Fascin-1 Promotes Cell Metastasis through Epithelial–Mesenchymal Transition in Canine Mammary Tumor Cell Lines
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Construction of Fascin-1 Overexpression and Knockdown Cell Line
2.3. Cell Viability Assay
2.4. Cell Migration Assay
2.5. Cell Adhesion Assay
2.6. Cell Invasion Assay
2.7. Immunocytochemistry (IHC) and Immunofluorescence Assay (IFA)
2.8. Tandem Mass Tags (TMT) Quantitative Proteome Analysis
2.9. Quantitative Reverse-Transcription PCR (RT-qPCR)
2.10. Western Blot
2.11. Observation of Migrasome Bodies by Scanning Electron Microscopy
2.12. Data Analysis
3. Results
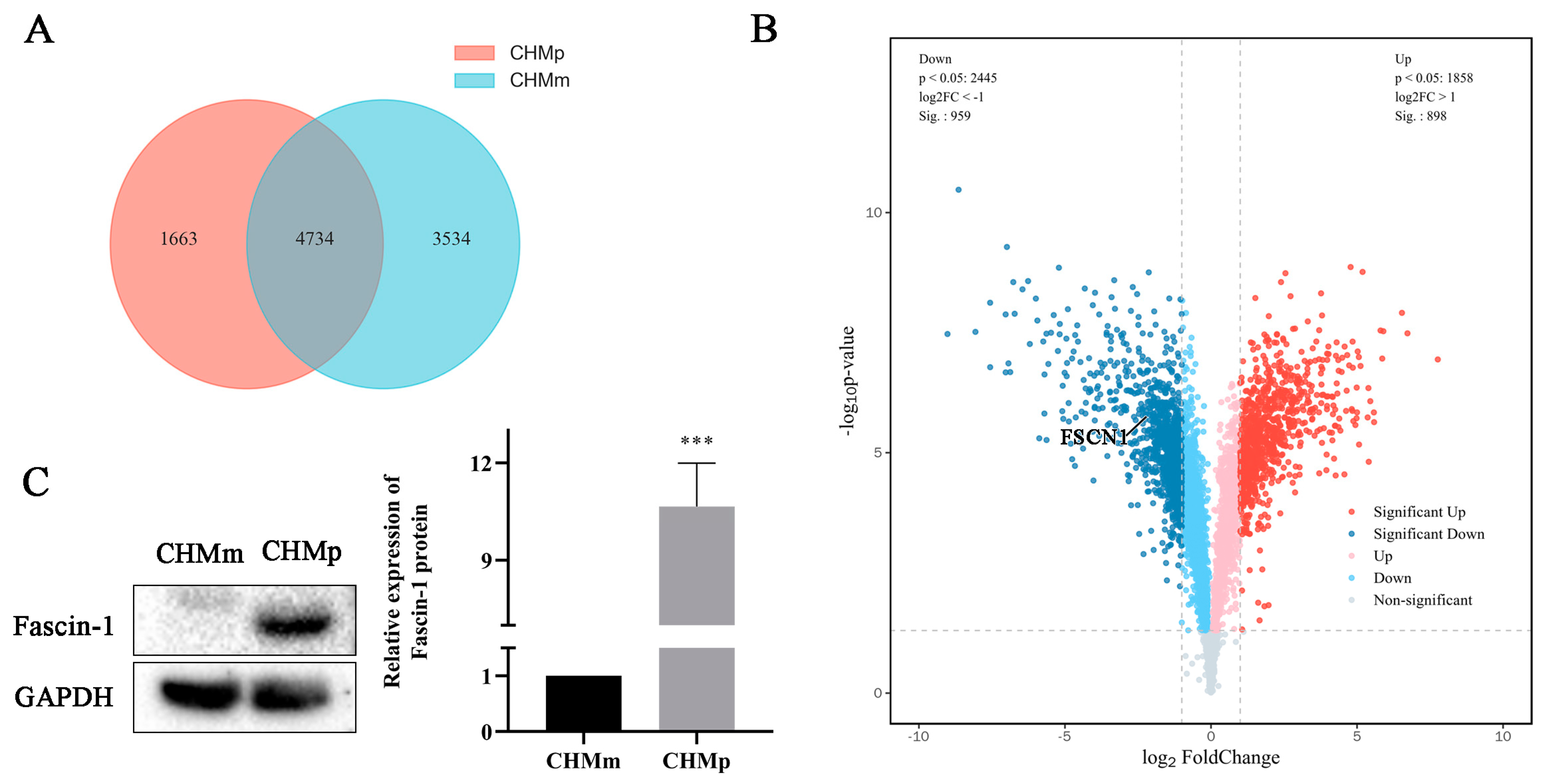
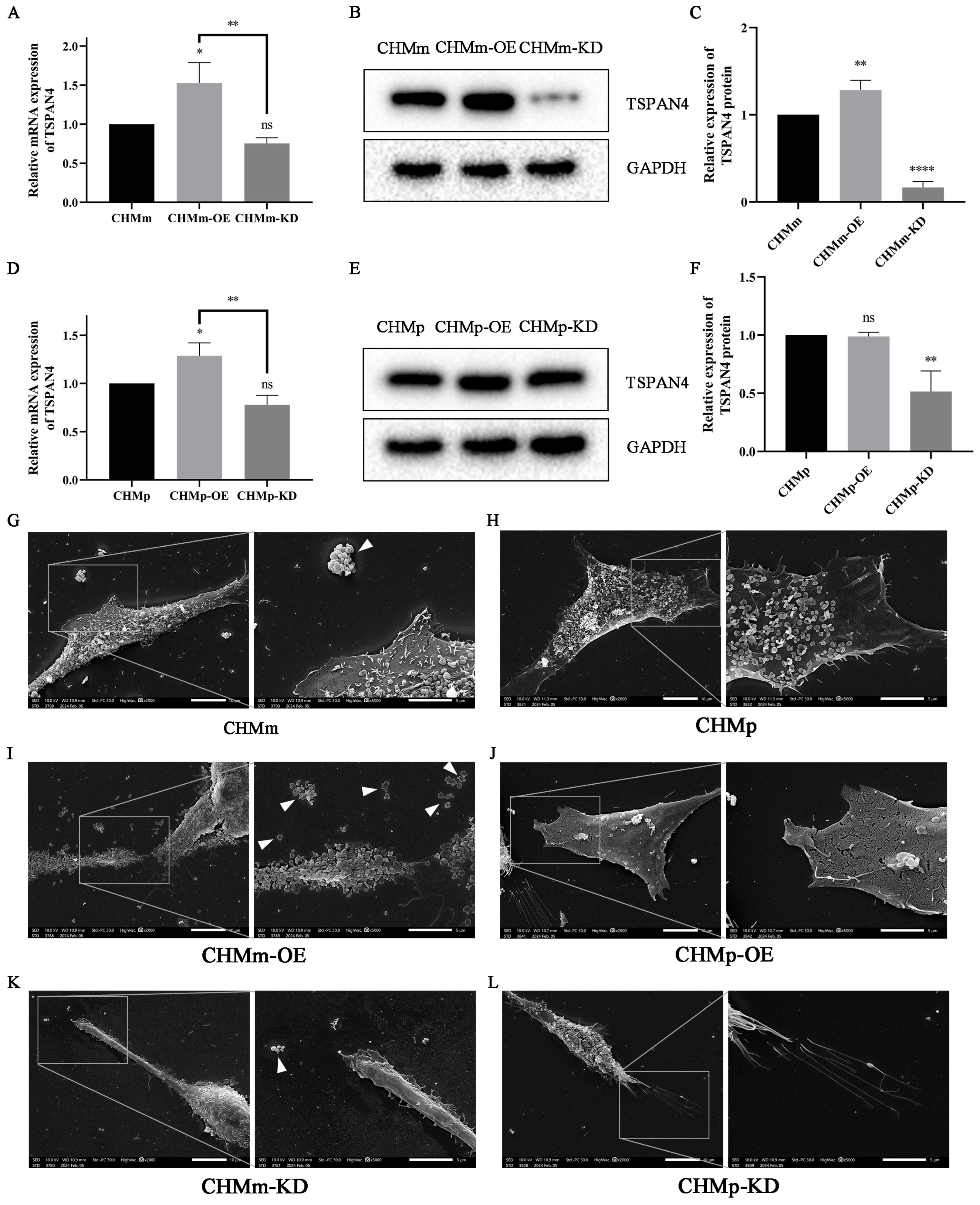
3.1. Difference of Fascin-1 Expression between CHMm and CHMp Cells
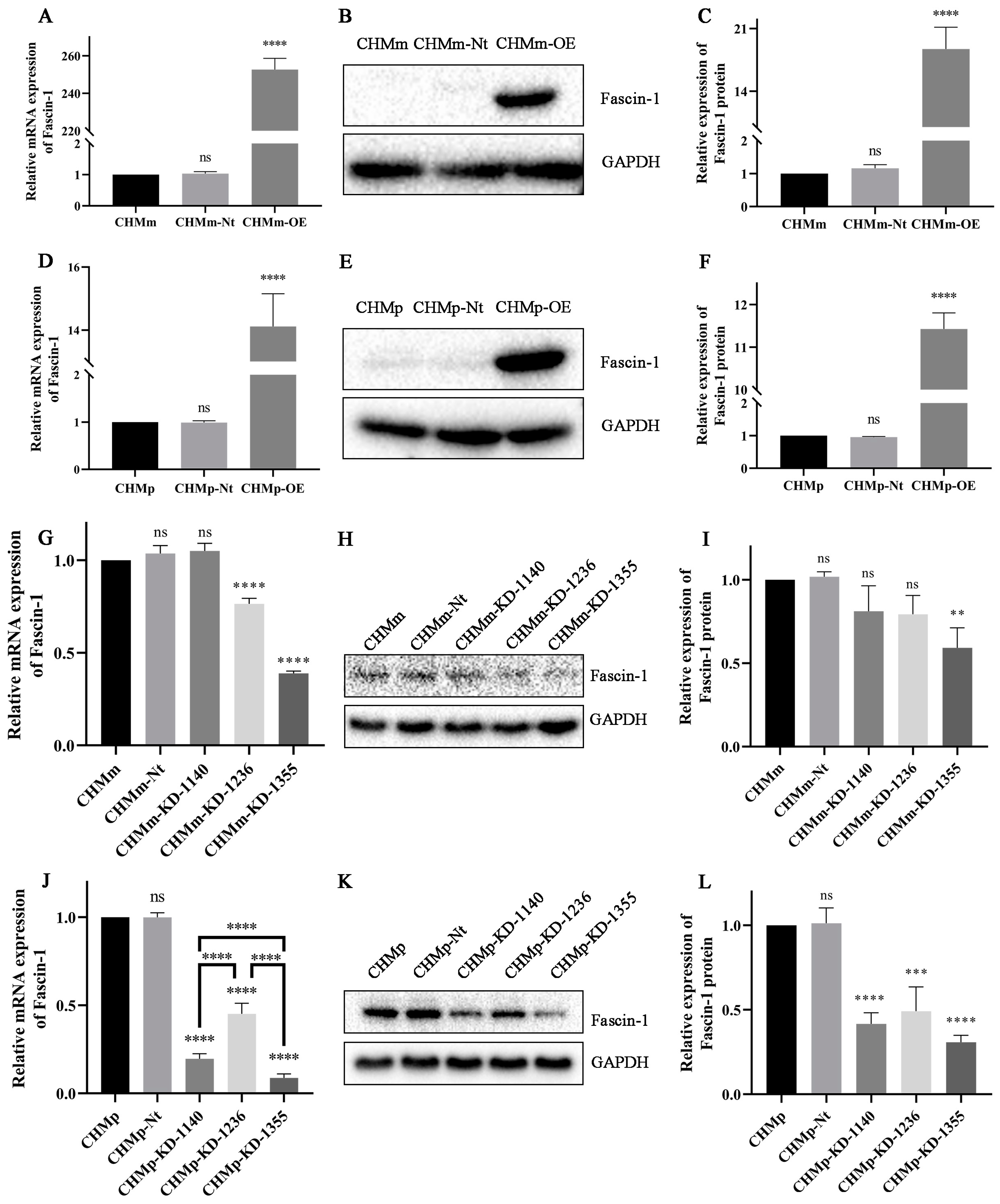
3.2. The Overexpression and Knockdown of Fascin-1 in CMT Cells
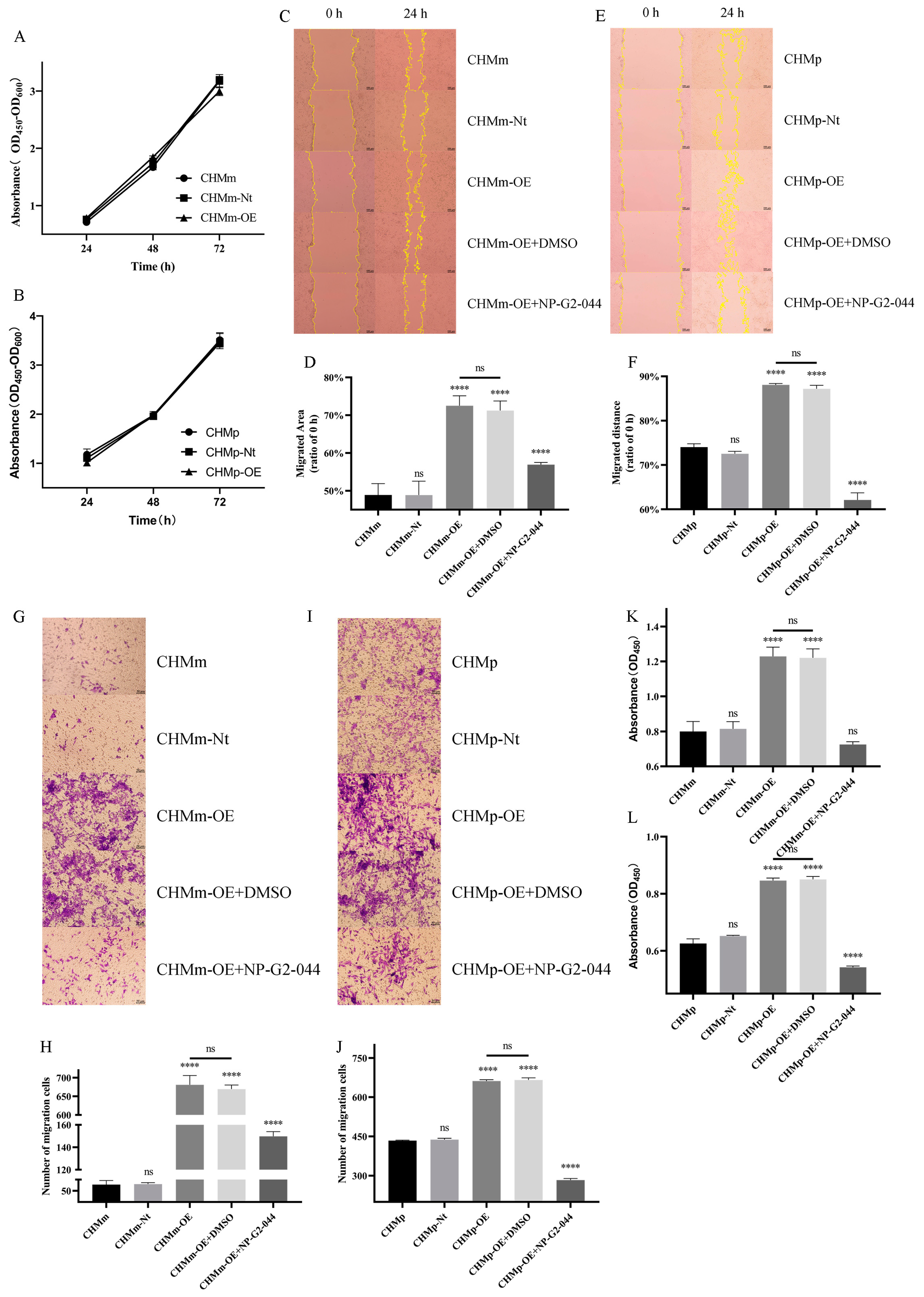
3.3. Cell Viability, Migration, Adhesion, and Invasion in CMT Cells
3.4. Fascin-1 Overexpression Enhances the Formation of Filopodia and Lamellipodia on the Surface of CHMm Cells
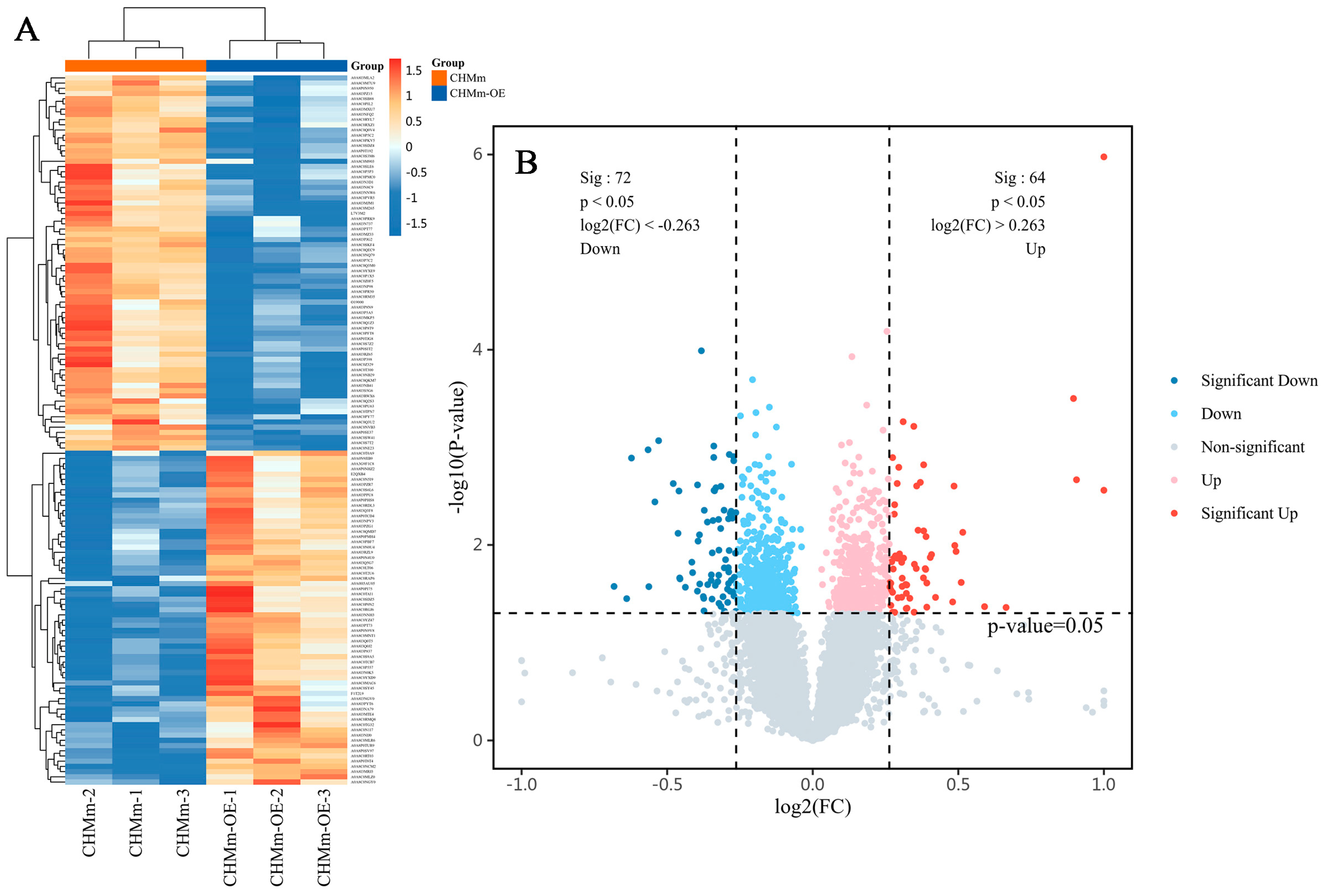
3.5. Differential Expression of the Proteins
3.6. Cluster Analyses of Differentially Expressed Proteins
3.7. Fascin-1 Overexpression Changes the Expression of Tumor Metastasis-Related Proteins
3.8. Effect of Fascin-1 Overexpression on the EMT-Related Protein Expression
3.9. Effect of Fascin-1 Expression on the Formation of Migration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sorenmo, K.U.; Kristiansen, V.M.; Cofone, M.A.; Shofer, F.S.; Breen, A.M.; Langeland, M.; Mongil, C.M.; Grondahl, A.M.; Teige, J.; Goldschmidt, M.H. Canine mammary gland tumours; a histological continuum from benign to malignant; clinical and histopathological evidence. Vet. Comp. Oncol. 2009, 7, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Merlo, D.F.; Rossi, L.; Pellegrino, C.; Ceppi, M.; Cardellino, U.; Capurro, C.; Ratto, A.; Sambucco, P.L.; Sestito, V.; Tanara, G.; et al. Cancer incidence in pet dogs: Findings of the Animal Tumor Registry of Genoa, Italy. J. Vet. Intern. Med. 2008, 22, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Ikebuchi, R.; Okagawa, T.; Adachi, M.; Takagi, S.; Kagawa, Y.; Nakajima, C.; Suzuki, Y.; Murata, S.; et al. Expression of PD-L1 on canine tumor cells and enhancement of IFN-γ production from tumor-infiltrating cells by PD-L1 blockade. PLoS ONE 2014, 9, e98415. [Google Scholar] [CrossRef] [PubMed]
