Isolation of the Initial Bovine Alphaherpesvirus 1 Isolate from Yanbian, China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Sample Collection
2.2. Cloning and Sequence Analysis of the gB Gene
2.3. Virus Isolation
2.4. Virus Titration (TCID50)
2.5. Virus Growth Curve
2.6. Indirect Immunofluorescence Assay (IFA)
2.7. Transmission Electron Microscopy (TEM)
2.8. Analysis of the Genetic Evolution of Amino Acids
2.9. BoAHV1-YBYJ Glycoprotein Gene Expression in MDBK Cells
3. Results
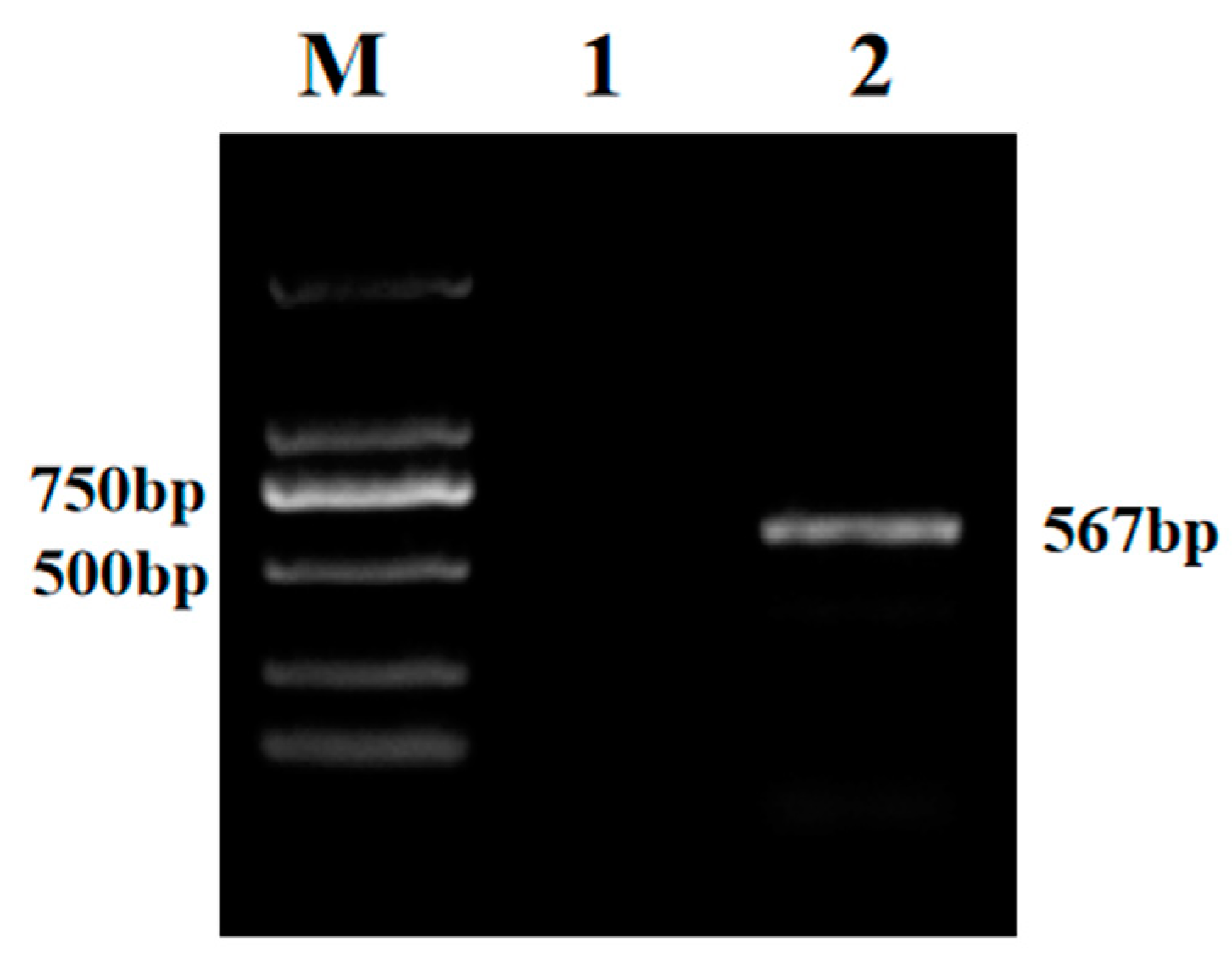
3.1. PCR Analysis of Test Samples
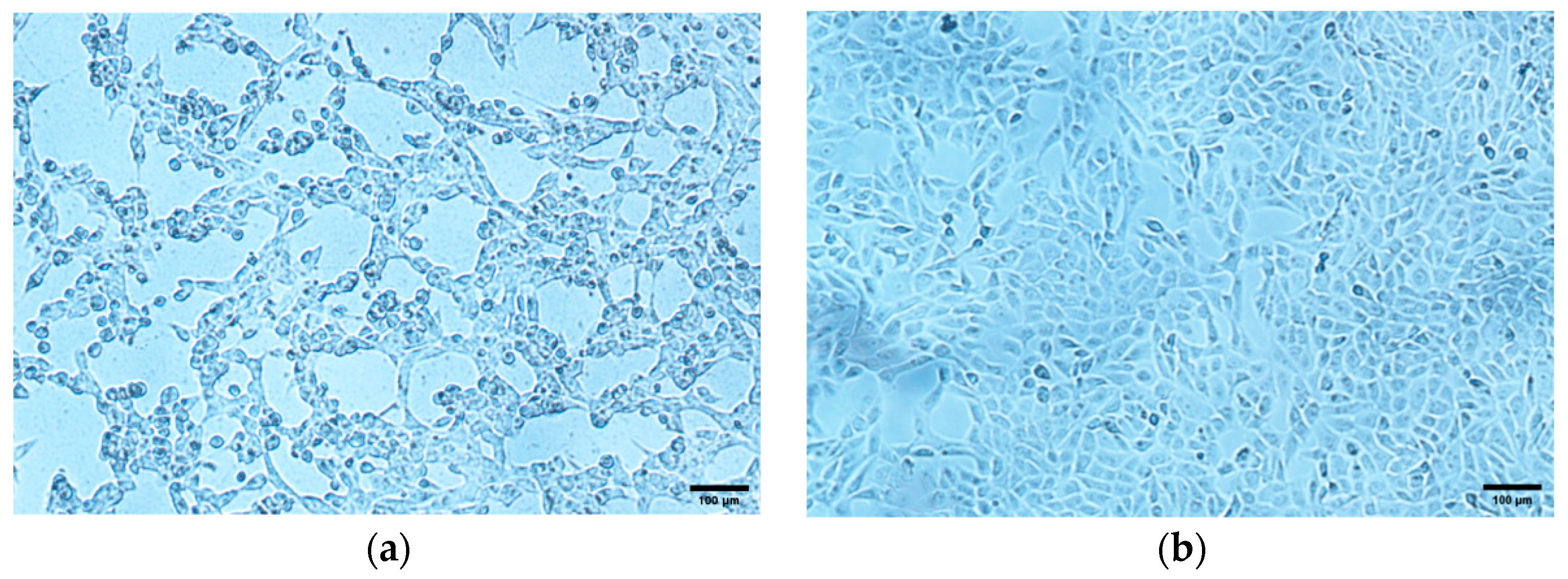
3.2. Virus Isolation
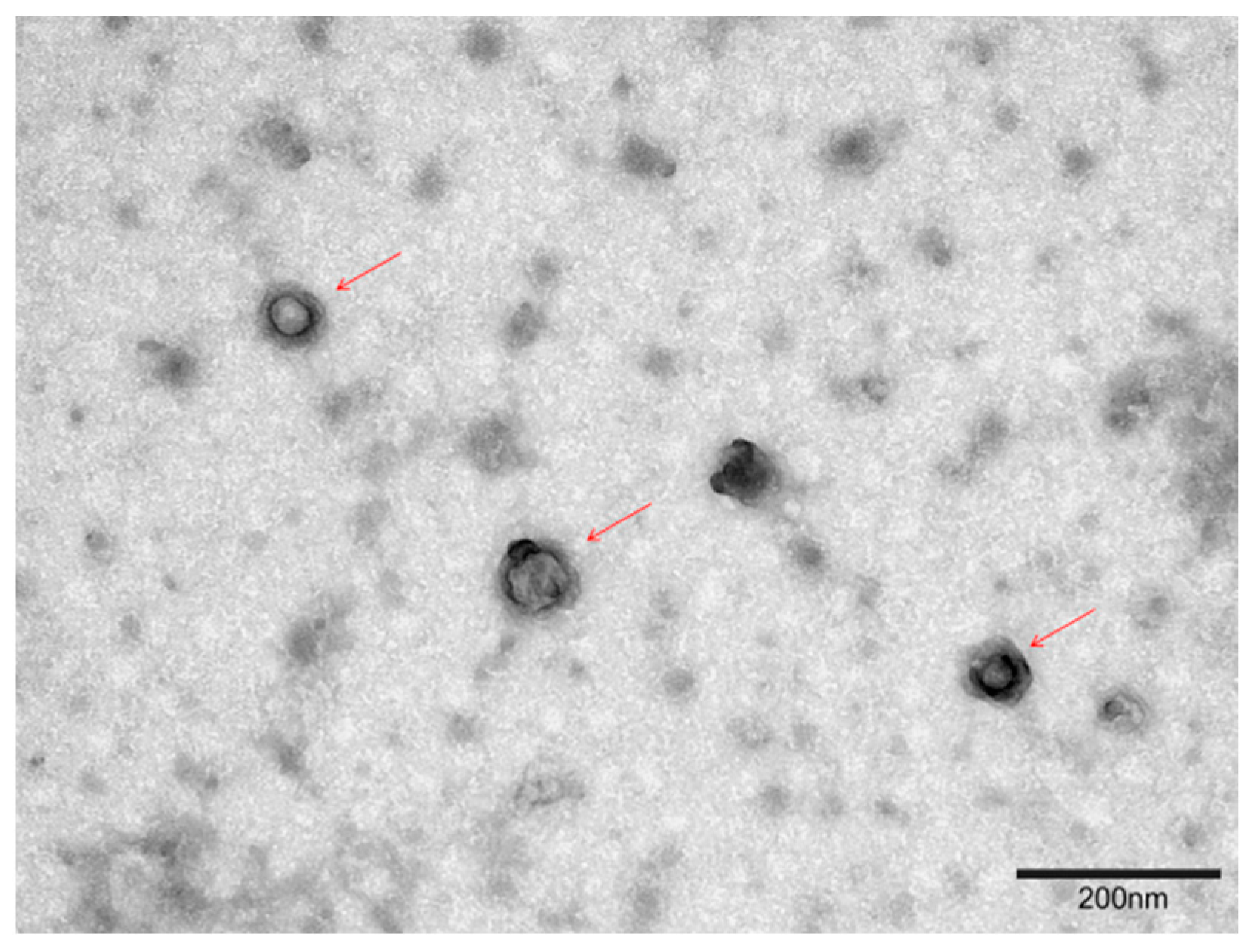
3.3. Transmission Electron Microscopy (TEM)
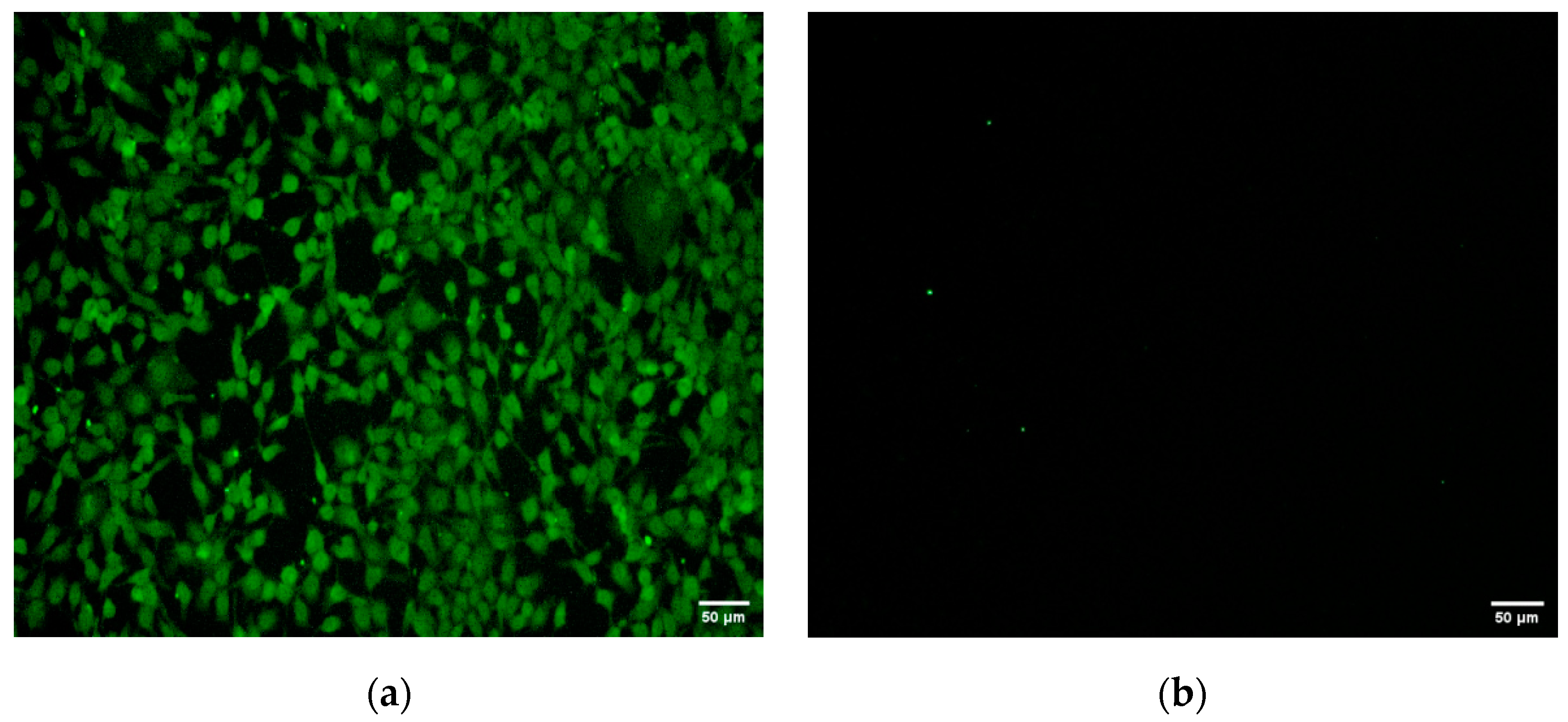
3.4. Indirect Immunofluorescence Assay (IFA)
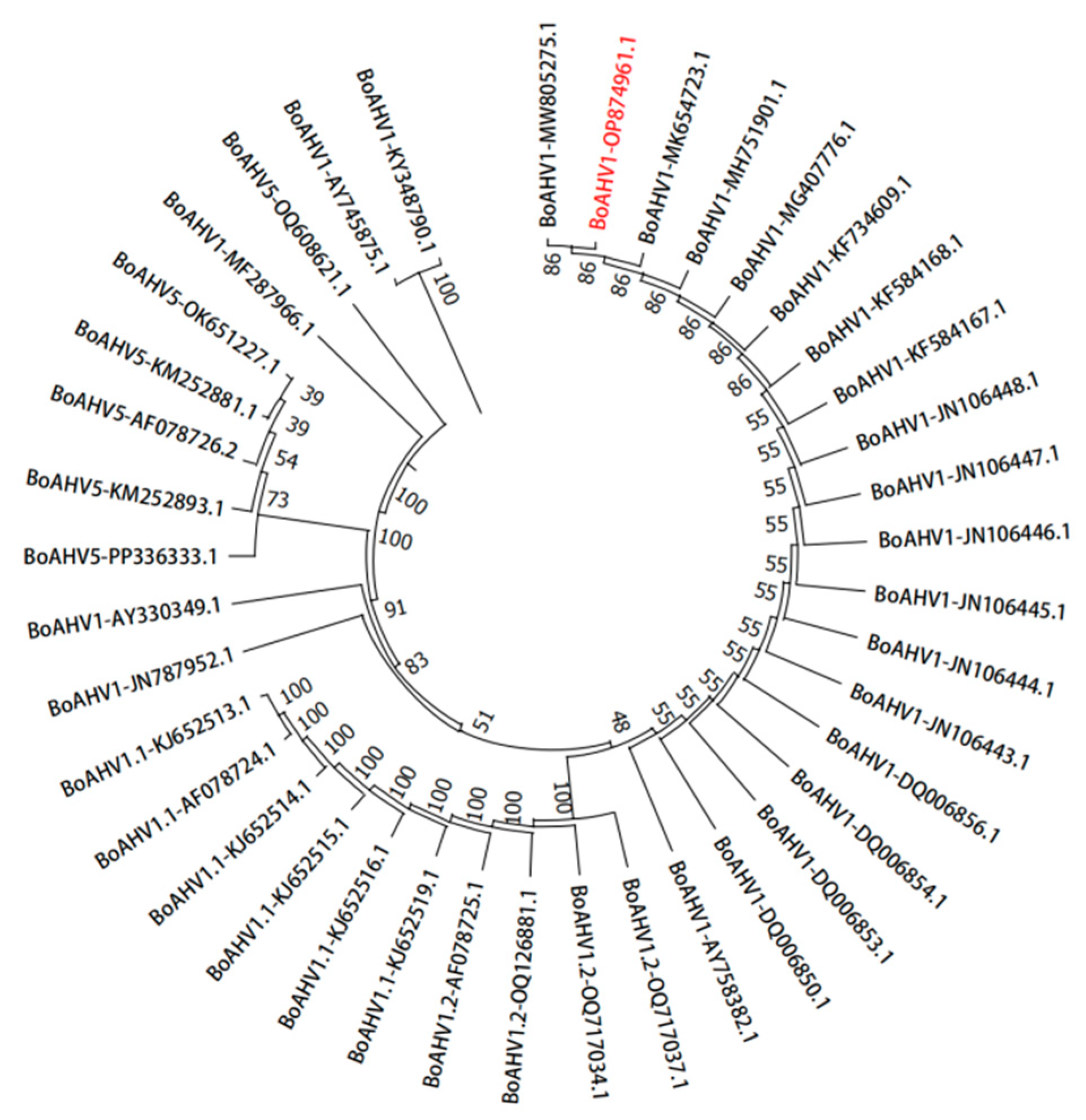
3.5. Phylogenetic Analysis of Amino Acid Evolution in Isolates
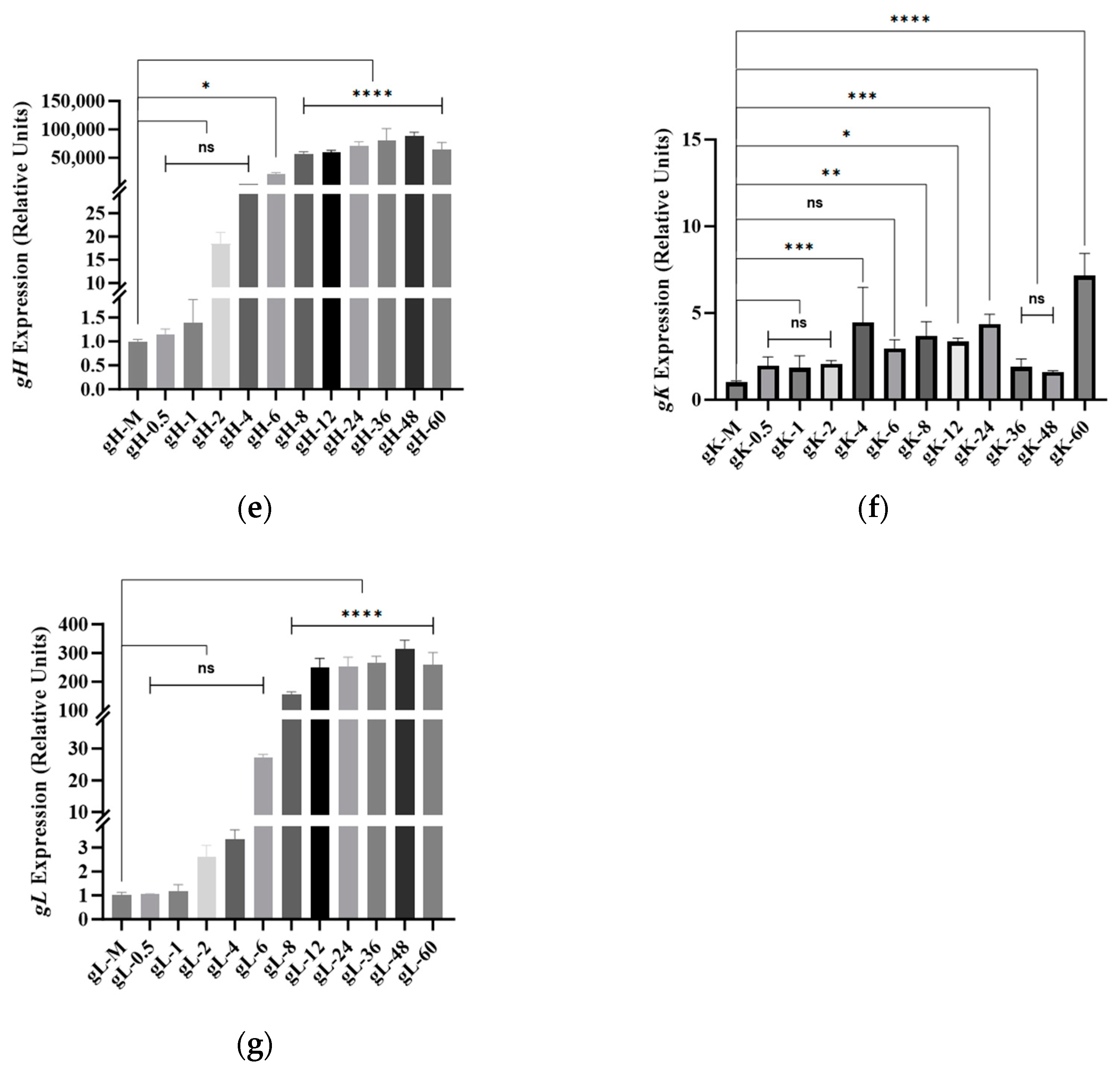
3.6. BoAHV1-YBYJ Glycoprotein Gene Expression in MDBK Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khattar, S.K.; van Drunen Littel-van den Harke, S.; Attah-Poku, S.K.; Babiuk, L.A.; Tikoo, S.K. Identification and characterization of a bovine herpesvirus-1 (BHV-1) glycoprotein gL which is required for proper antigenicity, processing, and transport of BHV-1 glycoprotein gH. Virology 1996, 219, 66–76. [Google Scholar] [CrossRef]
- Roberts, L.; Wood, D.A.; Hunter, A.R.; Munro, R.; Imray, S.W. Infectious bovine rhinotracheitis. Vet. Rec. 1981, 108, 107. [Google Scholar] [CrossRef]
- Durham, P.J. Infectious bovine rhinotracheitis virus and its role in bovine abortion. N. Z. Vet. J. 1974, 22, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Bitsch, V. Infectious bovine rhinotracheitis virus infection in bulls, with special reference to preputial infection. Appl. Microbiol. 1973, 26, 337–343. [Google Scholar] [CrossRef]
- O’Connor, A.M. Infectious Bovine Keratoconjunctivitis. Vet. Clin. N. Am. Food Anim. Pract. 2021, 37, xi–xii. [Google Scholar] [CrossRef]
