Simple Summary
Congenital defects have been described in almost every vertebrate group. In crocodiles, teratology alterations have been described in captive animals (pets, zoos, farms) such as Crocodylus niloticus and Gavialis gangeticus. The present study aimed to characterize congenital malformations of C. acutus from a farm in Lomas de Matunilla, Ballestas, Bolívar, Colombia. The analyzed eggs presented macroscopic malformations, with 42 different types of anomalies observed. Limb and tail malformations (29%) were the most common changes observed.
Abstract
The American crocodile (Crocodylus acutus, Cuvier, 1807) (Class Reptilia, Family Crocodylidae) is a crocodile species inhabiting the Neotropics. Congenital defects have been described in almost every vertebrate group. In crocodiles, teratology alterations have been described in captive animals (pets, zoos, farms) such as Crocodylus niloticus or Gavialis gangeticus. The present study aimed to characterize congenital malformations of C. acutus from a farm in Lomas de Matunilla, Ballestas, Bolívar, Colombia. A total of 550 unhatched eggs were examined after embryo death. A total of 61 embryos presented malformations, with 42 different types of anomalies observed. Limb and tail malformations (29%) were the most common malformations observed. Several malformations, such as cephalothoracopagus, thoracopagus, sternopagus, xiphopagus twins, campylorrachis scoliosa, and acrania, were documented in crocodiles for the first time. Research in teratology enhances our understanding of crocodile biology. It plays a role in their conservation and management, thus helping to ensure the long-term viability of these species in their natural habitats.
1. Introduction
American crocodile (Crocodylus acutus) (Class Reptilia, Family Crocodylidae) is a species of crocodile that inhabits the Neotropics like Belize, Colombia, Costa Rica, Cuba, the Dominican Republic, Ecuador, El Salvador, Guatemala, Haiti, Honduras, Jamaica, Mexico, Nicaragua, Panama, Peru, the United States (Florida), and Venezuela [1,2]. According to the International Union for Conservation of Nature (IUCN)—Red List, this species is considered Vulnerable [3]. Its habitat consists mainly of coastal areas [4]. C. acutus breed in late fall or early winter, and in February or March, the females lay 30 to 70 eggs in nests of sand, mud, and dead vegetation along the water bodies [5]. The incubation period is about 75–80 days. The temperature at which the eggs are incubated (dependent on the environment) can influence the sex of the hatchlings, a phenomenon known as temperature-dependent sex determination (TSD). Warm temperatures during incubation tend to produce females, while cooler temperatures produce males [6]. Alteration of the temperatures can alter the embryos [7].
Like other species, C. acutus faces various threats [3,8]. Expanding cities and towns into coastal and wetland areas leads to the loss of crucial nesting and basking sites. The conversion of wetlands and coastal regions into agricultural lands destroys crocodile habitats. Agricultural runoff, industrial waste, and urban pollution degrade the quality of water bodies where these animals live. Dam construction and water diversion for agriculture and urban use can alter the natural flow of rivers and streams [9]. In addition, illegal hunting exists due to the demand for crocodile skins, leather products, and meat [10]. Climate change leads to rising sea levels inundating nesting sites, and temperature changes can skew sex ratios, impacting population dynamics [11]. With the rise of human–crocodile conflicts, these animals sometimes prey on livestock or come to attack humans, leading to retaliatory killings [12].
Congenital defects have been described in almost every vertebrate group [13,14,15], but in wild animals, the descriptions of these anomalies are scarce, and the information is virtually non-existent [16]. They are more commonly observed in captive animals [17]. Most of the individuals who carry these malformations die before birth. If they survive, most malformations are only detected during a post-mortem exam [18]. Teratogens, agents, or factors that can disturb the normal development of an embryo can include environmental agents like drugs, infectious diseases (e.g., viruses, bacteria, and fungi), radiation, temperature, pollution (e.g., heavy metals, and pesticides), or certain maternal conditions [13,18,19].
In reptiles, a wide variety of congenital anomalies has been reported in several species [20]. For example, Martín-del-Campo et al. (2021) [21] showed the presence of malformation in three species of sea turtles associated with different environmental factors such as the incubation temperature, humidity, and the status of feeding areas [21]. Other studies in South American pit vipers (Bothrops jararaca) and South American rattlesnakes (Crotalus durissus) linked the malformation to the presence of chemical compounds such as pesticides and herbicides [16].
