Foot and Mouth Disease Vaccine Matching and Post-Vaccination Assessment in Abu Dhabi, United Arab Emirates
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Isolates Used for Vaccine Matching
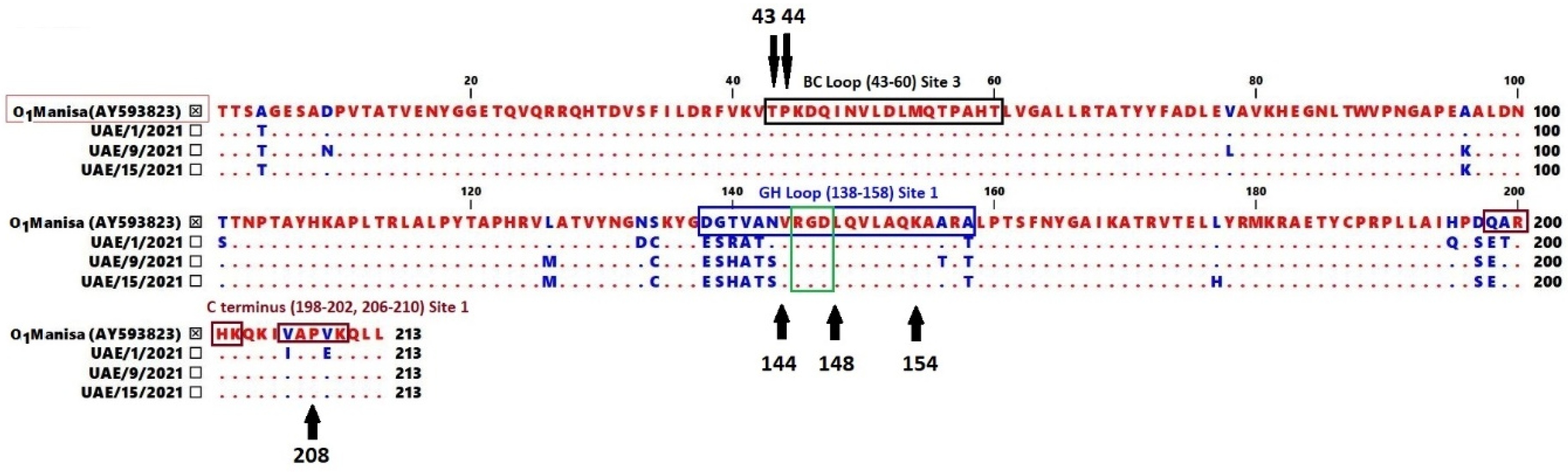
2.2. Analysis of Amino Acid Sequence Variability
2.3. Two-Dimensional Virus Neutralization Assay (2D-VNT) for Vaccine Matching
2.4. Post-Vaccination Assessment
2.4.1. Study Area
2.4.2. Vaccine and Vaccination
2.4.3. Sample Size and Collection
2.4.4. Serological Testing
3. Results
3.1. Phylogenetic Analysis
3.2. Amino Acid Sequence Variability Analysis for Identified Viruses
3.3. Vaccine Matching with 2 dm VNT
3.4. Evaluation of FMD Vaccination in 2023
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gloster, J.; Jones, A.; Redington, A.; Burgin, L.; Sørensen, J.H.; Turner, R.; Dillon, M.; Hullinger, P.; Simpson, M.; Astrup, P. Airborne spread of foot-and-mouth disease–model intercomparison. Vet. J. 2010, 183, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Sutmoller, P.; Olascoaga, R.C. Unapparent foot and mouth disease infection (sub-clinical infections and carriers): Implications for control. Rev. Sci. Tech.-Off. Int. Des Épizooties 2002, 21, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Grubman, M.J.; Baxt, B. Foot-and-mouth disease. Clin. Microbiol. Rev. 2004, 17, 465–493. [Google Scholar] [CrossRef] [PubMed]
- Baluka, S.A. Economic effects of foot and mouth disease outbreaks along the cattle marketing chain in Uganda. Vet. World 2016, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- El-Deen, N.A.N.; Neamat-Allah, A.N.; Rizk, L.G.; Fareed, R.S.G. Serological, hematological, biochemical and oxidative markers during foot and mouth disease serotype’O’infection, Egypt. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Vet. Med. 2017, 74, 2. [Google Scholar]
- Gizaw, D.; Tesfaye, Y.; Wood, B.A.; Di Nardo, A.; Shegu, D.; Muluneh, A.; Bilata, T.; Belayneh, R.; Fentie, A.; Asgdome, H.; et al. Molecular characterization of foot-and-mouth disease viruses circulating in Ethiopia between 2008 and 2019. Transbound. Emerg. Dis. 2020, 67, 2983–2992. [Google Scholar] [CrossRef]