- Gedon, J.; Wehrend, A.; Failing, K.; Kessler, M. Canine mammary tumours: Size matters-a progression from low to highly malignant subtypes. Vet. Comp. Oncol. 2021, 19, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Sleeckx, N.; De Rooster, H.; Veldhuis Kroeze, E.J.; Van Ginneken, C.; Van Brantegem, L. Canine mammary tumours, an overview. Reprod. Domest. Anim. 2011, 46, 1112–1131. [Google Scholar] [CrossRef] [PubMed]
- Gunnes, G.; Borge, K.S.; Lingaas, F. A statistical assessment of the biological relationship between simultaneous canine mammary tumours. Vet. Comp. Oncol. 2017, 15, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Sorenmo, K.U.; Rasotto, R.; Zappulli, V.; Goldschmidt, M.H. Development, anatomy, histology, lymphatic drainage, clinical features, and cell differentiation markers of canine mammary gland neoplasms. Vet. Pathol. 2011, 48, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L. Epidemiological Study of Mammary Tumors in Female Dogs Diagnosed during the Period 2002–2012: A Growing Animal Health Problem. PLoS ONE 2015, 10, e0127381. [Google Scholar] [CrossRef]
- Burrai, G.P.; Gabrieli, A.; Moccia, V.; Zappulli, V.; Porcellato, I.; Brachelente, C.; Pirino, S.; Polinas, M.; Antuofermo, E. A Statistical Analysis of Risk Factors and Biological Behavior in Canine Mammary Tumors: A Multicenter Study. Animals 2020, 10, 1687. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Pinho, S.; Carvalho, S.; Cabral, J.; Reis, C.A.; Gärtner, F. Canine tumors: A spontaneous animal model of human carcinogenesis. Transl. Res. 2012, 159, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ostrander, E.A.; Franklin, H. Epstein Lecture. Both ends of the leash--the human links to good dogs with bad genes. N. Engl. J. Med. 2012, 367, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Zamani-Ahmadmahmudi, M.; Nassiri, S.M.; Rahbarghazi, R. Serological proteome analysis of dogs with breast cancer unveils common serum biomarkers with human counterparts. Electrophoresis 2014, 35, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, S.M.; Mohammed, S. Canine mammary tumors as a model for human disease. Oncol. Lett. 2018, 15, 8195–8205. [Google Scholar] [CrossRef] [PubMed]
- Sleeckx, N.; Van Brantegem, L.; Van Den Eynden, G.; Fransen, E.; Casteleyn, C.; Van Cruchten, S.; Veldhuis Kroeze, E.; Van Ginneken, C. Lymphangiogenesis in canine mammary tumours: A morphometric and prognostic study. J. Comp. Pathol. 2014, 150, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Madej, J.A.; Madej, J.P.; Dziegiel, P.; Pula, B.; Nowak, M. Expression of hypoxia-inducible factor-1α and vascular density in mammary adenomas and adenocarcinomas in bitches. Acta Vet. Scand. 2013, 55, 73. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, S.; Yamakita, Y.; Ono, S.; Matsumura, F. Fascin, an actin-bundling protein, induces membrane protrusions and increases cell motility of epithelial cells. Mol. Biol. Cell 1998, 9, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Vignjevic, D.; Kojima, S.; Aratyn, Y.; Danciu, O.; Svitkina, T.; Borisy, G.G. Role of fascin in filopodial protrusion. J. Cell Biol. 2006, 174, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.E. Preparation and purification of polymerized actin from sea urchin egg extracts. J. Cell Biol. 1975, 66, 305–315. [Google Scholar] [CrossRef]
- Yamashiro-Matsumura, S.; Matsumura, F. Purification and characterization of an F-actin-bundling 55-kilodalton protein from HeLa cells. J. Biol. Chem. 1985, 260, 5087–5097. [Google Scholar] [CrossRef]
- Yao, J.; Qian, C.J.; Ye, B.; Zhao, Z.Q.; Wei, J.; Liang, Y.; Zhang, X. Signal transducer and activator of transcription 3 signaling upregulates fascin via nuclear factor-κB in gastric cancer: Implications in cell invasion and migration. Oncol. Lett. 2014, 7, 902–908. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Zhang, C.; Huang, D.; Wu, W.; Zhang, Y.; Shen, J.; Cai, Y.; Chen, W.; Yao, W. FSCN-1 increases doxorubicin resistance in hepatocellular carcinoma through promotion of epithelial-mesenchymal transition. Int. J. Oncol. 2018, 52, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Jayo, A.; Malboubi, M.; Antoku, S.; Chang, W.; Ortiz-Zapater, E.; Groen, C.; Pfisterer, K.; Tootle, T.; Charras, G.; Gundersen, G.G.; et al. Fascin Regulates Nuclear Movement and Deformation in Migrating Cells. Dev. Cell. 2016, 38, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Osanai, M.; Lee, G.H. Fascin-1 expression correlates with repression of E-cadherin expression in hepatocellular carcinoma cells and augments their invasiveness in combination with matrix metalloproteinases. Cancer Sci. 2011, 102, 1228–1235. [Google Scholar] [CrossRef]
- Xing, P.; Li, J.G.; Jin, F.; Zhao, T.T.; Liu, Q.; Dong, H.T.; Wei, X.L. Fascin, an actin-bundling protein, promotes breast cancer progression in vitro. Cell Biochem. Funct. 2011, 29, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Seki, N.; Kikkawa, N.; Fujimura, L.; Hoshino, I.; Akutsu, Y.; Chiyomaru, T.; Enokida, H.; Nakagawa, M.; Matsubara, H. miR-145, miR-133a and miR-133b: Tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int. J. Cancer 2010, 127, 2804–2814. [Google Scholar] [CrossRef]
- Ma, Y.; Machesky, L.M. Fascin1 in carcinomas: Its regulation and prognostic value. Int. J. Cancer 2015, 137, 2534–2544. [Google Scholar] [CrossRef]