- Yates, W.D. A review of infectious bovine rhinotracheitis, shipping fever pneumonia and viral-bacterial synergism in respiratory disease of cattle. Can. J. Comp. Med. Rev. Can. Med. Comp. 1982, 46, 225–263. [Google Scholar]
- Tikoo, S.K.; Campos, M.; Babiuk, L.A. Bovine herpesvirus 1 (BHV-1): Biology, pathogenesis, and control. Adv. Virus Res. 1995, 45, 191–223. [Google Scholar] [CrossRef] [PubMed]
- Tikoo, S.K.; Campos, M.; Popowych, Y.I.; van Drunen Littel-van den Hurk, S.; Babiuk, L.A. Lymphocyte proliferative responses to recombinant bovine herpes virus type 1 (BHV-1) glycoprotein gD (gIV) in immune cattle: Identification of a T cell epitope. Viral Immunol. 1995, 8, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Jones, C. Herpes simplex virus type 1 and bovine herpesvirus 1 latency. Clin. Microbiol. Rev. 2003, 16, 79–95. [Google Scholar] [CrossRef]
- Booker, C.W.; Guichon, P.T.; Jim, G.K.; Schunicht, O.C.; Harland, R.J.; Morley, P.S. Seroepidemiology of undifferentiated fever in feedlot calves in western Canada. Can. Vet. J. Rev. Vet. Can. 1999, 40, 40–48. [Google Scholar]
- Martin, S.W.; Bateman, K.G.; Shewen, P.E.; Rosendal, S.; Bohac, J.G.; Thorburn, M. A group level analysis of the associations between antibodies to seven putative pathogens and respiratory disease and weight gain in Ontario feedlot calves. Can. J. Vet. Res. Rev. Can. Rech. Vet. 1990, 54, 337–342. [Google Scholar]
- Yeşilbağ, K.; Güngör, B. Antibody prevalence against respiratory viruses in sheep and goats in North-Western Turkey. Trop. Anim. Health Prod. 2009, 41, 421–425. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, Y.; Jiang, X.; Yuan, W.; Zhu, G. First report of bovine herpesvirus 1 isolation from bull semen samples in China. Acta Virol. 2017, 61, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.C.; Ye, Z.M.; Li, S.Q.; Zhong, G.; Gu, Q.J. Infectious bovine rhinotracheitis virus was isolated from cows imported from New Zealand. Chin. Vet. Sci. 1981, 8–11+2. [Google Scholar] [CrossRef]
