Adhesion of Bacteroides vulgatus and Fusobacterium varium to the Colonic Mucosa of Healthy Beagles
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria and Growth Conditions
2.2. Colonic Tissue Samples
2.3. Collecting and Labeling Bacteria
2.4. Bacterial Adhesion to Colonic Mucosa
2.5. Bacterial Hydrophobicity
2.6. Statistical Analyses
3. Results
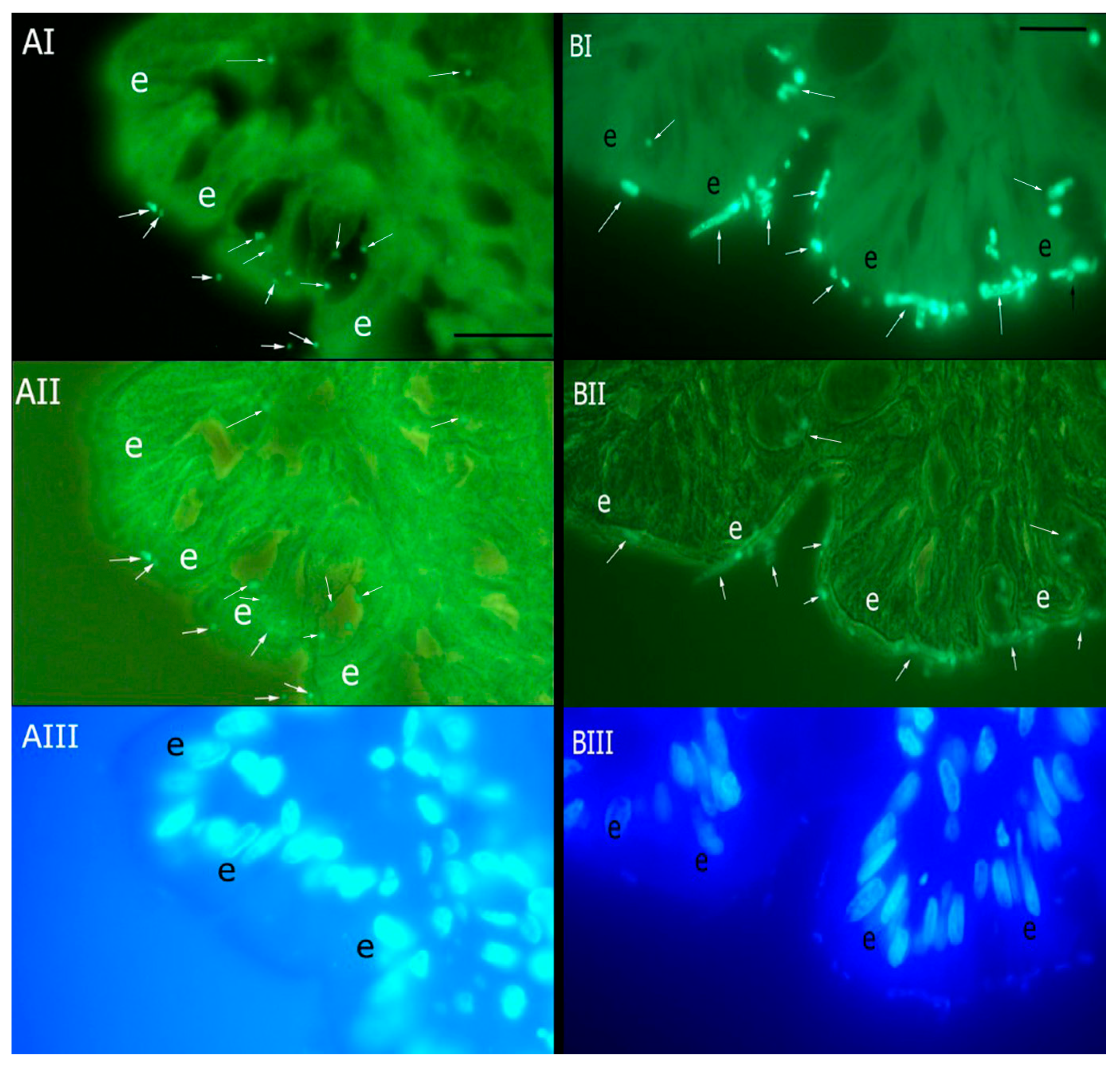
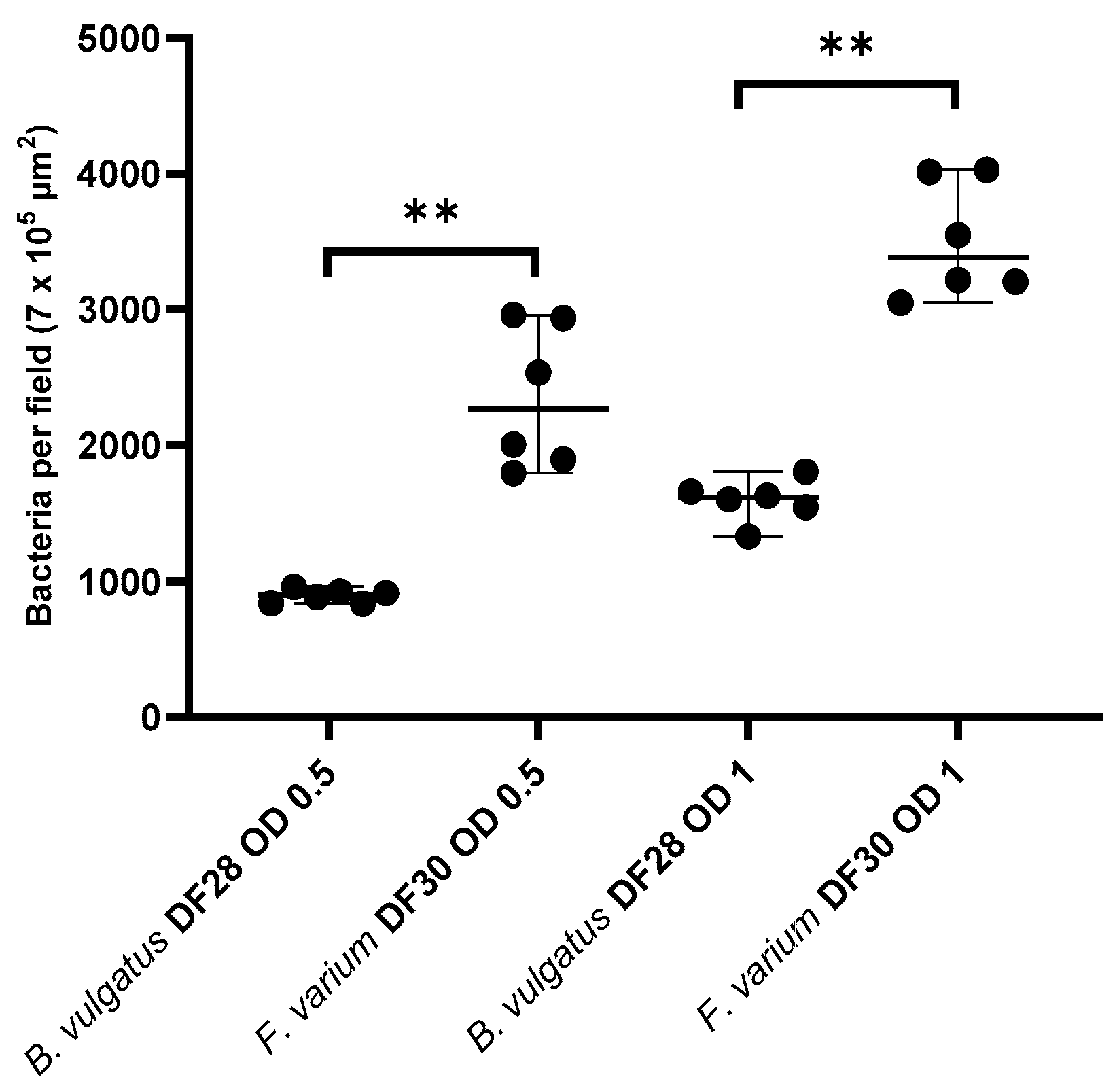
3.1. Adherence of B. vulgatus and F. varium to Canine Colonic Mucosa
3.2. Mucosal Adhesion of B. vulgatus and F. varium
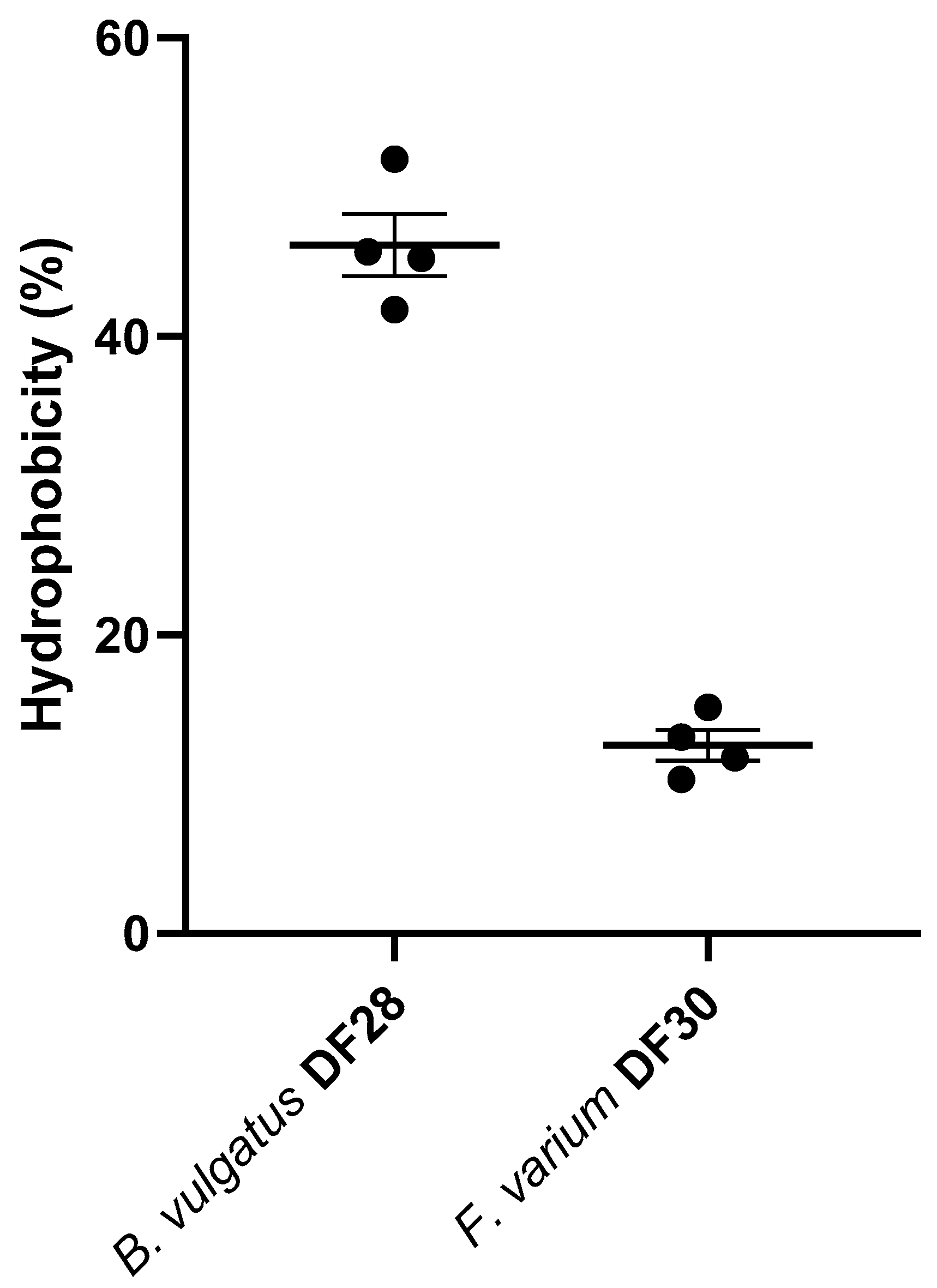
3.3. Hydrophobicity of B. vulgatus and F. varium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pilla, R.; Suchodolski, J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2019, 6, 498. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Camacho, J.; Steiner, J.M. Analysis of bacterial diversity in the canine duodenum, jejunum, ileum, and colon by comparative 16S rRNA gene analysis. FEMS Microbiol. Ecol. 2008, 66, 567–578. [Google Scholar] [CrossRef]
- Rigottier-Gois, L. Dysbiosis in inflammatory bowel diseases: The oxygen hypothesis. ISME J. 2013, 7, 1256–1261. [Google Scholar] [CrossRef]
- Jergens, A.E.; Heilmann, R.M. Canine chronic enteropathy—Current state-of-the-art and emerging concepts. Front. Vet. Sci. 2022, 9, 923013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, F.; Xue, J.; Lee, S.A.; Liu, L.; Riordan, S.M. Bacterial Species Associated with Human Inflammatory Bowel Disease and Their Pathogenic Mechanisms. Front. Microbiol. 2022, 13, 801892. [Google Scholar] [CrossRef]
- Pilla, R.; Guard, B.C.; Blake, A.B.; Ackermann, M.; Webb, C.; Hill, S.; Lidbury, J.A.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Long-Term Recovery of the Fecal Microbiome and Metabolome of Dogs with Steroid-Responsive Enteropathy. Animals 2021, 11, 2498. [Google Scholar] [CrossRef]
- Doulidis, P.G.; Galler, A.I.; Hausmann, B.; Berry, D.; Rodríguez-Rojas, A.; Burgener, I.A. Gut microbiome signatures of Yorkshire Terrier enteropathy during disease and remission. Sci. Rep. 2023, 13, 4337. [Google Scholar] [CrossRef]