- Hammond, J.M.; Maulidi, B.; Henning, N. Targeted FMD vaccines for Eastern Africa: The AgResults foot and mouth disease vaccine challenge project. Viruses 2021, 13, 1830. [Google Scholar] [CrossRef]
- Brito, B.; Rodriguez, L.; Hammond, J.; Pinto, J.; Perez, A. Review of the global distribution of foot-and-mouth disease virus from 2007 to 2014. Transbound. Emerg. Dis. 2017, 64, 316–332. [Google Scholar] [CrossRef]
- Di Nardo, A.; Knowles, N.; Paton, D. Combining livestock trade patterns with phylogenetics to help understand the spread of foot and mouth disease in sub-Saharan Africa, the Middle East and Southeast Asia. Rev. Sci. Tech. 2011, 30, 63. [Google Scholar] [CrossRef]
- Bachanek-Bankowska, K.; Di Nardo, A.; Wadsworth, J.; Henry, E.K.; Parlak, Ü.; Timina, A.; Mischenko, A.; Qasim, I.A.; Abdollahi, D.; Sultana, M. Foot-and-mouth disease in the middle east caused by an A/ASIA/G-VII virus lineage, 2015–2016. Emerg. Infect. Dis. 2018, 24, 1073. [Google Scholar] [CrossRef]
- Bachanek-Bankowska, K.; Di Nardo, A.; Wadsworth, J.; Mioulet, V.; Pezzoni, G.; Grazioli, S.; Brocchi, E.; Kafle, S.C.; Hettiarachchi, R.; Kumarawadu, P.L. Reconstructing the evolutionary history of pandemic foot-and-mouth disease viruses: The impact of recombination within the emerging O/ME-SA/Ind-2001 lineage. Sci. Rep. 2018, 8, 14693. [Google Scholar] [CrossRef] [PubMed]
- FAO. Foot-and-Mouth Disease: Quarterly Report. Rome. January–March 2023. 2023. Available online: https://www.fao.org/documents/card/en/c/cc6065en (accessed on 5 March 2023).
- Aslam, M.; Alkheraije, K.A. The prevalence of foot-and-mouth disease in Asia. Front. Vet. Sci. 2023, 10. [Google Scholar] [CrossRef]
- Chepkwony, E.C.; Gitao, G.C.; Muchemi, G.M.; Sangula, A.K. Isolation and molecular characterization of Foot and Mouth Disease virus serotype O circulated in Kenya during the period 2013–2018. Ger. J. Vet. Res. 2022, 2, 25–34. [Google Scholar] [CrossRef]
- WAHIS. 2023. Available online: https://wahis.woah.org/#/dashboards/country-or-disease-dashboard (accessed on 5 March 2023).