In crocodiles, teratology alterations have been described in captive animals (pets, zoos, farms) in species such as Crocodylus niloticus, Gavialis gangeticus, Osteolaemus tetraspis, Alligator mississippiensis, C. porosus, C. johnsoni, C. palustris, or C. moreletii [22,23,24]. Not all malformations can be considered as being from congenital origin, with some resulting from the exposure of embryos to environmental stressors [18]. Pollution is a major concern, particularly chemical contaminants such as pesticides, heavy metals, and endocrine-disrupting compounds that can accumulate in aquatic ecosystems [25]. These substances can interfere with normal developmental processes, leading to physical abnormalities. Habitat degradation due to urbanization, agriculture, and industrial activities can also play a role. Destruction of nesting sites and changes in water quality can create stressful conditions that increase the likelihood of malformations. Climate change and associated effects, like temperature fluctuations and altered precipitation patterns, can also disrupt embryonic development since crocodile sex determination and growth rates are temperature-dependent [22,26].
The present study aimed to characterize congenital malformations observed in American crocodiles (Crocodylus acutus) from a farm in Colombia.
2. Materials and Methods
Eggs of the American crocodile (Crocodylus acutus Cuvier, 1807) from a farm located in Lomas de Matunilla, Ballestas, Bolívar, Colombia (10°10′06″ N 75°28′46″ W) (Figure 1), were analyzed during Colombia’s dry season (December to March) in 2023.
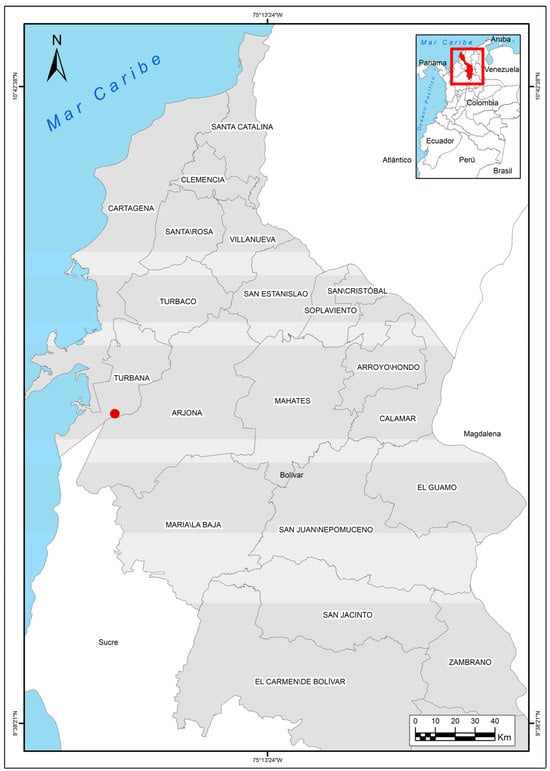
Figure 1.
Colombia map with the location of the American crocodile (Crocodylus acutus Cuvier, 1807) farm.
From 61 nests across 15 lakes, 1773 eggs were laid from 61 females. The eggs were incubated and hatched from 25 April 2023 to 31 May 2023. Of the 1773 eggs, 1242 were incubated, with 48 eggs broken and 470 infertile eggs. Table 1 shows the information regarding nests, lakes, offspring, collection date, incubated eggs, laid eggs, broken eggs, infertile eggs, collected eggs, and dead embryos.

Table 1.
Information regarding nests (n = 61), lakes (n = 15), offspring, collection date, incubated eggs (n = 1242), laid eggs (n = 1773), broken eggs (n = 48), infertile eggs (n = 470), hatches (n = 705), and dead embryos (n = 505) from American crocodile (Crocodylus acutus Cuvier, 1807) from a farm in Colombia.
All the eggs that did not hatch in the expected period (75–80 days) were analyzed. After embryo death, stillborn crocodiles were removed from the eggs and measured, systematically examined, and photographed with a scale. All the gross malformations were recorded in an Excel file and then categorized by different anatomical regions into skin, spine, head (excluding eye and nose), nose, limbs, tail, and other malformations.
3. Results
From the 1773 eggs laid, 550 eggs with dead embryos were analyzed from those 61 embryos that presented malformations. Overall, 95.7% (n = 57) presented more than one malformation. Forty-two different types of anomalies were observed (Table 2).

Table 2.