- Goldschmidt, M.; Peña, L.; Rasotto, R.; Zappulli, V. Classification and grading of canine mammary tumors. Vet. Pathol. 2011, 48, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Peña, L.; Gama, A.; Goldschmidt, M.H.; Abadie, J.; Benazzi, C.; Castagnaro, M.; Díez, L.; Gärtner, F.; Hellmén, E.; Kiupel, M.; et al. Canine mammary tumors: A review and consensus of standard guidelines on epithelial and myoepithelial phenotype markers, HER2, and hormone receptor assessment using immunohistochemistry. Vet. Pathol. 2014, 51, 127–145. [Google Scholar] [CrossRef]
- Duffy, M.J.; Harbeck, N.; Nap, M.; Molina, R.; Nicolini, A.; Senkus, E.; Cardoso, F. Clinical use of biomarkers in breast cancer: Updated guidelines from the European Group on Tumor Markers (EGTM). Eur. J. Cancer 2017, 75, 284–298. [Google Scholar] [CrossRef]
- Elkhatib, N.; Neu, M.B.; Zensen, C.; Schmoller, K.M.; Louvard, D.; Bausch, A.R.; Betz, T.; Vignjevic, D.M. Fascin plays a role in stress fiber organization and focal adhesion disassembly. Curr. Biol. 2014, 24, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Huang, F.K.; Huang, J.; Chen, S.; Jakoncic, J.; Leo-Macias, A.; Diaz-Avalos, R.; Chen, L.; Zhang, J.J.; Huang, X.Y. Molecular mechanism of fascin function in filopodial formation. J. Biol. Chem. 2013, 288, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Borisy, G.G.; Svitkina, T.M. Actin machinery: Pushing the envelope. Curr. Opin. Cell Biol. 2000, 12, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Mogilner, A.; Rubinstein, B. The physics of filopodial protrusion. Biophys. J. 2005, 89, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Atilgan, E.; Wirtz, D.; Sun, S.X. Mechanics and dynamics of actin-driven thin membrane protrusions. Biophys. J. 2006, 90, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef]
- Jacquemet, G.; Baghirov, H.; Georgiadou, M.; Sihto, H.; Peuhu, E.; Cettour-Janet, P.; He, T.; Perälä, M.; Kronqvist, P.; Joensuu, H.; et al. L-type calcium channels regulate filopodia stability and cancer cell invasion downstream of integrin signalling. Nat. Commun. 2016, 7, 13297. [Google Scholar] [CrossRef]
- Cheng, K.W.; Mullins, R.D. Initiation and disassembly of filopodia tip complexes containing VASP and lamellipodin. Mol. Biol. Cell. 2020, 31, 2021–2034. [Google Scholar] [CrossRef]
- Sherer, L.A.; Courtemanche, N. Cooperative bundling by fascin generates actin structures with architectures that depend on filament length. Front. Cell Dev. Biol. 2022, 10, 974047. [Google Scholar]
- Winkelman, J.D.; Bilancia, C.G.; Peifer, M.; Kovar, D.R. Ena/VASP Enabled is a highly processive actin polymerase tailored to self-assemble parallel-bundled F-actin networks with Fascin. Proc. Natl. Acad. Sci. USA 2014, 111, 4121–4126. [Google Scholar] [CrossRef]
