Comparison of Nanoparticles and Single-Layer Centrifugation for Separation of Dead from Live Stallion Spermatozoa
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Semen Collection
2.2. Media
2.3. Semen Evaluation
2.4. Sperm Sorting Procedures
2.5. Experimental Design
- SLC pellet: Sperm concentrated at the bottom of the conical tube after centrifugation with EquiPure™, subsequently reconstituted with INRA 96;
- SLC Supernatant: Sperm remaining suspended in solution above the pellet after centrifugation with EquiPure™;
- NP Supernatant: Sperm suspended in solution, not bound to nanoparticles;
- NP Remnant: Sperm bound to nanoparticles and held in the conical tube by the magnet after decanting off the supernatant;
- Control: Sperm diluted in INRA 96 extender only.
2.6. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aurich, J.; Aurich, C. Developments in european horse breeding and consequences for veterinarians in equine reproduction. Reprod. Domest. Anim. 2006, 41, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Love, C.C. Semen collection techniques. Vet. Clin. N. Am. Equine Pract. 1992, 8, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Loomis, P.R. Advanced methods for handling and preparation of stallion semen. Vet. Clin. N. Am. Equine Pract. 2006, 22, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Loomis, P.R. The equine frozen semen industry. Anim. Reprod. Sci. 2001, 68, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Baumber, J.; Ball, B.A.; Linfor, J.J.; Meyers, S.A. Reactive oxygen species and cryopreservation promote DNA fragmentation in equine spermatozoa. J. Androl. 