- Eltahir, Y.M.; Ishag, H.Z.A.; Wadsworth, J.; Hicks, H.M.; Knowles, N.J.; Mioulet, V.; King, D.P.; Mohamed, M.S.; Bensalah, O.K.; Yusof, M.F. Molecular Epidemiology of Foot-and-Mouth Disease Viruses in the Emirate of Abu Dhabi, United Arab Emirates. Vet. Sci. 2024, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- WOAH. Infection with foot and mouth disease virus. In OIE Terrestrial Animal Health Code; World Organisation for Animal Health: Paris, France, 2021; pp. 1–24. [Google Scholar]
- El Damaty, H.M.; Fawzi, E.M.; Neamat-Allah, A.N.; Elsohaby, I.; Abdallah, A.; Farag, G.K.; El-Shazly, Y.A.; Mahmmod, Y.S. Characterization of foot and mouth disease virus serotype SAT-2 in swamp water buffaloes (Bubalus bubalis) under the Egyptian smallholder production system. Animals 2021, 11, 1697. [Google Scholar] [CrossRef] [PubMed]
- Willems, T.; De Vleeschauwer, A.; Perez-Filgueira, M.; Li, Y.; Ludi, A.; Lefebvre, D.; Wilsden, G.; Statham, B.; Haas, B.; Mattion, N. FMD vaccine matching: Inter laboratory study for improved understanding of r1 values. J. Virol. Methods 2020, 276, 113786. [Google Scholar] [CrossRef] [PubMed]
- Brehm, K.; Kumar, N.; Thulke, H.-H.; Haas, B. High potency vaccines induce protection against heterologous challenge with foot-and-mouth disease virus. Vaccine 2008, 26, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- FAO. Foot and Mouth Disease Vaccination and Post-Vaccination Monitoring: Guidelines; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Gray, A.R.; Wood, B.A.; Henry, E.; King, D.P.; Mioulet, V. Elimination of Non-cytopathic Bovine Viral Diarrhea Virus From the LFBK-αvβ6 Cell Line. Front. Vet. Sci. 2021, 8, 715120. [Google Scholar] [CrossRef] [PubMed]
- LaRocco, M.; Krug, P.W.; Kramer, E.; Ahmed, Z.; Pacheco, J.M.; Duque, H.; Baxt, B.; Rodriguez, L.L. A continuous bovine kidney cell line constitutively expressing bovine αvβ6 integrin has increased susceptibility to foot-and-mouth disease virus. J. Clin. Microbiol. 2013, 51, 1714–1720. [Google Scholar] [CrossRef]
- WOAH. Principles and Methods of Validation of Diagnostic Assays for Infectious Diseases; OIE: Paris, France, 2018; Chapter 1.1.6. [Google Scholar]
- Thrusfield, M. Veterinary Epidemiology, 3rd ed.; Blackwell Publishing Ltd.: Oxford, UK; John Wiley & Sons: Hoboken, NJ, USA, 2007; p. 610. [Google Scholar]
- Lycett, S.; Tanya, V.; Hall, M.; King, D.; Mazeri, S.; Mioulet, V.; Knowles, N.; Wadsworth, J.; Bachanek-Bankowska, K.; Ngu Ngwa, V. The evolution and phylodynamics of serotype A and SAT2 foot-and-mouth disease viruses in endemic regions of Africa. Sci. Rep. 2019, 9, 5614. [Google Scholar] [CrossRef]
- WOAH. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 12th ed.; Volume 2 Foot and Mouth Disease; Office International des Epizooties: Paris, France, 2023; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.01.08_FMD.pdf (accessed on 15 February 2024).
- Doel, T. FMD vaccines. Virus Res. 2003, 91, 81–99. [Google Scholar] [CrossRef]
- Tesfaye, Y.; Khan, F.; Yami, M.; Wadsworth, J.; Knowles, N.J.; King, D.P.; Gelaye, E. A vaccine-matching assessment of different genetic variants of serotype O foot-and-mouth disease virus isolated in Ethiopia between 2011 and 2014. Arch. Virol. 2020, 165, 1749–1757. [Google Scholar] [CrossRef]
- Crowther, J.; Farias, S.; Carpenter, W.; Samuel, A. Identification of a fifth neutralizable site on type O foot-and-mouth disease virus following characterization of single and quintuple monoclonal antibody escape mutants. J. Gen. Virol. 1993, 74, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- McCahon, D.; Crowther, J.; Belsham, G.; Kitson, J.; Duchesne, M.; Have, P.; Meloen, R.; Morgan, D.; De Simone, F. Evidence for at least four antigenic sites on type O foot-and-mouth disease virus involved in neutralization; identification by single and multiple site monoclonal antibody-resistant mutants. J. Gen. Virol. 1989, 70, 639–645. [Google Scholar] [CrossRef]