- Jaiswal, R.; Breitsprecher, D.; Collins, A.; Corrêa, I.R., Jr.; Xu, M.Q.; Goode, B.L. The formin Daam1 and fascin directly collaborate to promote filopodia formation. Curr. Biol. 2013, 23, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Vignjevic, D.; Schoumacher, M.; Gavert, N.; Janssen, K.P.; Jih, G.; Laé, M.; Louvard, D.; Ben-Ze’ev, A.; Robine, S. Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res. 2007, 67, 6844–6853. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.P.; Massagué, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Dawson, J.C.; Forero-Vargas, M.; Spence, H.J.; Yu, X.; König, I.; Anderson, K.; Machesky, L.M. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr. Biol. 2010, 20, 339–345. [Google Scholar] [CrossRef]
- Jacquemet, G.; Hamidi, H.; Ivaska, J. Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr. Opin. Cell Biol. 2015, 36, 23–31. [Google Scholar] [CrossRef]
- Huang, F.K.; Han, S.; Xing, B.; Huang, J.; Liu, B.; Bordeleau, F.; Reinhart-King, C.A.; Zhang, J.J.; Huang, X.Y. Targeted inhibition of fascin function blocks tumour invasion and metastatic colonization. Nat. Commun. 2015, 6, 7465. [Google Scholar] [CrossRef]
- Scott, K.L.; Nogueira, C.; Heffernan, T.P.; Van Doorn, R.; Dhakal, S.; Hanna, J.A.; Min, C.; Jaskelioff, M.; Xiao, Y.; Wu, C.J.; et al. Proinvasion metastasis drivers in early-stage melanoma are oncogenes. Cancer Cell 2011, 20, 92–103. [Google Scholar] [CrossRef]
- Li, A.; Morton, J.P.; Ma, Y.; Karim, S.A.; Zhou, Y.; Faller, W.J.; Woodham, E.F.; Morris, H.T.; Stevenson, R.P.; Juin, A.; et al. Fascin is regulated by slug, promotes progression of pancreatic cancer in mice, and is associated with patient outcomes. Gastroenterology 2014, 146, 1386–1396. [Google Scholar] [CrossRef]
- Barnawi, R.; Al-Khaldi, S.; Majed Sleiman, G.; Sarkar, A.; Al-Dhfyan, A.; Al-Mohanna, F.; Ghebeh, H.; Al-Alwan, M. Fascin Is Critical for the Maintenance of Breast Cancer Stem Cell Pool Predominantly via the Activation of the Notch Self-Renewal Pathway. Stem Cells 2016, 34, 2799–2813. [Google Scholar] [CrossRef]
- Nakagawa, T.; Watanabe, M.; Ohashi, E.; Uyama, R.; Takauji, S.; Mochizuki, M.; Nishimura, R.; Ogawa, H.; Sugano, S.; Sasaki, N. Cyclopedic protein expression analysis of cultured canine mammary gland adenocarcinoma cells from six tumours. Res. Vet. Sci. 2006, 80, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, Y.; Wang, L.; Cong, Y.; Huang, K.; Pan, X.; Liu, G.; Li, W.; Dai, C.; Xu, P.; et al. LncRNA IDH1-AS1 sponges miR-518c-5p to suppress proliferation of epithelial ovarian cancer cell by targeting RMB47. J. Biomed. Res. 2023, 38, 51–65. [Google Scholar] [CrossRef]