2003, 24, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.E.; Andara, K.; Briones, E.; Felmer, R. Bovine sperm separation by swim-up and density gradients (Percoll and BoviPure): Effect on sperm quality, function and gene expression. Reprod. Biol. 2017, 17, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Yoshioka, K.; Hikono, H.; Iwagami, G.; Suzuki, C.; Kikuchi, K. Centrifugation on percoll density gradient enhances motility, membrane integrity and in vitro fertilizing ability of frozen–thawed boar sperm. Zygote 2013, 23, 68–75. [Google Scholar] [CrossRef]
- Hoogewijs, M.; Morrell, J.; Van Soom, A.; Govaere, J.; Johannisson, A.; Piepers, S.; De Schauwer, C.; de Kruif, A.; De Vliegher, S. Sperm selection using single layer centrifugation prior to cryopreservation can increase thawed sperm quality in stallions. Equine Vet. J. 2011, 43, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Husna, A.U.; Ejaz, R.; Qadeer, S.; Azam, A.; Rakha, B.A.; Ansari, M.S.; Shahzad, Q.; Javed, M.; Vazquez-Levin, M.H.; Akhter, S. A comparative analysis of sperm selection procedures prior to cryopreservation for Nili-Ravi buffalo bull (Bubalus bubalis) semen-: Assessment of its impact on post-thaw sperm functional quality. Anim. Reprod. Sci. 2016, 174, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Durfey, C.L.; Burnett, D.D.; Liao, S.F.; Steadman, C.S.; Crenshaw, M.A.; Clemente, H.J.; Willard, S.T.; Ryan, P.L.; Feugang, J.M. Nanotechnology-based selection of boar spermatozoa: Growth development and health assessments of produced offspring. Livest. Sci. 2017, 205, 137–142. [Google Scholar] [CrossRef]
- Durfey, C.L.; Swistek, S.E.; Liao, S.F.; Crenshaw, M.A.; Clemente, H.J.; Thirunalai, R.V.K.G.; Steadman, C.S.; Ryan, P.L.; Willard, S.T.; Feugang, J.M. Nanotechnology-based approach for safer enrichment of semen with best spermatozoa. J. Anim. Sci. Biotechnol. 2019, 10, 14. [Google Scholar] [CrossRef]