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef] [PubMed]
- Minamoto, Y.; Minamoto, T.; Isaiah, A.; Sattasathuchana, P.; Buono, A.; Rangachari, V.R.; McNeely, I.H.; Lidbury, J.; Steiner, J.M.; Suchodolski, J.S. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 2019, 33, 1608–1618. [Google Scholar] [CrossRef]
- Alessandri, G.; Milani, C.; Mancabelli, L.; Mangifesta, M.; Lugli, G.A.; Viappiani, A.; Duranti, S.; Turroni, F.; Ossiprandi, M.C.; van Sinderen, D.; et al. The impact of human-facilitated selection on the gut microbiota of domesticated mammals. FEMS Microbiol. Ecol. 2019, 95, fiz121. [Google Scholar] [CrossRef]
- Alessandri, G.; Argentini, C.; Milani, C.; Turroni, F.; Cristina Ossiprandi, M.; van Sinderen, D.; Ventura, M. Catching a glimpse of the bacterial gut community of companion animals: A canine and feline perspective. Microb. Biotechnol. 2020, 13, 1708–1732. [Google Scholar] [CrossRef] [PubMed]
- Doron, L.; Coppenhagen-Glazer, S.; Ibrahim, Y.; Eini, A.; Naor, R.; Rosen, G.; Bachrach, G. Identification and characterization of fusolisin, the Fusobacterium nucleatum autotransporter serine protease. PLoS ONE 2014, 9, e111329. [Google Scholar] [CrossRef] [PubMed]
- Potrykus, J.; White, R.L.; Bearne, S.L. Proteomic investigation of amino acid catabolism in the indigenous gut anaerobe Fusobacterium varium. Proteomics 2008, 8, 2691–2703. [Google Scholar] [CrossRef]
- Ohkusa, T.; Sato, N.; Ogihara, T.; Morita, K.; Ogawa, M.; Okayasu, I. Fusobacterium varium localized in the colonic mucosa of patients with ulcerative colitis stimulates species-specific antibody. J. Gastroenterol. Hepatol. 2002, 17, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Byndloss, M.X.; Bäumler, A.J. The germ-organ theory of non-communicable diseases. Nat. Rev. Microbiol. 2018, 16, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Petrof, E.O.; Gloor, G.B.; Vanner, S.J.; Weese, S.J.; Carter, D.; Daigneault, M.C.; Brown, E.M.; Schroeter, K.; Allen-Vercoe, E. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome 2013, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Niina, A.; Kibe, R.; Suzuki, R.; Yuchi, Y.; Teshima, T.; Matsumoto, H.; Kataoka, Y.; Koyama, H. Fecal microbiota transplantation as a new treatment for canine inflammatory bowel disease. Biosci. Microbiota Food Health 2021, 40, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Toresson, L.; Spillmann, T.; Pilla, R.; Ludvigsson, U.; Hellgren, J.; Olmedal, G.; Suchodolski, J.S. Clinical Effects of Faecal Microbiota Transplantation as Adjunctive Therapy in Dogs with Chronic Enteropathies—A Retrospective Case Series of 41 Dogs. Vet. Sci. 2023, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Pengo, G.; Caldin, M.; Palumbo Piccionello, A.; Steiner, J.M.; Cohen, N.D.; Jergens, A.E.; Suchodolski, J.S. Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS ONE 2014, 9, e94699. [Google Scholar] [CrossRef]
- White, R.; Atherly, T.; Guard, B.; Rossi, G.; Wang, C.; Mosher, C.; Webb, C.; Hill, S.; Ackermann, M.; Sciabarra, P.; et al. Randomized, controlled trial evaluating the effect of multi-strain probiotic on the mucosal microbiota in canine idiopathic inflammatory bowel disease. Gut Microbes 2017, 8, 451–466. [Google Scholar] [CrossRef]
- Juntunen, M.; Kirjavainen, P.V.; Ouwehand, A.C.; Salminen, S.J.; Isolauri, E. Adherence of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infection. Clin. Diagn. Lab. Immunol. 2001, 8, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Kainulainen, V.; Tang, Y.; Spillmann, T.; Kilpinen, S.; Reunanen, J.; Saris, P.E.J.; Satokari, R. The canine isolate Lactobacillus acidophilus LAB20 adheres to intestinal epithelium and attenuates LPS-induced IL-8 secretion of enterocytes in vitro. BMC Microbiol. 2015, 15, 4. [Google Scholar] [CrossRef]