- Gao, Y.; Li, W.; Wang, Z.; Zhang, C.; He, Y.; Liu, X.; Tang, K.; Zhang, W.; Long, Q.; Liu, Y.; et al. SEL1L preserves CD8(+) T-cell survival and homeostasis by fine-tuning PERK signaling and the IL-15 receptor-mediated mTORC1 axis. Cell Mol. Immunol. 2023, 20, 1232–1250. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, L.X.; Zhang, C.Y.; Bai, N.; Feng, C.; Zhang, Z.M.; Wang, L.; Gao, Z.Z. LncRNA CRNDE promotes cell proliferation, migration and invasion of ovarian cancer via miR-423-5p/FSCN1 axis. Mol. Cell Biochem. 2022, 477, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Leonel, C.; Borin, T.F.; De Carvalho Ferreira, L.; Moschetta, M.G.; Bajgelman, M.C.; Viloria-Petit, A.M.; De Campos Zuccari, D.A. Inhibition of Epithelial-Mesenchymal Transition and Metastasis by Combined TGFbeta Knockdown and Metformin Treatment in a Canine Mammary Cancer Xenograft Model. J. Mammary Gland. Biol. Neoplasia. 2017, 22, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.I.; Utturkar, S.; Lee, M.; Yang, H.H.; Cui, Z.; Atallah Lanman, N.; Zhang, G.; Ramos Cardona, X.E.; Mittal, S.K.; Miller, M.A. Ductal Carcinoma In Situ Progression in Dog Model of Breast Cancer. Cancers 2020, 12, 418. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Wu, G. The rejuvenated scenario of epithelial-mesenchymal transition (EMT) and cancer metastasis. Cancer Metastasis Rev. 2012, 31, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yoshida, S.; Choisunirachon, N.; Saito, T.; Matsumoto, K.; Saeki, K.; Mochizuki, M.; Nishimura, R.; Sasaki, N.; Nakagawa, T. The relationship between clinicopathological features and expression of epithelial and mesenchymal markers in spontaneous canine mammary gland tumors. J. Vet. Med. Sci. 2014, 76, 1321–1327. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Vora, H.H.; Patel, N.A.; Rajvik, K.N.; Mehta, S.V.; Brahmbhatt, B.V.; Shah, M.J.; Shukla, S.N.; Shah, P.M. Cytokeratin and vimentin expression in breast cancer. Int. J. Biol. Markers 2009, 24, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.M.; Sato, O.; Ikebe, R.; Lee, S.; Ikebe, M.; Jung, H.S. Structural Analysis of Human Fascin-1: Essential Protein for Actin Filaments Bundling. Life 2022, 12, 843. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Siton, O.; Bernheim-Groswasser, A. Reconstitution of actin-based motility by vasodilator-stimulated phosphoprotein (VASP) depends on the recruitment of F-actin seeds from the solution produced by cofilin. J. Biol. Chem. 2014, 289, 31274–31286. [Google Scholar] [CrossRef] [PubMed]