Malformations in American crocodile (Crocodylus acutus) from a farm in Colombia.
Regarding body regions, 4.9% (10/202) affected the skin, 9.8% (20/202) the spine, 29% (61/204) the limbs and tail, and 25% (51/202) the head. Other malformations were 27.9% (Figure 2). The main malformation by region observed were as described below:
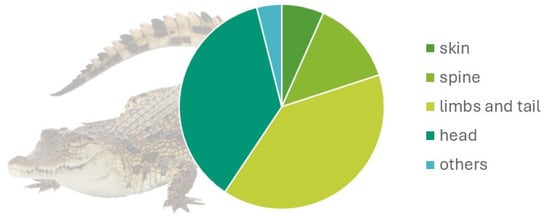
Figure 2.
Malformation distribution by anatomical region in 61 embryos of the American crocodile (Crocodylus acutus Cuvier, 1807).
3.1. Skin Malformations
Ephitheliogenesis imperfecta (a localized region of abnormal skin) was observed in five animals, two of which had incomplete scale formation (Figure 3). Alterations of scale color were leucism (n = 2), where the scales were white crocodiles with dark markings and regional depigmentation to white (n = 3) (Figure 4).

Figure 3.
Ephitheliogenesis imperfecta in the lower jaw (↑) (A), eyes (↑) (B), absence of scales on the entire body (C) in Crocodylus acutus embryos. Scale bar = 5 cm.
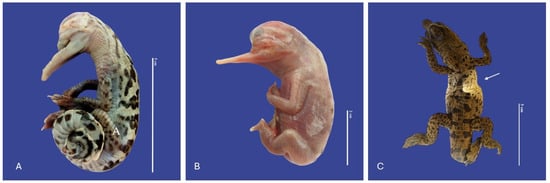
Figure 4.
Leucism (A,B), depigmentation (↑) (C) Crocodylus acutus embryos. Scale bar = 2 cm and 5 cm.
3.2. Spine Malformations
The main spine malformations were observed in fourteen animals with scoliosis (lateral curvature of the spinal column), three with lordosis (ventrodorsal deviation, curvature of the spinal column with a ventral convexity) (Figure 5), two with kyphosis (Increased dorsal convexity in the curvature of the spinal column as viewed from the side) (Figure 6), and one with campylorrachis scoliosa (curvature of the spine a lack of vertebrae and spinal cord caudal to the thoracic region) (Figure 7).

Figure 5.
Lordosis, kinked tail (↑), and yolk sac retention with non-closure of the abdominal wall in a Crocodylus acutus embryo ((A,B)—Lateral view, (C)—dorsoventral view). Scale bar = 5 cm.
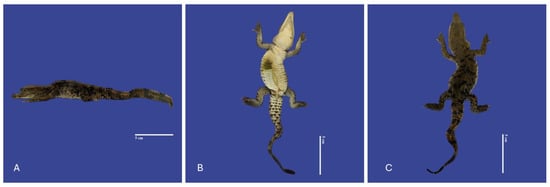
Figure 6.
Yolk sac retention with non-closure of the abdominal wall, kinked tail, scoliosis and kyphosis in a Crocodylus acutus embryo. ((A)—Lateral view, (B)—ventral view, (C)—dorsoventral view). Scale bar = 5 cm.
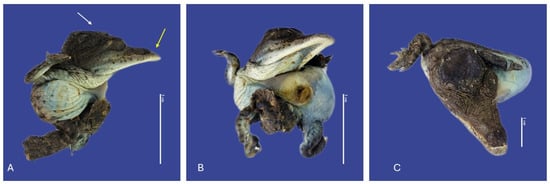
Figure 7.
Yolk sac retention with non-closure of the abdominal wall, maxillary macrognathia (↑ yellow), acrania (↑ white), curved tail, and campylorrachis scoliosa in Crocodylus acutus embryo. (A,B)—Lateral view, (C)—cranial view). Scale bar = 3 cm and 1 cm.
3.3. Head
The main malformations observed in the head were one anencephaly/prosencephalic hypoplasia (absence of the cranial region of the head, with the brain absent or reduced), twelve laterognathia (mandible pointing up and jaw pointing down), three meningoencephalocete (herniation of the brain and meninges through a cranial opening covered by skin), one brachygnathia (short lower mandible), four microcephaly (small head), three maxillary micrognathia (short upper jaw), one external hydrocephalus (abnormal accumulation of cerebrospinal fluid within the ventricles and/or subarachnoid spaces, increase in head circumference, combined with enlarged subarachnoid spaces), seven maxillary macrognathia (longer low jaw), three acrania (absence skull), one acephaslostomia, one maxilla agnathia (absent upper jaw), and one brachycephalic skull (shape of a skull shorter than average in its species) (Figure 8, Figure 9, Figure 10, Figure 11, Figure 12 and Figure 13).