- Said, T.M.; Agarwal, A.; Grunewald, S.; Rasch, M.; Glander, H.-J.; Paasch, U. Evaluation of sperm recovery following annexin V magnetic-activated cell sorting separation. Reprod. BioMedicine Online 2006, 13, 336–339. [Google Scholar] [CrossRef]
- Falchi, L.; Khalil, W.A.; Hassan, M.; Marei, W.F.A. Perspectives of nanotechnology in male fertility and sperm function. Int. J. Vet. Sci. Med. 2018, 6, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Said, T.M.; Agarwal, A.; Zborowski, M.; Grunewald, S.; Glander, H.-J.; Paasch, U. Utility of magnetic cell separation as a molecular sperm preparation technique. J. Androl. 2007, 29, 134–142. [Google Scholar] [CrossRef]
- Odhiambo, J.F.; DeJarnette, J.M.; Geary, T.W.; Kennedy, C.E.; Suarez, S.S.; Sutovsky, M.; Sutovsky, P. Increased conception rates in beef cattle inseminated with nanopurified bull semen. Biol. Reprod. 2014, 91, 97. [Google Scholar] [CrossRef]
- Morris, L.H.; de Haan, T.; Landriscina, L.G.; Wilsher, S.; Gibb, Z. The effects of nanoparticle semen purification on semen quality parameters in stallions. J. Equine Vet. Sci. 2018, 66, 75. [Google Scholar] [CrossRef]
- Coutinho da Silva, M.A.; Pinto, C.R.F.; Young, J.M.; Cole, K. 8 The use of annexin V magnetic-activated cell storing to separate apoptotic sperm from the ejaculate of stallions. Reprod. Fertil. Dev. 2011, 23, 110–111. [Google Scholar] [CrossRef]
- Domínguez, E.; Moreno-Irusta, A.; Castex, H.R.; Bragulat, A.F.; Ugaz, C.; Clemente, H.; Giojalas, L.; Losinno, L. Sperm sexing mediated by magnetic nanoparticles in donkeys, a preliminary in vitro study. J. Equine Vet. Sci. 2018, 65, 123–127. [Google Scholar] [CrossRef]
- Rateb, S.A. Purification of cryopreserved camel spermatozoa following protease-based semen liquefaction by lectin-functionalized DNA-defrag magnetic nanoparticles. Reprod. Domest. Anim. 2020, 56, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.K. Assessment of sperm quality: A flow cytometric approach. Anim. Reprod. Sci. 2001, 68, 239–247. [Google Scholar] [CrossRef]