- Xie, Q.; McCahon, D.; Crowther, J.; Belsham, G.; McCullough, K. Neutralization of foot-and-mouth disease virus can be mediated through any of at least three separate antigenic sites. J. Gen. Virol. 1987, 68, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.; Clark, S.; Berryman, S.; Burman, A.; Cambier, S.; Mu, D.; Nishimura, S.; King, A.M. Integrin αvβ8 functions as a receptor for foot-and-mouth disease virus: Role of the β-chain cytodomain in integrin-mediated infection. J. Virol. 2004, 78, 4533–4540. [Google Scholar] [CrossRef]
- Jackson, T.; King, A.; Stuart, D.; Fry, E. Structure and receptor binding. Virus Res. 2003, 91, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Logan, D.; Abu-Ghazaleh, R.; Blakemore, W.; Curry, S.; Jackson, T.; King, A.; Lea, S.; Lewis, R.; Newman, J.; Parry, N. Structure of a major immunogenic site on foot-and-mouth disease virus. Nature 1993, 362, 566–568. [Google Scholar] [CrossRef]
- Balinda, S.N.; Sangula, A.K.; Heller, R.; Muwanika, V.B.; Belsham, G.J.; Masembe, C.; Siegismund, H.R. Diversity and transboundary mobility of serotype O foot-and-mouth disease virus in East Africa: Implications for vaccination policies. Infect. Genet. Evol. 2010, 10, 1058–1065. [Google Scholar] [CrossRef]
- Sahle, M.; Venter, E.; Dwarka, R.; Vosloo, W. Molecular epidemiology of serotype O foot-and-mouth disease virus isolated from cattle in Ethiopia between 1979–2001. Onderstepoort J. Vet. Res. 2004, 71, 129–138. [Google Scholar] [CrossRef]
- Das, B.; Sanyal, A.; Subramaniam, S.; Mohapatra, J.K.; Pattnaik, B. Field outbreak strains of serotype O foot-and-mouth disease virus from India with a deletion in the immunodominant βG-βH loop of the VP1 protein. Arch. Virol. 2012, 157, 1967–1970. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, M.; Yuvaraj, S.; Madhanmohan, M.; Subramaniam, S.; Pattnaik, B.; Paton, D.; Srinivasan, V.; Parida, S. Antigenic and genetic comparison of foot-and-mouth disease virus serotype O Indian vaccine strain, O/IND/R2/75 against currently circulating viruses. Vaccine 2015, 33, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Mohapatra, J.K.; Das, B.; Sanyal, A.; Pattnaik, B. Genetic and antigenic analysis of foot-and-mouth disease virus serotype O responsible for outbreaks in India during 2013. Infect. Genet. Evol. 2015, 30, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Ulziibat, G.; Raizman, E.; Lkhagvasuren, A.; Bartels, C.J.; Oyun-Erdene, O.; Khishgee, B.; Browning, C.; King, D.P.; Ludi, A.B.; Lyons, N.A. Comparison of vaccination schedules for foot-and-mouth disease among cattle and sheep in Mongolia. Front. Vet. Sci. 2023, 10, 990043. [Google Scholar] [CrossRef] [PubMed]
- Peta, F.R.; Sirdar, M.; van Bavel, P.; Mutowembwa, P.; Visser, N.; Olowoyo, J.; Seheri, M.; Heath, L. Evaluation of potency and duration of immunity elicited by a multivalent FMD vaccine for use in South Africa. Front. Vet. Sci. 2021, 8, 750223. [Google Scholar] [CrossRef]
- Kharatyan, S.; Sargsyan, K.; Elbakyan, H.; Markosyan, T.; Tumanyan, P.; Hakobyan, V.; Sargsyan, V.; Badalyan, M.; Chobanyan, G.; Achenbach, J.E. Evaluation of the effectiveness of foot-and-mouth disease vaccination of animals in the buffer zone of the Republic of Armenia in 2016–2020. BMC Vet. Res. 2023, 19, 176. [Google Scholar] [CrossRef]