- Feng, Q.M.; Yang, B.Q.; Zhang, R.Z.; Yang, H.Z.; Li, C.L.; Wang, G.X.; Hong, S.W.; Wang, S.Y. The regression test report of infectious bovine rhinotracheitis virus strains isolated from imported New Zealand dairy cows. China Anim. Health Insp. 1982, 20–25. [Google Scholar]
- Li, S.G.; Li, Z.G.; Chen, B.W. Report on an outbreak of infectious bovine rhinotracheitis in dairy cows imported from New Zealand. Chin. J. Prev. Vet. 1984, 3, 33–34+2. [Google Scholar]
- Metzler, A.E.; Matile, H.; Gassmann, U.; Engels, M.; Wyler, R. European isolates of bovine herpesvirus 1: A comparison of restriction endonuclease sites, polypeptides, and reactivity with monoclonal antibodies. Arch. Virol. 1985, 85, 57–69. [Google Scholar] [CrossRef]
- Muylkens, B.; Thiry, J.; Kirten, P.; Schynts, F.; Thiry, E. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet. Res. 2007, 38, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Favier, P.A.; Marin, M.S.; Pérez, S.E. Role of bovine herpesvirus type 5 (BoHV-5) in diseases of cattle. Recent findings on BoHV-5 association with genital disease. Open Vet. J. 2012, 2, 46–53. [Google Scholar] [CrossRef]
- Marawan, M.A.; Deng, M.; Wang, C.; Chen, Y.; Hu, C.; Chen, J.; Chen, X.; Chen, H.; Guo, A. Characterization of BoHV-1 gG-/tk-/gE-Mutant in Differential Protein Expression, Virulence, and Immunity. Vet. Sci. 2021, 8, 253. [Google Scholar] [CrossRef]
- Barber, K.A.; Daugherty, H.C.; Ander, S.E.; Jefferson, V.A.; Shack, L.A.; Pechan, T.; Nanduri, B.; Meyer, F. Protein Composition of the Bovine Herpesvirus 1.1 Virion. Vet. Sci. 2017, 4, 11. [Google Scholar] [CrossRef]
- Romera, S.A.; Perez, R.; Marandino, A.; LuciaTau, R.; Campos, F.; Roehe, P.M.; Thiry, E.; Maidana, S.S. Whole-genome analysis of natural interspecific recombinant between bovine alphaherpesviruses 1 and 5. Virus Res. 2022, 309, 198656. [Google Scholar] [CrossRef] [PubMed]