- Harker, A.J.; Katkar, H.H.; Bidone, T.C.; Aydin, F.; Voth, G.A.; Applewhite, D.A.; Kovar, D.R. Ena/VASP processive elongation is modulated by avidity on actin filaments bundled by the filopodia cross-linker fascin. Mol. Biol. Cell 2019, 30, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Tucker, P.K.; Evans, I.R.; Wood, W. Ena drives invasive macrophage migration in Drosophila embryos. Dis. Model. Mech. 2011, 4, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.L.; Crawford, A.; Houdusse, A.; Titus, M.A. VASP-mediated actin dynamics activate and recruit a filopodia myosin. Elife 2021, 10, e68082. [Google Scholar] [CrossRef] [PubMed]
- Pruyne, D. Revisiting the Phylogeny of the Animal Formins: Two New Subtypes, Relationships with Multiple Wing Hairs Proteins, and a Lost Human Formin. PLoS ONE 2016, 11, e0164067. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Parsons, M.; Adams, J.C. Dual actin-bundling and protein kinase C-binding activities of fascin regulate carcinoma cell migration downstream of Rac and contribute to metastasis. Mol. Biol. Cell 2007, 18, 4591–4602. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, S.; Ren, H.; Sun, W.; Zhang, H.; Sun, J.; Yang, S.; Hao, J. Hypoxia-inducible factor-1 promotes pancreatic ductal adenocarcinoma invasion and metastasis by activating transcription of the actin-bundling protein fascin. Cancer Res. 2014, 74, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta. 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yu, H.; Liu, S.Y.; Xiao, X.S.; Dong, W.H.; Chen, Y.N.; Xu, W.; Zhu, T. Prognostic value of tissue inhibitor of metalloproteinase-2 expression in patients with non-small cell lung cancer: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0124230. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.B.; Hsieh, M.J.; Hsieh, Y.H.; Chien, M.H.; Chiou, H.L.; Yang, S.F. Antimetastatic effects of norcantharidin on hepatocellular carcinoma by transcriptional inhibition of MMP-9 through modulation of NF-kB activity. PLoS ONE 2012, 7, e31055. [Google Scholar] [CrossRef] [PubMed]
- Acloque, H.; Adams, M.S.; Fishwick, K.; Bronner-Fraser, M.; Nieto, M.A. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J. Clin. Investig. 2009, 119, 1438–1449. [Google Scholar] [CrossRef]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.L.; Mainez, J.; Vega, S.; Sancho, P.; Murillo, M.M.; De Frutos, C.A.; Del Castillo, G.; López-Blau, C.; Fabregat, I.; Nieto, M.A. Snail1 suppresses TGF-beta-induced apoptosis and is sufficient to trigger EMT in hepatocytes. J. Cell Sci. 2010, 123, 3467–3477. [Google Scholar] [CrossRef] [PubMed]
- Han, S.P.; Kim, J.H.; Han, M.E.; Sim, H.E.; Kim, K.S.; Yoon, S.; Baek, S.Y.; Kim, B.S.; Oh, S.O. SNAI1 is involved in the proliferation and migration of glioblastoma cells. Cell Mol. Neurobiol. 2011, 31, 489–496. [Google Scholar] [CrossRef]