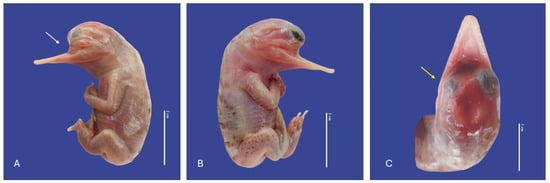
Figure 8.
Maxilla agnathia (↑ white), microphthalmia (↑ yellow), acaudia, and leucism in Crocodylus acutus embryo. ((A,B)—Lateral view, (C)—cranial view). Scale bar = 2 cm and 1 cm.

Figure 9.
(A) Maxillary micrognathia and atresia; (B) maxillary micrognathia; (C) maxillary macrognathia and laterognathia; (D) Laterognathia in Crocodylus acutus embryos. Scale bar = 2 cm and 3 cm.
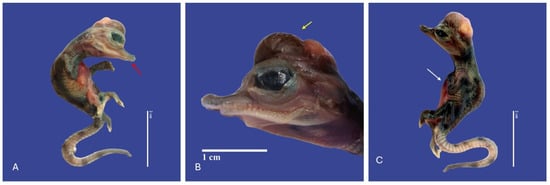
Figure 10.
Depigmentation of the skin, mandibular micrognathia (↑ red), microcephaly, gastroschisis, ectopia cordis (↑ white), schistosomia, kyphosis, meningoencephalocele (↑ yellow), bend tail, epitheliogenesis imperfecta in all the body in Crocodylus acutus embryo ((A,C)—Lateral view, (B)—Head). Scale bar = 2 cm and 1 cm.
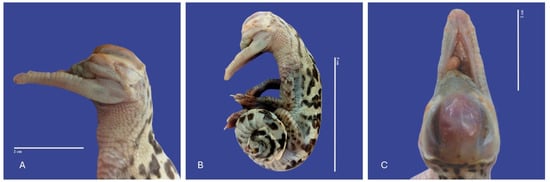
Figure 11.
Leucism, acephalostomia, anophthalmia, atresia, and curly tail in Crocodylus acutus embryo. ((A)—Lateral head view, (B)—Lateral body view, (C)—cranial view). Scale bar = 2 cm and 5 cm.

Figure 12.
Yolk sac retention, meningoencephalocele (↑ white), kinked tail, exophthalmia, and brachygnathia in Crocodylus acutus embryo. ((A)—Lateral body view, (B)—Cranial view, (C)—Lateral head view, (D)—dorsoventral view). Scale bar = 5 cm.

Figure 13.
Kinked tail, maxillary micrognathia (↑ white), exophthalmos in both eyes, brachycephalic skull, ankylodactyly of the two digits fused in the left posterior member (↑ yellow), congenital cataract in both eyes, tubercles on top of the skull (↑ red), and epitheliogenesis imperfecta on the palpebra Crocodylus acutus embryo. ((A)—Lateral body view, (B)—Cranial view, (C)—ventrodoral head view, (D)—lateral head view, (E)—ventrodorsal view). Scale bar = 5 cm and 2 cm.
3.4. Limbs and Tail Malformations
In the limbs, both anterior and posterior, observed micromeli (short member) (n = 1), polydactyly (extra digit) (n = 2) (Figure 14), carpal flexure (n = 1), malroted members (n = 1) and arthrogryposis (n = 1).
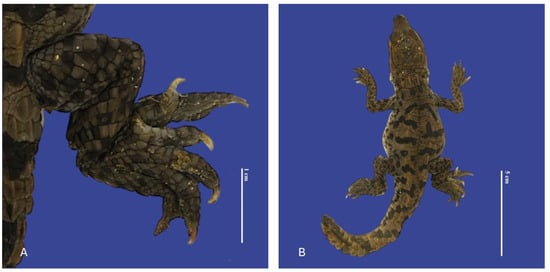
Figure 14.