- Morrell, J.M.; Dalin, A.-M.; Rodriguez-Martinez, H. Comparison of density gradient and single layer centrifugation of stallion spermatozoa: Yield, motility and survival. Equine Vet. J. 2009, 41, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, P.; De Lamirande, E.; Gagnon, C. Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic. Biol. Med. 1997, 22, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Blondin, P.; Coenen, K.; Sirard, M. The impact of reactive oxygen species on bovine sperm fertilizing ability and oocyte maturation. J. Androl. 1997, 18, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Ball, B.A.; Vo, A.T.; Baumber, J. Generation of reactive oxygen species by equine spermatozoa. Am. J. Vet. Res. 2001, 62, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.; Johannisson, A.; Dalin, A.-M.; Rodriguez-Martinez, H. Morphology and chromatin integrity of stallion spermatozoa prepared by density gradient and single layer centrifugation through silica colloids. Reprod. Domest. Anim. 2009, 44, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, A.-S.; Johannisson, A.; Bäckgren, L.; Dalin, A.-M.; Rodriguez-Martinez, H.; Morrell, J. Single layer centrifugation of stallion spermatozoa through Androcoll™-E does not adversely affect their capacitation-like status, as measured by CTC staining. Reprod. Domest. Anim. 2011, 46, e74–e78. [Google Scholar] [CrossRef] [PubMed]
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef] [PubMed]
- Makker, K.; Agarwal, A.; Sharma, R.K. Magnetic activated cell sorting (MACS): Utility in assisted reproduction. Indian J. Exp. Biol. 2008, 46, 491–497. [Google Scholar]