- van Drunen Littel-van den Hurk, S.; Myers, D.; Doig, P.A.; Karvonen, B.; Habermehl, M.; Babiuk, L.A.; Jelinski, M.; Van Donkersgoed, J.; Schlesinger, K.; Rinehart, C. Identification of a mutant bovine herpesvirus-1 (BHV-1) in post-arrival outbreaks of IBR in feedlot calves and protection with conventional vaccination. Can. J. Vet. Res. Rev. Can. Rech. Vet. 2001, 65, 81–88. [Google Scholar]
- Wu, C.T.; Li, Y.M.; Tang, S.G. Prokaryotic expression of gB gene of bovine herpesvirus-1and establishment of an indirect ELISA based on the recombinant fusion protein. Chin. Vet. Sci. 2010, 40, 1259–1264. [Google Scholar] [CrossRef]
- Chowdhury, S.I.; Wei, H.; Weiss, M.; Pannhorst, K.; Paulsen, D.B. A triple gene mutant of BoHV-1 administered intranasally is significantly more efficacious than a BoHV-1 glycoprotein E-deleted virus against a virulent BoHV-1 challenge. Vaccine 2014, 32, 4909–4915. [Google Scholar] [CrossRef]
- Wang, H.J.; Wang, S.H.; Chang, L.L.; Chen, H.J. Development of taqman probe real-time quantitative pcr to detect gb gene of bovine infectious rhinotracheitis virus. Chin. J. Anim. Infect. Dis. 2017, 25, 64–67. [Google Scholar]
- Xu, N.; Yang, F.; Lei, Y.; Li, P.A.; Guan, T.Y. Establishment of duplex real-time PCR assay for detection of infectious bovine rhinotracheitis virus gB and gE genes. Chin. J. Prev. Vet. Med. 2017, 39, 556–559. [Google Scholar]
- Granátová, M.; Psikal, I. [Cell-mediated immunity in calves immunized against or infected with the bovine rhinotracheitis virus]. Vet. Med. 1989, 34, 385–394. [Google Scholar]
- Hou, L.N.; Wu, Z.W.; Zhang, K.; Gao, H.J. Current Status, Issues, and Countermeasures of Conservation Work for the Yellow Cattle in Yanbian Region. J. Jilin Agric. Univ. 2023, 45, 396–401. [Google Scholar] [CrossRef]
- Ludwig, G.V.; Letchworth, G.J., 3rd. Temporal control of bovine herpesvirus 1 glycoprotein synthesis. J. Virol. 1987, 61, 3292–3294. [Google Scholar] [CrossRef]