Polydactyly in the posterior right limb with two extra fingers, distended celomic cavity, tail blunt-tipped, bent tail, and brachyury in Crocodylus acutus embryo. ((A)—Limb view, (B)—ventrodorsal view). Scale bar = 1 cm and 5 cm.
Alterations of the tail observed were curled tail (curved into nearly a full circle) (n = 17), kinked tail (localized undulation(s) of the tail) (n = 18), acaudia (absent tail) (n = 16), brachyuri (n = 3), and bent tail (n = 2) (Figure 15).

Figure 15.
(A) Kinked tail; (B) acaudia; (C) curved tail Crocodylus acutus embryos. Scale bar = 5 cm.
3.5. Other Malformations
In Achondroplasia (dwarfism), the long bones are abnormally short, although the trunk is of normal length and the abdomen is large (round)—the size of normal individuals. The head is moderately enlarged and flattened. This malformation was observed in four individuals (Figure 16). Four cases of twins were observed. Cephalothoracopagus (Figure 17) were symmetrical monozygotic twin crocodiles that conjoined from the head down to the thorax with one head, one face, one neck, a single thorax, two abdomen, two umbilical cords, eight limbs, and two tails. There were three monozygotic symmetrical twins (identical twins) (Figure 18) and one thoracopagus, sternopagus, and xiphopagus twin (Twins attached by the thorax, sternum, and xiphoid process). We also observed one case of a distended celomic cavity, one case of Ectopia cordis (externalized heart), one case of gastroschisis (externalization of the internal organs abdomen), and 47 cases of yolk sac retention.
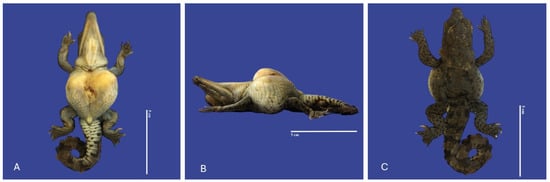
Figure 16.
Achondroplasia, scoliosis, yolk sac retention, micromelia of the left upper member, and curly tail Crocodylus acutus embryo. ((A)—Ventrodorsally view, (B)—Lateral view, (C)—Dorsal ventral view). Scale bar = 5 cm.
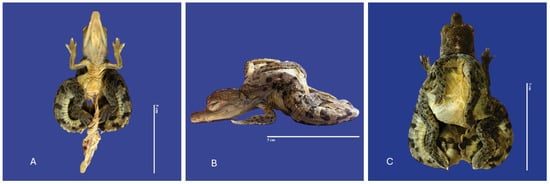
Figure 17.
Cephalothoracopagus twins, curly tail, epitheliogenesis imperfecta at the top of the skull, and yolk sac retention in Crocodylus acutus embryo. ((A,B)—Lateral view, (C)—Ventrodorsal view). Scale bar = 5 cm.

Figure 18.
Monozygotic Symmetrical Twins of Crocodylus acutus. ((A)—Ventra dorsal view, (B)—Lateral view, (C)—Dorsal ventral view). Scale bar = 5 cm.
4. Discussion
In this work, the authors describe the congenital deformities in American crocodiles (Crocodylus acutus). To the the authors’ knowledge, the present study is the first of this kind. According to studies performed [27], crocodiles have a low prevalence of deformities even in captivity. In Gavialis gangeticus, deformities were detected in 6% of the 1061 hatchlings examined [28].
In this study, all the animals were born in captivity and died during gestation, except for one individual. This animal was born without a tail; it would be difficult for it to survive in the wild since it could not swim to the surface [24], but it is more likely to survive in captivity. Limbs and tail malformations (29%) were the most common changes observed, followed by head (25%). These results are similar to what has been described by other authors [6,24,29]. Almost every congenital malformation observed in this study has been reported in other species of crocodiles [23,24,26]. In the cases described were observed malformations such as cephalothoracopagus, thoracopagus, sternopagus, xiphopagus twins, campylorrachis scoliosa, acephaslostomia, and acrania, that were not described in crocodiles to the moment.
Malformations in wild animals are rarely described [22,27]. These congenital malformations can be associated with many factors, such as hatchlings from very young and very old females, genetic causes, malnutrition of the parents, defective incubation, and carcinogenic agents [30].