- Abed, J.; Emgard, J.E.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A.; et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe 2016, 20, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, B.; Hadad, T.; Glasner, A.; Gur, C.; Granot, Z.; Bachrach, G.; Mandelboim, O. Stromal Cell-Derived Factor 1 Mediates Immune Cell Attraction upon Urinary Tract Infection. Cell Rep. 2017, 20, 40–47. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, N.; Prete, R.; Battista, N.; Corsetti, A. Adhesion Properties of Food-Associated Lactobacillus plantarum Strains on Human Intestinal Epithelial Cells and Modulation of IL-8 Release. Front. Microbiol. 2018, 9, 2392. [Google Scholar] [CrossRef]
- Alp, D.; Kuleasan, H. Adhesion mechanisms of lactic acid bacteria: Conventional and novel approaches for testing. World J. Microbiol. Biotechnol. 2019, 35, 156. [Google Scholar] [CrossRef]
- Hanifeh, M.; Spillmann, T.; Huhtinen, M.; Sclivagnotis, Y.S.; Grönthal, T.; Hynönen, U. Ex-Vivo Adhesion of Enterococcus faecalis and Enterococcus faecium to the Intestinal Mucosa of Healthy Beagles. Animals 2021, 11, 3283. [Google Scholar] [CrossRef]
- Lauková, A.; Strompfová, V.; Ouwehand, A. Adhesion Properties of Enterococci to Intestinal Mucus of Different Hosts. Vet. Res. Commun. 2004, 28, 647–655. [Google Scholar] [CrossRef]
- Rinkinen, M.; Jalava, K.; Westermarck, E.; Salminen, S.; Ouwehand, A.C. Interaction between probiotic lactic acid bacteria and canine enteric pathogens: A risk factor for intestinal Enterococcus faecium colonization? Vet. Microbiol. 2003, 92, 111–119. [Google Scholar] [CrossRef]
- Rinkinen, M.; Mättö, J.; Salminen, S.; Westermarck, E.; Ouwehand, A.C. In vitro adhesion of lactic acid bacteria to canine small intestinal mucus. J. Anim. Physiol. Anim. Nutr. 2000, 84, 43–47. [Google Scholar] [CrossRef]
- Rinkinen, M.; Westermarck, E.; Salminen, S.; Ouwehand, A.C. Absence of host specificity for in vitro adhesion of probiotic lactic acid bacteria to intestinal mucus. Vet. Microbiol. 2003, 97, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, B.; Hadad, T.; Bachrach, G.; Mandelboim, O. Quantification of Bacterial Attachment to Tissue Sections. Bio-Protoc. 2018, 8, e2741. [Google Scholar] [CrossRef] [PubMed]