| Farm Name | Date of Sample Collection | Sample Type | No. of Samples | WRLFMD Label | Animal Species Infected | FMD Vaccination | GenBank Accession Number |
|---|---|---|---|---|---|---|---|
| A | April 2021 | Mouth swab | 1 | UAE/1/2021 | Arabian oryx | No | OR425051 |
| B | November 2021 | Mouth swab | 1 | UAE/9/2021 | Goat | No | OR425057 |
| C | December 2021 | Heart tissue | 1 | UAE/15/2021 | Sheep | Yes-single dose * | OR425053 |
| Isolate | Lineage (Sublineage) | Vaccine Strain Compared to | Number of Amino Acid Substitutions | Variability at Site 1 | Variability at Site 3 | ||
|---|---|---|---|---|---|---|---|
| GH Loop | C-Terminus | BC Loop | |||||
| (138–156) | (198–202) | (206–210) | (43–60) | ||||
| UAE/1/2021 | O/ME-SA/PanAsia-2 (ANT-10) | O1 Manisa (AY593823) | 16 | 6 | 2 | 2 | - |
| UAE/9/2021 | O/ME-SA/SA-2018 | 16 | 8 | - | - | - | |
| UAE/15/2021 | 14 | 7 | - | - | - | ||
| Vaccine | O/UAE/1/2021 O/ME-SA/PanAsia-2/ANT-10 | O/UAE/15/2021 O/ME-SA/SA-2018 | O/UAE/9/2021 O/ME-SA/SA-2018 |
|---|---|---|---|
| O1 Campos, Biogénesis Bagó | 0.43, 2.56 | 0.51, 2.63 | 0.60, 2.70 |
| O-3039, Boehringer Ingelheim | 0.38, 1.64 | 0.75, 1.94 | 0.59, 1.83 |
| O1 Campos, Boehringer Ingelheim | 0.19, 2.02 | 0.28, 2.19 | 0.23, 2.10 |
| O1 Manisa, Boehringer Ingelheim | 0.48, 2.08 | 0.56, 2.15 | 0.44, 2.04 |
| O PanAsia-2, Boehringer Ingelheim | 0.32, 2.14 | 0.47, 2.30 | 0.32, 2.13 |
| O/TUR/5/09, MSD Animal Health | 0.44, 2.13 | 0.69, 2.32 | 0.68, 2.32 |
| Period | Result | Serotype A | Serotype O | Serotype A in Total Sheep and Goats | Serotype O in Total Sheep and Goats | ||
|---|---|---|---|---|---|---|---|
| Sheep | Goat | Sheep | Goat | ||||
| Five months after the 1st FMD vaccination dose | Total tested | 230 | 166 | 230 | 166 | 396 | 396 |
| No. of positive | 123 | 64 | 166 | 106 | 187 | 272 | |
| No. of negative | 107 | 102 | 64 | 60 | 209 | 124 | |
| % Of immunity coverage | 53% | 39% | 72% | 64% | 47% | 69% | |
| Total tested | 230 | 166 | 230 | 166 | 396 | 396 | |
| 28 days after the 2nd FMD vaccination dose | No. of positive | 179 | 142 | 211 | 136 | 321 | 347 |
| No. of negative | 51 | 24 | 19 | 30 | 75 | 105 | |
| % of immunity coverage | 78% | 86% | 92% | 82% | 81% | 88% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eltahir, Y.M.; Ishag, H.Z.A.; Parekh, K.; Wood, B.A.; Ludi, A.; King, D.P.; Bensalah, O.K.; Khan, R.A.; Shah, A.A.M.; Kayaf, K.; et al. Foot and Mouth Disease Vaccine Matching and Post-Vaccination Assessment in Abu Dhabi, United Arab Emirates. Vet. Sci. 2024, 11, 272. https://doi.org/10.3390/vetsci11060272
Eltahir YM, Ishag HZA, Parekh K, Wood BA, Ludi A, King DP, Bensalah OK, Khan RA, Shah AAM, Kayaf K, et al. Foot and Mouth Disease Vaccine Matching and Post-Vaccination Assessment in Abu Dhabi, United Arab Emirates. Veterinary Sciences. 2024; 11(6):272. https://doi.org/10.3390/vetsci11060272
Chicago/Turabian StyleEltahir, Yassir M., Hassan Zackaria Ali Ishag, Krupali Parekh, Britta A. Wood, Anna Ludi, Donald P. King, Oum Keltoum Bensalah, Rashid A. Khan, Asma Abdi Mohamed Shah, Kaltham Kayaf, and et al. 2024. "Foot and Mouth Disease Vaccine Matching and Post-Vaccination Assessment in Abu Dhabi, United Arab Emirates" Veterinary Sciences 11, no. 6: 272. https://doi.org/10.3390/vetsci11060272
APA StyleEltahir, Y. M., Ishag, H. Z. A., Parekh, K., Wood, B. A., Ludi, A., King, D. P., Bensalah, O. K., Khan, R. A., Shah, A. A. M., Kayaf, K., & Mohamed, M. S. (2024). Foot and Mouth Disease Vaccine Matching and Post-Vaccination Assessment in Abu Dhabi, United Arab Emirates. Veterinary Sciences, 11(6), 272. https://doi.org/10.3390/vetsci11060272