For example, Alligator mississippiensis from Lake Apopka (USA) embryos presented a large number of malformations associated with a chemical spill of sulfuric acid, dicofol, organochlorine pesticides, and Polychlorinated biphenyls (PCBs) [29,31]. South American pit vipers (Bothrops jararaca) and South American rattlesnakes (Crotalus durissus) malformations have been linked to the increased use of pesticides and herbicides in the agriculture fields near their habitat [16]. Benzo(a)pyrene and dibenzo(a)anthracene, polychlorinated biphenyls (PCBs), and heavy metals (As, Cu, Cd, Hg, Pb) were related to the development of malformations in the semi-aquatic turtles such as Chelydra serpentina and Chrysemy spicta from the John Heinz National Wildlife Refuge (USA) [32].
There is the possibility that genes can be associated with these malformations if both parents are carriers. In gharial populations from Nepal, a ‘blind’ gene has been suspected to occur [28]. In spite of our study, we did not confirm the etiology of the congenital malformations; a combination of many factors, such as the females’ age, inbreeding, incubation errors, and chemical pollutants in the water (the farms’ water originates in very polluted rivers in Columbia) should be considered. Although no samples were collected from the water in the farm lakes, it is very possible that pesticides like Organochlorine and heavy metals are present in such water since these compounds have been detected in other rivers in Colombia [29].
Although other studies have reported the occurrence of malformations in crocodiles and other reptiles, there is still a large gap concerning the classification of these malformations and defining the frequency with which they occur. Crocodiles are ectothermic carnivores that live in aquatic environments and may act as sentinel species [33,34]. In the future, more detailed studies should be performed to find the potential role of genetic and environmental factors in the occurrence of the reported malformations in these species.
5. Conclusions
This study describes congenital deformities in American crocodiles (Crocodylus acutus). It is important to note that research in teratology not only enhances our understanding of the biology of crocodiles but also plays a role in their conservation and management, helping to ensure the long-term viability of these species in their natural habitats. Identifying the causes of developmental abnormalities can contribute to conservation efforts by addressing potential threats and improving management strategies. Although other studies have reported the occurrence of malformations in crocodiles, there is still a large gap concerning the classification of these malformations and the frequency at which they occur. Examining abnormal development can also offer insights into the genetic diversity of crocodile populations and potential genetic stressors.
Author Contributions
Conceptualization, O.S.S. and A.G.; methodology, O.S.S., A.G. and J.A.C.M.; software, J.M.O., O.S.S. and A.G.; validation, J.J.D. and I.P.; formal analysis, J.J.D. and I.P.; investigation, A.G., O.S.S. and J.A.C.M.; resources, O.S.S., J.A.C.M. and J.J.D.; data curation, A.G. and O.S.S.; writing—original draft preparation, A.G. and O.S.S.; writing—review and editing, A.G., O.S.S. and I.P.; visualization, O.S.S. and J.A.C.M.; supervision, I.P. and J.J.D.; project administration, A.G. and O.S.S.; funding acquisition, A.G. and I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the projects UIDB/00772/2020 (Doi:10.54499/UIDB/00772/2020) and funded by the Portuguese Foundation for Science and Technology. (FCT).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Balaguera-Reina, S.A.; Jennings, N.D.; Godfrey, S.T.; Brandt, L.A.; Daykin, B.; Squires, M.A.; Mazzotti, F.J. Hematology and Biochemistry Reference Intervals for American Crocodiles (Crocodylus acutus) in South Florida. Front. Vet. Sci. 2022, 9, 919488. [Google Scholar] [CrossRef] [PubMed]
- American Crocodile (Crocodylus acutus). Available online: https://www.inaturalist.org/taxa/26085-Crocodylus-acutus (accessed on 30 December 2023).