- Baranowski, E.; Keil, G.; Lyaku, J.; Rijsewijk, F.A.; van Oirschot, J.T.; Pastoret, P.P.; Thiry, E. Structural and functional analysis of bovine herpesvirus 1 minor glycoproteins. Vet. Microbiol. 1996, 53, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Karimi, O.; Bitaraf Sani, M.; Bakhshesh, M.; Zareh Harofteh, J.; Poormirzayee-Tafti, H. Prevalence of bovine herpesvirus 1 antibodies and risk factors in dairy cattle of Iran’s central desert. Trop. Anim. Health Prod. 2022, 55, 23. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.S.; Franco, A.C.; Hübner, S.O.; Oliveira, M.T.; Silva, A.D.; Esteves, P.A.; Roehe, P.M.; Rijsewijk, F.A. High prevalence of co-infections with bovine herpesvirus 1 and 5 found in cattle in southern Brazil. Vet. Microbiol. 2009, 139, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.G.; Denwood, M.J.; de Sousa Américo Batista Santos, C.; Alves, C.J.; Pituco, E.M.; de Campos Nogueira Romaldini, A.H.; De Stefano, E.; Nielsen, S.S.; de Azevedo, S.S. Bayesian estimation of herd-level prevalence and risk factors associated with BoHV-1 infection in cattle herds in the State of Paraíba, Brazil. Prev. Vet. Med. 2019, 169, 104705. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.T.; Campos, F.S.; Dias, M.M.; Velho, F.A.; Freneau, G.E.; Brito, W.M.; Rijsewijk, F.A.; Franco, A.C.; Roehe, P.M. Detection of bovine herpesvirus 1 and 5 in semen from Brazilian bulls. Theriogenology 2011, 75, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.; Lane, E.; Lozano, J.M.; O’Keeffe, K.; Byrne, A.W. Bovine Herpes Virus Type 1 (BoHV-1) seroprevalence, risk factor and Bovine Viral Diarrhoea (BVD) co-infection analysis from Ireland. Sci. Rep. 2024, 14, 867. [Google Scholar] [CrossRef] [PubMed]