- de Wouters, T.; Jans, C.; Niederberger, T.; Fischer, P.; Rühs, P.A. Adhesion Potential of Intestinal Microbes Predicted by Physico-Chemical Characterization Methods. PLoS ONE 2015, 10, e0136437. [Google Scholar] [CrossRef]
- Wirth, R.; Bellack, A.; Bertl, M.; Bilek, Y.; Heimerl, T.; Herzog, B.; Leisner, M.; Probst, A.; Rachel, R.; Sarbu, C.; et al. The Mode of Cell Wall Growth in Selected Archaea Is Similar to the General Mode of Cell Wall Growth in Bacteria as Revealed by Fluorescent Dye Analysis. Appl. Environ. Microbiol. 2011, 77, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Kline, K.A.; Fälker, S.; Dahlberg, S.; Normark, S.; Henriques-Normark, B. Bacterial Adhesins in Host-Microbe Interactions. Cell Host Microbe 2009, 5, 580–592. [Google Scholar] [CrossRef]
- Nakano, V.; Piazza, R.M.F.; Cianciarullo, A.M.; Bueris, V.; Santos, M.F.; Menezes, M.A.; Mendes-Ledesma, M.R.B.; Szulczewski, V.; Elias, W.P.; Pumbwe, L.; et al. Adherence and invasion of Bacteroidales isolated from the human intestinal tract. Clin. Microbiol. Infect. 2008, 14, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kumita, W.; Ode, T.; Ichinose, S.; Ando, A.; Fujiyama, Y.; Chida, T.; Okamura, N. OmpA variants affecting the adherence of ulcerative colitis-derived Bacteroides vulgatus. J. Med. Dent. Sci. 2010, 57, 55–64. [Google Scholar]
- Pumbwe, L.; Skilbeck, C.A.; Wexler, H.M. The Bacteroides fragilis cell envelope: Quarterback, linebacker, coach-or all three? Anaerobe 2006, 12, 211–220. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Chen, Z.; Wong, M.C.S.; Hui, M.; Yu, J.; Ng, S.C.; Sung, J.J.Y.; Chan, F.K.L.; Chan, P.K.S. Southern Chinese populations harbour non-nucleatum Fusobacteria possessing homologues of the colorectal cancer-associated FadA virulence factor. Gut 2020, 69, 1998–2007. [Google Scholar] [CrossRef]
