; Ferguson, J.; Adams, D.H.; Pallen, M.; et al. The gut-adherent microbiota of PSC–IBD is distinct to that of IBD. Gut 2017, 66, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bao, X.; Goel, A.; Colombel, J.F.; Pekow, J.; Jabri, B.; Williams, K.M.; Castillo, A.; Odin, J.A.; Meckel, K.; et al. The features of mucosa-associated microbiota in primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 2016, 43, 790–801. [Google Scholar] [CrossRef]
- Duboc, H.; Rajca, S. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 2013, 62, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Vavassori, P.; Mencarelli, A. Secondary bile acids and gut microbiota in the pathogenesis of inflammatory bowel diseases. Pharmacol. Res. 2014, 85, 58–64. [Google Scholar]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016, 7, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.; Bernstein, C.; Payne, C.M.; Dvorakova, K.; Garewal, H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat. Res./Rev. Mutat. Res. 2005, 589, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.B.; Fukiya, S.; Hagio, M.; Fujii, N.; Ishizuka, S.; Ooka, T.; Ogura, Y.; Hayashi, T.; Yokota, A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011, 141, 1773–1781. [Google Scholar] [CrossRef]
- Joyce, S.A.; MacSharry, J.; Casey, P.G.; Kinsella, M.; Murphy, E.F.; Shanahan, F.; Hill, C.; Gahan, C.G. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl. Acad. Sci. USA 2014, 111, 7421–7426. [Google Scholar] [CrossRef]
- Schaap, F.G.; Trauner, M.; Jansen, P.L. Bile acid receptors as targets for drug development. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J. Hepatol. 2013, 58, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.; Marchesi, J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 13580–13585. [Google Scholar] [CrossRef]
- Begley, M.; Gahan, C.G.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wu, X.; Nawaz, M.; Li, J.; Yu, P.; Moore, J.E.; Xu, J. Molecular characterization of fecal microbiota in patients with viral diarrhea. Curr. Microbiol. 2018, 75, 811–816. [Google Scholar] [CrossRef]
| Type of Addictive (per Kg) | Component |
|---|---|
| Vitamins |
|
| Microbial component and yeast |
|
| Organoleptic additives |
|
| Other |
|
| Demographics | T0 | T1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Group | Breed | Sex | Age (y) | Diagnosis | ALT | GI Signs | ALT | GI Signs |
| 1 | TG | Mix-breed | M | 10.1 | Degenerative h. | 107 | Yes | 59 | no |
| 2 | TG | Maltese | MN | 13.3 | CCHBD | 1693 | No | 409 | no |
| 3 | TG | CKCS | M | 9.9 | CCHBD | 299 | No | 118 | no |
| 4 | TG | Cocker Spaniel | M | 7.8 | CCHBD | 45 | No | 33 | no |
| 5 | TG | Mix-breed | FN | 2.6 | Chronic hepatitis | 465 | No | 329 | no |
| 6 | TG | Golden Retriever | M | 8.8 | CCHBD | 25 | Yes | 34 | no |
| 7 | TG | Dachshund | F | 14.7 | CCHBD | 38 | No | 44 | no |
| 8 | TG | Shih Tzu | F | 14.6 | CCHBD | 37 | No | 45 | no |
| 9 | TG | French bulldog | FN | 3 | Chronic hepatitis | 683 | No | 273 | no |
| 10 | TG | Mix-breed | M | 13.4 | CCHBD | 51 | Yes | 56 | no |
| 11 | TG | Yorkshire | M | 11.7 | CCHBD | 713 | No | 943 | no |
| 12 | TG | Mix-breed | FN | 10.2 | Chronic hepatitis | 46 | Yes | 956 | no |
| 13 | TG | Toy poodle | F | 13.5 | CCHBD | 30 | No | 27 | no |
| 14 | TG | Mix-breed | FN | 8.9 | CCHBD | 690 | Yes | 53 | no |
| 15 | TG | Mix-breed | M | 9.4 | Chronic hepatitis | 877 | Yes | 413 | yes |
| Median age (range) | 10 (2.6–14.7) | ||||||||
| 16 | CG | Mix-breed | M | 7.6 | CCHBD | 61 | No | 52 | no |
| 17 | CG | Mix-breed | FN | 8.6 | Chronic hepatitis | 178 | No | 230 | yes |
| 18 | CG | Mix-breed | MN | 10.3 | CCHBD | 57 | No | 61 | no |
| 19 | CG | English Setter | M | 9.2 | CCHBD | 126 | No | 120 | no |
| 20 | CG | Jack Russel Terrier | F | 14.0 | Degenerative h. | 56 | No | 107 | no |
| 21 | CG | WHWT | F | 10.3 | Chronic hepatitis | 103 | No | 91 | no |
| 22 | CG | Breton | F | 7.6 | Degenerative h. | 48 | No | 68 | no |