- Dagalp, S.B.; Farzani, T.A.; Dogan, F.; Alkan, F.; Ozkul, A. Molecular and antigenic characterization of bovine herpesvirus type 1 (BoHV-1) strains from cattle with diverse clinical cases in Turkey. Trop. Anim. Health Prod. 2020, 52, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Pchelnikov, A.V.; Yatsenyuk, S.P.; Krasnikova, M.S. Circulation of bovine herpesvirus (Herpesviridae: Varicellovirus) and bovine viral diarrhea virus (Flaviviridae: Pestivirus) among wild artiodactyls of the Moscow region. Vopr. Virusol. 2023, 68, 142–151. [Google Scholar] [CrossRef]
- Esposito, C.; Fiorito, F.; Miletti, G.; Serra, F.; Balestrieri, A.; Cioffi, B.; Cerracchio, C.; Galiero, G.; De Carlo, E.; Amoroso, M.G.; et al. Involvement of herpesviruses in cases of abortion among water buffaloes in southern Italy. Vet. Res. Commun. 2022, 46, 719–729. [Google Scholar] [CrossRef]
- Whetstone, C.A.; Evermann, J.F. Characterization of bovine herpesviruses isolated from six sheep and four goats by restriction endonuclease analysis and radioimmunoprecipitation. Am. J. Vet. Res. 1988, 49, 781–785. [Google Scholar]
- Menvík, J.; Pospísil, Z.; Suchánková, A.; Cepicá, A.; Rozkosný, V.; Machatková, M. Activation of latent infectious bovine rhinotracheitis after experimental infection with parainfluenza 3 virus in young calves. Zentralblatt Fur Vet. Reihe B J. Vet. Med. Ser. B 1976, 23, 854–864. [Google Scholar] [CrossRef]
- Porter, D.D.; Larsen, A.E.; Cox, N.A. Isolation of infectious bovine rhinotracheitis virus from Mustelidae. J. Clin. Microbiol. 1975, 1, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Chowdhury, S. A review of the biology of bovine herpesvirus type 1 (BHV-1), its role as a cofactor in the bovine respiratory disease complex and development of improved vaccines. Anim. Health Res. Rev. 2007, 8, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Keil, G.M.; Klopfleisch, C.; Giesow, K.; Veits, J. Protein display by bovine herpesvirus type 1 glycoprotein B. Vet. Microbiol. 2010, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Fujii, S.; Takada, A.; Kida, H. The amino-terminal residue of glycoprotein B is critical for neutralization of bovine herpesvirus 1. Virus Res. 2006, 115, 105–111. [Google Scholar] [CrossRef]