- Grunewald, S.; Paasch, U.; Said, T.M.; Rasch, M.; Agarwal, A.; Glander, H.-J. Magnetic-activated cell sorting before cryopreservation preserves mitochondrial integrity in human spermatozoa. Cell Tissue Bank. 2006, 7, 99–104. [Google Scholar] [CrossRef]
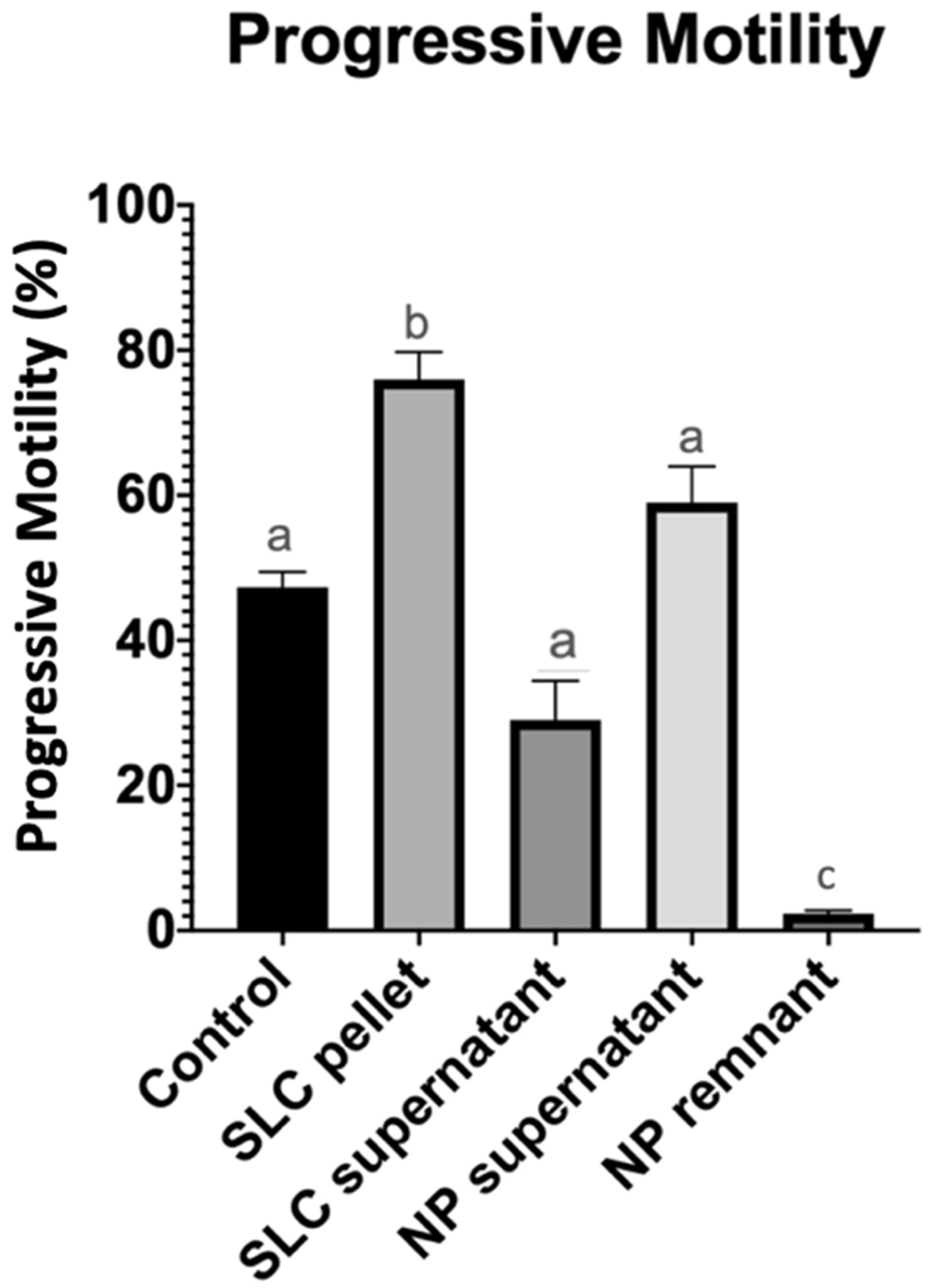
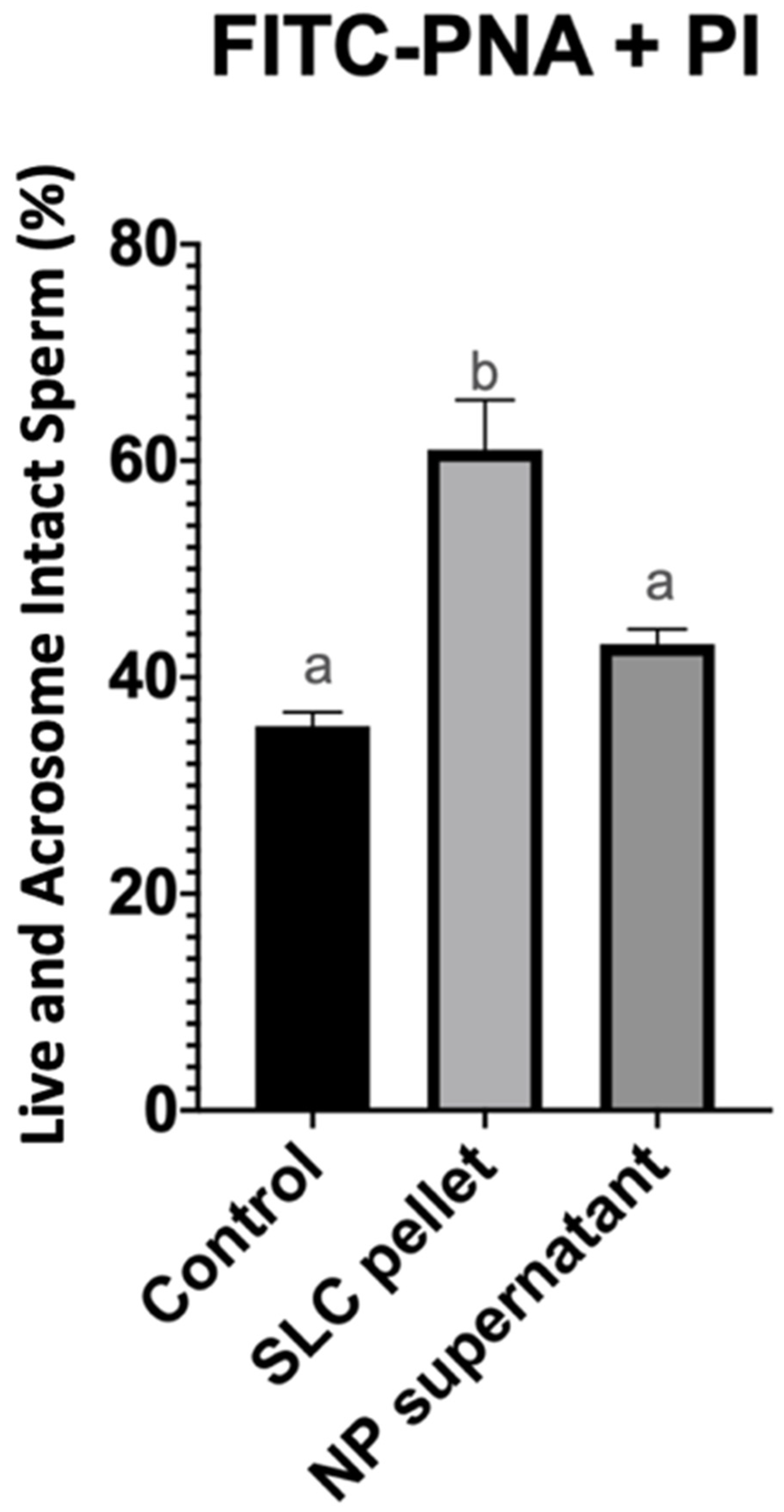
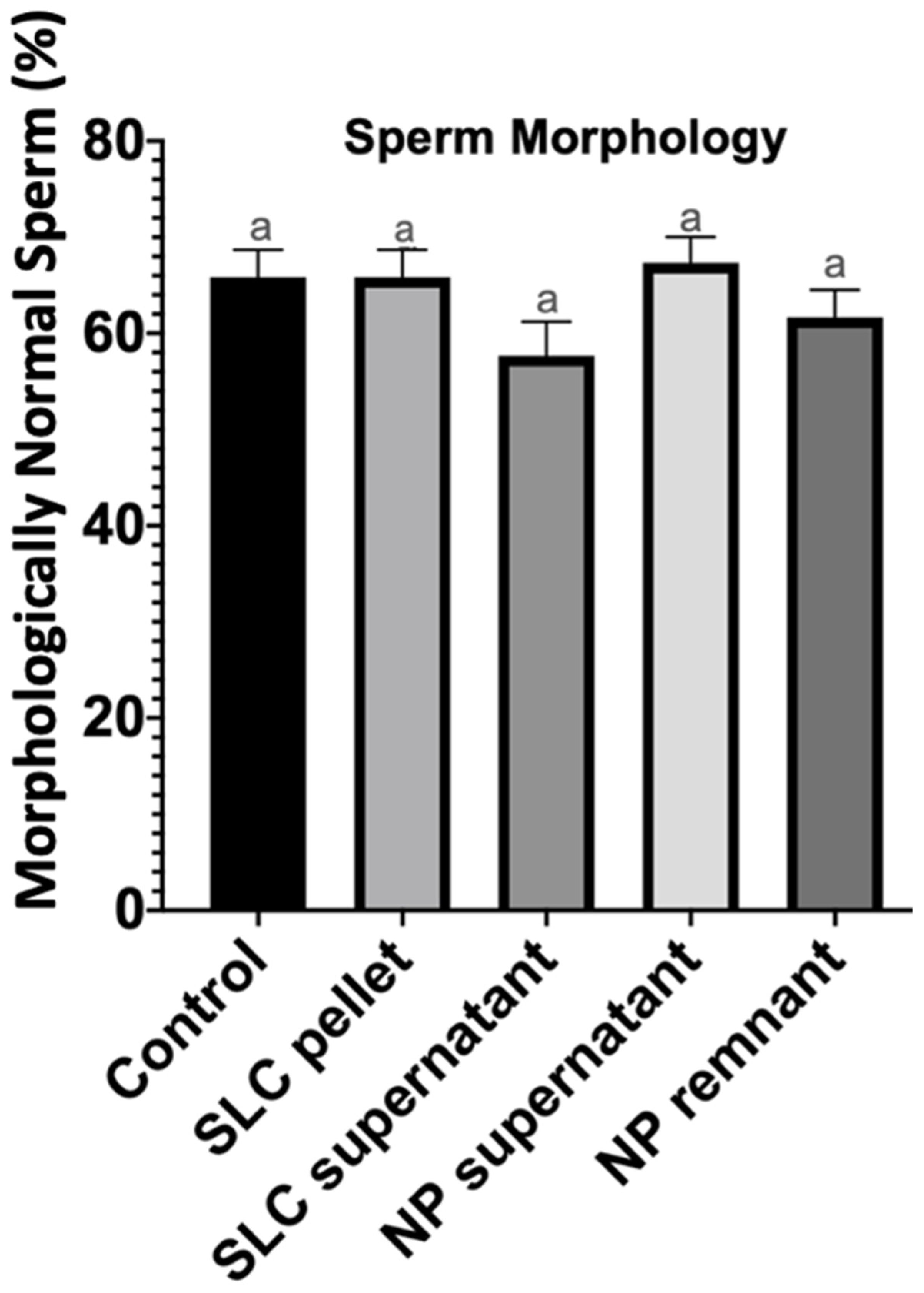
| Treatment Groups | Total Motility (%) | Progressive Motility (%) | Total Number of Sperm Recovered (Millions) | Total Number of Progressively Motile Sperm Recovered (Millions) |
|---|---|---|---|---|
| Control | 50.3 ± 6.3 a | 47.3 ± 5.1 a | 620.2 ± 51.8 a | 293.7 ± 41.9 a |
| NP supernatant | 62.3 ± 11.3 a | 59 ± 12.2 a | 482.5 ± 137.3 a | 317.6 ± 109 a |
| SLC pellet | 82.7 ± 8.3 b | 76 ± 9.2 b | 229.8 ± 66.9 b | 157.8 ± 43.6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisiau, C.; Moffett, P.; Graham, J.; McCue, P. Comparison of Nanoparticles and Single-Layer Centrifugation for Separation of Dead from Live Stallion Spermatozoa. Vet. Sci. 2024, 11, 307. https://doi.org/10.3390/vetsci11070307
Bisiau C, Moffett P, Graham J, McCue P. Comparison of Nanoparticles and Single-Layer Centrifugation for Separation of Dead from Live Stallion Spermatozoa. Veterinary Sciences. 2024; 11(7):307. https://doi.org/10.3390/vetsci11070307
Chicago/Turabian StyleBisiau, Christian, Paula Moffett, James Graham, and Patrick McCue. 2024. "Comparison of Nanoparticles and Single-Layer Centrifugation for Separation of Dead from Live Stallion Spermatozoa" Veterinary Sciences 11, no. 7: 307. https://doi.org/10.3390/vetsci11070307
APA StyleBisiau, C., Moffett, P., Graham, J., & McCue, P. (2024). Comparison of Nanoparticles and Single-Layer Centrifugation for Separation of Dead from Live Stallion Spermatozoa. Veterinary Sciences, 11(7), 307. https://doi.org/10.3390/vetsci11070307