- Rainwater, K.L.; Wiederhold, N.P.; Sutton, D.A.; Garner, M.M.; Maguire, C.; Sanders, C.; Gibas, C.; Cano, J.F.; Guarro, J.; Stchigel, A.M. Novel Paranannizziopsis Species in a Wagler’s Viper (Tropidolaemus wagleri), Tentacled Snakes (Erpeton tentaculatum), and a Rhinoceros Snake (Rhynchophis boulengeri) in a Zoological Collection. Med. Mycol. 2019, 57, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.M. Tolerance of Sea Water by the American Crocodile, Crocodylus acutus. J. Herpetol. 1981, 15, 187–192. [Google Scholar] [CrossRef]
- Ogden, J.C. Status and Nesting Biology of the American Crocodile, Crocodylus acutus, (Reptilia, Crocodilidae) in Florida. J. Herpetol. 1978, 12, 183–196. [Google Scholar] [CrossRef]
- Platt, S.G.; Thorbjarnarson, J.B. Nesting Ecology of the American Crocodile in the Coastal Zone of Belize. Copeia 2000, 2000, 869–873. [Google Scholar] [CrossRef]
- Barragán Lara, R.; Grajales, J.G.; Martínez Ramírez, E. Nest Temperature Assessment in an American Crocodile (Crocodylus acutus) Population on the Central Coast of Oaxaca, Mexico. J. Therm. Biol. 2021, 99, 103012. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, J.G.; Ankley, G.; Bell, H.; Carpenter, H.; Fort, D.; Gardiner, D.; Gardner, H.; Hale, R.; Helgen, J.C.; Jepson, P.; et al. Strategies for Assessing the Implications of Malformed Frogs for Environmental Health. Environ. Health Perspect. 2000, 108, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Briggs-Gonzalez, V.S.; Basille, M.; Cherkiss, M.S.; Mazzotti, F.J. American Crocodiles (Crocodylus acutus) as Restoration Bioindicators in the Florida Everglades. PLoS ONE 2021, 16, e0250510. [Google Scholar] [CrossRef] [PubMed]
- Platt, S.G.; Thorbjarnarson, J.B. Status and Conservation of the American Crocodile, Crocodylus acutus, in Belize. Biol. Conserv. 2000, 96, 13–20. [Google Scholar] [CrossRef]
- Rodgers, E.M.; Schwartz, J.J.; Franklin, C.E. Diving in a Warming World: The Thermal Sensitivity and Plasticity of Diving Performance in Juvenile Estuarine Crocodiles (Crocodylus porosus). Conserv. Physiol. 2015, 3, cov054. [Google Scholar] [CrossRef]
- Brien, M.L.; Gienger, C.M.; Browne, C.A.; Read, M.A.; Joyce, M.J.; Sullivan, S. Patterns of Human–Crocodile Conflict in Queensland: A Review of Historical Estuarine Crocodile (Crocodylus porosus) Management. Wildl. Res. 2017, 44, 281–290. [Google Scholar] [CrossRef]
- Garcês, A.; Pires, I.; Rodrigues, P. Teratological Effects of Pesticides in Vertebrates: A Review. J. Environ. Sci. Health Part B 2020, 55, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Garcês, A.; Pires, I. Teratological Effects of Pesticides in Reptiles—A Review. In Bird and Reptile Species in Environmental Risk Assessment Strategies; Liwszyc, G., Larramendy, M.L., Eds.; The Royal Society of Chemistry: London, UK, 2023; pp. 97–109. ISBN 978-1-83916-710-2. [Google Scholar]
- Bárcenas-Ibarra, A.; de la Cueva, H.; Rojas-Lleonart, I.; Abreu-Grobois, F.A.; Lozano-Guzmán, R.I.; Cuevas, E.; García-Gasca, A. First Approximation to Congenital Malformation Rates in Embryos and Hatchlings of Sea Turtles. Birth Defects Res. A Clin. Mol. Teratol. 2015, 103, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, S.S.; Grego, K.F.; Lorigados, C.A.B.; Fonseca-Pinto, A.C.B.C.; Fernandes, W.; Sá-Rocha, L.C.; Catão-Dias, J.L. Malformations in Neotropical Viperids: Qualitative and Quantitative Analysis. J. Comp. Pathol. 2013, 149, 503–508. [Google Scholar] [CrossRef] [PubMed]
- MacGeady, T.; Quinn, P.J.; Fiztpatrick, E.; Ryan, M.; Kilroy, D.; Lonergan, P. Veterinary Embryology, 2nd ed.; Wiley-Blackwell: Hoboken, NY, USA, 2017. [Google Scholar]
- Seifer, R. Teratology. In Encyclopedia of Infant and Early Childhood Development; Haith, M.M., Benson, J.B., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 333–343. ISBN 978-0-12-370877-9. [Google Scholar]