- Li, Y.; van Drunen Littel-van den Hurk, S.; Liang, X.; Babiuk, L.A. The cytoplasmic domain of bovine herpesvirus 1 glycoprotein B is important for maintaining conformation and the high-affinity binding site of gB. Virology 1996, 222, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; van Drunen Littel-van den Hurk, S.; Liang, X.; Babiuk, L.A. Functional analysis of the transmembrane anchor region of bovine herpesvirus 1 glycoprotein gB. Virology 1997, 228, 39–54. [Google Scholar] [CrossRef][Green Version]


| Primers | Primer Sequences (5′-3′) | Product Size (bp) |
|---|---|---|
| BoAHV1-gB | BoAHV1-gB-F: GCCGTGAAGCGGAAGTT BoAHV1-gB-R: CCTGGTGGACAAGAAGTGG | 567 |
| BoAHV1-qgB | BoAHV1-qgB-F: GGCTCGCCAACTTCTTTCA BoAHV1-qgB-R: AACGGGTTCGCAATAAACG | 124 |
| BoAHV1-qgC | BoAHV1-qgC-F: CCCGTGCTGCTGTTCGTAG BoAHV1-qgC-R: GACTTGGTGCCCATGTCGC | 176 |
| BoAHV1-qgD | BoAHV1-qgD-F: GGATTACGAGCAAAAGAAGGTT BoAHV1-qgD-R: CAAAATACGGCGGAACGAC | 125 |
| BoAHV1-qgE | BoAHV1-qgE-F: GACATCCTCAACCCCTTCG BoAHV1-qgE-R: CTGTCGTCATCCGCAAAAG | 125 |
| BoAHV1-qgH | BoAHV1-qgH-F: CCTACTGCGGCAGCGTGTT BoAHV1-qgH-R: GAGGCGAGGGTTGAAGACG | 137 |
| BoAHV1-qgK | BoAHV1-qgK-F: CGCTTGCTGTCAACTTCCG BoAHV1-qgK-R: AACCCACGCCCAGATTTTC | 188 |
| BoAHV1-qgL | BoAHV1-qgL-F: GGCAACTTATTGCTCGCAGAC BoAHV1-qgL-R: GGCAAGCACCCGCCTTATA | 189 |
| GAPDH | GAPDH-F: GACCTGCCGCCTGGAGAA GAPDH-R: GAAGAGTGAGTGTCGCTGTTGA | 144 |
| Sequence Name | GenBank ID | Location |
|---|---|---|
| BoAHV1 | OP874961 | Yanji |
| BoAHV1 | AY330349 | Brazil |
| BoAHV1 | AY745875 | Brazil |
| BoAHV1 | AY758382 | Brazil |
| BoAHV1 | DQ006850 | Brasil |
| BoAHV1 | DQ006853 | Brasil |
| BoAHV1 | DQ006854 | Brasil |
| BoAHV1 | DQ006856 | Brasil |
| BoAHV1 | JN787952 | Inner Mongolia |
| BoAHV1 | KF584167 | Israel |
| BoAHV1 | KF584168 | Israel |
| BoAHV1 | KF734609 | India |
| BoAHV1 | KY348790 | Xinjiang |