| 23 | CG | Bull terrier | MN | 10.0 | CCHBD | 30 | No | 31 | no |
| 24 | CG | CKCS | FN | 8.8 | Chronic hepatitis | 52 | No | 72 | no |
| 25 | CG | CKCS | FN | 7.7 | CCHBD | 2652 | No | 41 | no |
| 26 | CG | WHWT | F | 10.7 | Degenerative h. | 54 | Yes | 57 | no |
| 27 | CG | Labrador | M | 9.6 | CCHBD | 311 | Yes | 350 | no |
| 28 | CG | Toy poodle | F | 5.7 | CCHBD | 204 | Yes | 122 | yes |
| 29 | CG | Boxer | F | 9.3 | Degenerative h. | 467 | No | 435 | yes |
| 30 | CG | Mix-breed | FN | 11.3 | CCHBD | 114 | No | 108 | yes |
| 31 | CG | Yorkshire | FN | 11.6 | CCHBD | 127 | No | 195 | no |
| Median age (range) | 9.4 (5.7–14) | ||||||||
| Biochemical Parameter | Treatment Group | Control Group | Reference Range | p-Value |
|---|---|---|---|---|
| ALP (U/L) | 512 (86–8907) | 576 (74–8839) | 45–250 | 0.82 |
| GGT (U/L) | 3.9 (1.1–375) | 5.4 (2.2–70) | 2–11 | 0.59 |
| AST (U/L) | 37 (18–324) | 35 (20–1493) | 15–40 | 0.94 |
| ALT (U/L) | 107 (25–1697) | 108 (30–2652) | 20–70 | 0.90 |
| Tot Bil (mg/dL) | 0.22 (0.1–1.55) | 0.2 (0.07–6.55) | 0.07–0.3 | 0.27 |
| TP (g/dL) | 6.9 (4.4–9.1) | 7 (5.2–9.8) | 5.8–7.8 | 0.54 |
| Alb (g/dL) | 4 (2.7–4.6) | 3.9 (2.8–5.4) | 2.6–4.1 | 0.91 |
| Chol (mg/dL) | 297 (111–673) | 318 (90–677) | 120–280 | 0.57 |
| Trig (mg/dL) | 130 (46–1475) | 114 (45–654) | 25–90 | 0.91 |
| Treatment Group | Control Group | |||
|---|---|---|---|---|
| Timepoint | T0 | T1 | T0 | T2 |
| Alpha diversity | 2.407 (0.831−3.163) | 2.533 (1.501−3.197) | 16,000 (9000−32,000) | 39,000 (26,000−48,000) |
| N species | 15,000 (11,000−19,000) | 16,000 (13,000−23,000) | 2.200 (0.839−3.491) | 3.292 (2.207−4.098) |
| Treatment Group | Control Group | |||||
|---|---|---|---|---|---|---|
| T0 | T1 | p-Value | T0 | T1 | p-Value | |
| CDCA | 162.9 (56.6–705.3) | 396 (75.4–999.7) | 0.06 | 391 (12.9–861.1) | 458.3 (47.6–1390) | 0.27 |
| UDCA | 197.1 (32.5–689.3) | 114.8 (18.7–669.6) | 0.85 | 277.3 (112.7–1734) | 246.1 (5.8–1332) | 0.81 |
| CA | 377.6 (9.1–1305) | 245.3 (18.9–1513) | 0.36 | 239.3 (22.5–1303) | 100.7 (16.6–726.5) | 0.34 |
| DCA | 238 (7.5–2351) | 412.1 (7.15–1578) | 0.24 | 407.4 (9.05–1928) | 283.4 (3.5–1416) | 0.48 |
| LCA | 538.4 (48.4–1209) | 458.7 (30.39–1392) | 0.81 | 605.5 (155.8–1195) | 372 (23.3–1077) | 0.22 |
| Total Primary | 656.6 (69.3–1472) | 665.4 (331–1943) | 0.09 | 512.8 (146.4–1823) | 655.1 (88.7–2104) | 0.98 |
| Total Secondary | 910.1 (168.5–3705) | 1011 (139.7–3031) | 0.48 | 1198 (379.7–3456) | 948.2 (33.7–2820) | 0.46 |
| Ratio P/S | 0.35 (0.06–2.3) | 0.7 (0.2–2.4) | 0.94 | 0.5515 (0.07–1.56) | 0.598 (0.2–2.634162) | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habermaass, V.; Biolatti, C.; Bartoli, F.; Gori, E.; Bruni, N.; Olivero, D.; Marchetti, V. Effects of Synbiotic Administration on Gut Microbiome and Fecal Bile Acids in Dogs with Chronic Hepatobiliary Disease: A Randomized Case–Control Study. Vet. Sci. 2024, 11, 364. https://doi.org/10.3390/vetsci11080364
Habermaass V, Biolatti C, Bartoli F, Gori E, Bruni N, Olivero D, Marchetti V. Effects of Synbiotic Administration on Gut Microbiome and Fecal Bile Acids in Dogs with Chronic Hepatobiliary Disease: A Randomized Case–Control Study. Veterinary Sciences. 2024; 11(8):364. https://doi.org/10.3390/vetsci11080364
Chicago/Turabian StyleHabermaass, Verena, Corrado Biolatti, Francesco Bartoli, Eleonora Gori, Natascia Bruni, Daniela Olivero, and Veronica Marchetti. 2024. "Effects of Synbiotic Administration on Gut Microbiome and Fecal Bile Acids in Dogs with Chronic Hepatobiliary Disease: A Randomized Case–Control Study" Veterinary Sciences 11, no. 8: 364. https://doi.org/10.3390/vetsci11080364
APA StyleHabermaass, V., Biolatti, C., Bartoli, F., Gori, E., Bruni, N., Olivero, D., & Marchetti, V. (2024). Effects of Synbiotic Administration on Gut Microbiome and Fecal Bile Acids in Dogs with Chronic Hepatobiliary Disease: A Randomized Case–Control Study. Veterinary Sciences, 11(8), 364. https://doi.org/10.3390/vetsci11080364