- Bellairs, A.A. Cleft palate, microphthalmia and other malformations in embryos of lizards and snakes. Proc. Zool. Soc. Lond. 1965, 144, 239–252. [Google Scholar] [CrossRef]
- Palmieri, C.; Selleri, P.; Di Girolamo, N.; Montani, A.; Della Salda, L. Multiple Congenital Malformations in a Dicephalic Spur-Thighed Tortoise (Testudo graeca ibera). J. Comp. Pathol. 2013, 149, 368–371. [Google Scholar] [CrossRef]
- Martín-del-Campo, R.; Calderón-Campuzano, M.F.; Rojas-Lleonart, I.; Briseño-Dueñas, R.; García-Gasca, A. Congenital Malformations in Sea Turtles: Puzzling Interplay between Genes and Environment. Animals 2021, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Rainwater, T.R.; Platt, S.G.; McMurry, S.T.; Anderson, T.A. Organochlorine Contaminants in MoreletÕs Crocodile (Crocodylus moreletii) Eggs from Belize. Chemosphere 2000, 40, 671–678. [Google Scholar] [CrossRef]
- Vyas, R. Case of Polydactyly Limb in Juvenile Mugger Crocodile (Crocodylus palustris). Russ. J. Herpetol. 2018, 25, 139–142. [Google Scholar] [CrossRef]
- Huchzermeyer, F.W. Chapter 4—Diseases of Eggs and Hatchlings. In Crocodiles Biology Husbandry and Disease; CAB International: Wallingford, UK, 2003; pp. 139–156. [Google Scholar] [CrossRef]
- Charruau, P.; Niño-Torres, C. A Third Case of Amelia in Morelet’s Crocodile from the Yucatan Peninsula. Dis. Aquat. Org. 2014, 109, 263–267. [Google Scholar] [CrossRef]
- Brandon-Pliego, J.; Vannini, F. Anomalies and Growth of a Crocodylus acutus in Puerto Escondido, Oaxaca, Mexico. Crocodile Spec. Group Newsl. 2009, 28, 1–20. [Google Scholar]
- Boete, E.E.; Sogbe, E. Enfermedades en caimanes del Orinoco (Crocodylus intermedius) y caimanes de la costa (Crocodylus acutus) mantenidos en zoocriaderos venezolanos. Rev. Cient. 1991, 10, 328–338. [Google Scholar]
- Singh, L.A.K.; Bustard, H.R. Congenital Defects in the Gharial Gavialis Gangeticus (Gmelin). Br. J. Herpetol. 1982, 6, 215–219. [Google Scholar]
- Sepúlveda, M.S.; Wiebe, J.J.; Honeyfield, D.C.; Rauschenberger, H.R.; Hinterkopf, J.P.; Johnson, W.E.; Gross, T.S. Organochlorine Pesticides and Thiamine in Eggs of Largemouth Bass and American Alligators and Their Relationship with Early Life-Stage Mortality. J. Wildl. Dis. 2004, 40, 782–786. [Google Scholar] [CrossRef]
- González, L.M.; Margalida, A.; Mañosa, S.; Sánchez, R.; Oria, J.; Molina, J.I.; Caldera, J.; Aranda, A.; Prada, L. Causes and Spatio-Temporal Variations of Non-Natural Mortality in the Vulnerable Spanish Imperial Eagle Aquila adalberti during a Recovery Period. Oryx 2007, 41, 495–502. [Google Scholar] [CrossRef]
- Rauschenberger, R.H. Developmental Mortality in American Alligators (Alligator mississippiensis) Exposed to Organochlorine Pesticides. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2004. [Google Scholar]
- Bell, B.; Spotila, J.R.; Congdon, J. High Incidence of Deformity in Aquatic Turtles in the John Heinz National Wildlife Refuge. Environ. Pollut. 2006, 142, 457–465. [Google Scholar] [CrossRef]
- Board on Environmental Studies and Toxicology; National Research Council; Division on Earth and Life Studies; Commission on Life Sciences; Committee on Animals as Monitors of Environmental Hazard. Animals as Sentinels of Environmental Health Hazards: Committee on Animals as Monitors of Environmental Hazards, Board on Environmental Studies and Toxicology, Commission on Life Sciences, National Re; National Academies Press: Washington, DC, USA, 1991; ISBN 978-0-309-59489-9. [Google Scholar]
- Tavalieri, Y.E.; Galoppo, G.H.; Canesini, G.; Luque, E.H.; Muñoz-de-Toro, M.M. Effects of Agricultural Pesticides on the Reproductive System of Aquatic Wildlife Species, with Crocodilians as Sentinel Species. Mol. Cell. Endocrinol. 2020, 518, 110918. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).