| BoAHV1 | MG407776 | USA |
| BoAHV1 | MH751901 | USA |
| BoAHV1 | MK654723 | Sichuan |
| BoAHV1 | MW805275 | Egypt |
| BoAHV1 | JN106443 | Beijing |
| BoAHV1 | JN106444 | Beijing |
| BoAHV1 | JN106445 | Beijing |
| BoAHV1 | JN106446 | Beijing |
| BoAHV1 | JN106447 | Beijing |
| BoAHV1 | JN106448 | Beijing |
| BoAHV1 | MF287966 | Hebei |
| BoAHV1.1 | AF078724 | Sweden |
| BoAHV1.1 | KJ652513 | USA |
| BoAHV1.1 | KJ652514 | USA |
| BoAHV1.1 | KJ652515 | USA |
| BoAHV1.1 | KJ652516 | USA |
| BoAHV1.1 | KJ652519 | Egypt |
| BoAHV1.2 | AF078725 | Sweden |
| BoAHV1.2 | OQ126881 | Sichuan |
| BoAHV1.2 | OQ717034 | Sichuan |
| BoAHV1.2 | OQ717037 | Xinjiang |
| BoAHV5 | AF078726 | Switzerland |
| BoAHV5 | KM252881 | Brazil |
| BoAHV5 | KM252893 | Brazil |
| BoAHV5 | OK651227 | Russia |
| BoAHV5 | OQ608621 | India |
| BoAHV5 | PP336333 | Turkey |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.; Fu, J.; Yu, K.; Gao, X.; Zang, K.; Ma, H.; Xue, H.; Song, Y.; Zhu, K.; Yang, M.; et al. Isolation of the Initial Bovine Alphaherpesvirus 1 Isolate from Yanbian, China. Vet. Sci. 2024, 11, 348. https://doi.org/10.3390/vetsci11080348
Hao J, Fu J, Yu K, Gao X, Zang K, Ma H, Xue H, Song Y, Zhu K, Yang M, et al. Isolation of the Initial Bovine Alphaherpesvirus 1 Isolate from Yanbian, China. Veterinary Sciences. 2024; 11(8):348. https://doi.org/10.3390/vetsci11080348
Chicago/Turabian StyleHao, Jingrui, Jingfeng Fu, Kai Yu, Xu Gao, Keyan Zang, Haoyuan Ma, Haowen Xue, Yanhao Song, Kunru Zhu, Meng Yang, and et al. 2024. "Isolation of the Initial Bovine Alphaherpesvirus 1 Isolate from Yanbian, China" Veterinary Sciences 11, no. 8: 348. https://doi.org/10.3390/vetsci11080348
APA StyleHao, J., Fu, J., Yu, K., Gao, X., Zang, K., Ma, H., Xue, H., Song, Y., Zhu, K., Yang, M., & Zhang, Y. (2024). Isolation of the Initial Bovine Alphaherpesvirus 1 Isolate from Yanbian, China. Veterinary Sciences, 11(8), 348. https://doi.org/10.3390/vetsci11080348