- Okamoto, A.C.; Gaetti-Jardim, E., Jr.; Cai, S.; Avila-Campos, M.J. Influence of antimicrobial subinhibitory concentrations on hemolytic activity and bacteriocin-like substances in oral Fusobacterium nucleatum. New Microbiol. 2000, 23, 137–142. [Google Scholar] [PubMed]
- Lei, Q.; Mingchao, X.; Xiaoying, L.; Suping, Z.; Gui, Z.; Jing, Y.; Hui, S.; Liyun, L.; Jianguo, X. Evaluation of the probiotic properties of Bacteroides vulgatus Bv46. Dis. Surveill. 2022, 37, 579–584. (In Chinese) [Google Scholar] [CrossRef]
- Reis, A.C.M.; Silva, J.O.; Laranjeira, B.J.; Pinheiro, A.Q.; Carvalho, C.B.M. Virulence factors and biofilm production by isolates of Bacteroides fragilis recovered from dog intestinal tracts. Braz. J. Microbiol. 2014, 45, 647–650. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oyston, P.C.; Handley, P.S. Surface structures, haemagglutination and cell surface hydrophobicity of Bacteroides fragilis strains. J. Gen. Microbiol. 1990, 136, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Roos, S.; Jonsson, H. A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiol. 2002, 148, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Botes, M.; Guigas, C.; Schillinger, U.; Wiid, I.; Wachsman, M.B.; Holzapfel, W.H.; Dicks, L.M. Boza, a natural source of probiotic lactic acid bacteria. J. Appl. Microbiol. 2008, 104, 465–477. [Google Scholar] [CrossRef]
- Douillard, F.P.; de Vos, W.M. Biotechnology of health-promoting bacteria. Biotechnol. Adv. 2019, 37, 107369. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.C.; Almeida, D.; Domingos, M.; Seabra, C.L.; Machado, D.; Freitas, A.C.; Gomes, A.M. Commensal Obligate Anaerobic Bacteria and Health: Production, Storage, and Delivery Strategies. Front. Bioeng. Biotechnol. 2020, 8, 550. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Diagnosis and interpretation of intestinal dysbiosis in dogs and cats. Vet. J. 2016, 215, 30–37. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, Y.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Protective effects of different Bacteroides vulgatus strains against lipopolysaccharide-induced acute intestinal injury, and their underlying functional genes. J. Adv. Res. 2022, 36, 27–37. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Y.; Wang, X.; Rao, L.; Yan, X.; Gao, R.; Shen, T.; Zhou, Y.; Kong, C.; Zhou, L. Probiotic Cocktail Alleviates Intestinal Inflammation through Improving Gut Microbiota and Metabolites in Colitis Mice. Front. Cell. Infect. Microbiol. 2022, 12, 886061. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Gueimonde, M.; Gomez-Gallego, C.; Delfederico, L.; Salminen, S. Correlation between in vitro and in vivo assays in selection of probiotics from traditional species of bacteria. Trends Food Sci. Technol. 2017, 68, 83–90. [Google Scholar] [CrossRef]
| Strain | Origin | |
|---|---|---|
| Bacteroides vulgatus | DF28 | Dog feces; isolated at the Microbiome Laboratory, Orion Corporation, Orion Pharma, R&D, Turku, Finland. |
| Fusobacterium varium | DF30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanifeh, M.; Huhtinen, M.; Sclivagnotis, Y.S.; Lyhs, U.; Grönthal, T.; Spillmann, T. Adhesion of Bacteroides vulgatus and Fusobacterium varium to the Colonic Mucosa of Healthy Beagles. Vet. Sci. 2024, 11, 319. https://doi.org/10.3390/vetsci11070319
Hanifeh M, Huhtinen M, Sclivagnotis YS, Lyhs U, Grönthal T, Spillmann T. Adhesion of Bacteroides vulgatus and Fusobacterium varium to the Colonic Mucosa of Healthy Beagles. Veterinary Sciences. 2024; 11(7):319. https://doi.org/10.3390/vetsci11070319
Chicago/Turabian StyleHanifeh, Mohsen, Mirja Huhtinen, Yannes S. Sclivagnotis, Ulrike Lyhs, Thomas Grönthal, and Thomas Spillmann. 2024. "Adhesion of Bacteroides vulgatus and Fusobacterium varium to the Colonic Mucosa of Healthy Beagles" Veterinary Sciences 11, no. 7: 319. https://doi.org/10.3390/vetsci11070319
APA StyleHanifeh, M., Huhtinen, M., Sclivagnotis, Y. S., Lyhs, U., Grönthal, T., & Spillmann, T. (2024). Adhesion of Bacteroides vulgatus and Fusobacterium varium to the Colonic Mucosa of Healthy Beagles. Veterinary Sciences, 11(7), 319. https://doi.org/10.3390/vetsci11070319