- Simeonov, K.P.; Byrns, C.N.; Clark, M.L.; Norgard, R.J.; Martin, B.; Stanger, B.Z.; Shendure, J.; Mckenna, A.; Lengner, C.J. Single-cell lineage tracing of metastatic cancer reveals selection of hybrid EMT states. Cancer Cell 2021, 39, 1150–1162.e1159. [Google Scholar] [CrossRef]
- Huang, Y.; Zucker, B.; Zhang, S.; Elias, S.; Zhu, Y.; Chen, H.; Ding, T.; Li, Y.; Sun, Y.; Lou, J.; et al. Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains. Nat. Cell Biol. 2019, 21, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Jiang, Z.; Lu, D.; Wang, X.; Liang, H.; Zhang, J.; Meng, Y.; Li, Y.; Wu, D.; Huang, Y.; et al. Migrasomes provide regional cues for organ morphogenesis during zebrafish gastrulation. Nat. Cell Biol. 2019, 21, 966–977. [Google Scholar] [CrossRef]
- Zhu, M.; Zou, Q.; Huang, R.; Li, Y.; Xing, X.; Fang, J.; Ma, L.; Li, L.; Yang, X.; Yu, L. Lateral transfer of mRNA and protein by migrasomes modifies the recipient cells. Cell Res. 2021, 31, 237–240. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Ding, Y.; Zhang, J.; Xu, Y.; Xu, J.; Zheng, S.; Yang, H. Migrasome and Tetraspanins in Vascular Homeostasis: Concept, Present, and Future. Front. Cell Dev. Biol. 2020, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Jiang, D.; Hu, X.; Du, W.; Ji, L.; Yang, Y.; Li, X.; Sho, T.; Wang, X.; Li, Y.; et al. Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell 2021, 184, 2896–2910.e2813. [Google Scholar] [CrossRef] [PubMed]
- Deberardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef]
- Hung, J.J.; Yang, M.H.; Hsu, H.S.; Hsu, W.H.; Liu, J.S.; Wu, K.J. Prognostic significance of hypoxia-inducible factor-1alpha, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax 2009, 64, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.J.; Russell, R.C.; Roche, O.; Burry, T.N.; Fish, J.E.; Chow, V.W.; Kim, W.Y.; Saravanan, A.; Maynard, M.A.; Gervais, M.L.; et al. VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and snail. Mol. Cell Biol. 2007, 27, 157–169. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.; Jonkers, J.; et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef]
- Tian, M.; Chen, K.; Huang, J.; Chu, D.; Li, J.; Huang, K.; Ma, C. Asiatic acid inhibits angiogenesis and vascular permeability through the VEGF/VEGFR2 signaling pathway to inhibit the growth and metastasis of breast cancer in mice. Phytother. Res. 2021, 35, 6389–6400. [Google Scholar] [CrossRef]
- Wang, L.; Shi, H.; Liu, Y.; Zhang, W.; Duan, X.; Li, M.; Shi, X.; Wang, T. Cystathionine-γ-lyase promotes the metastasis of breast cancer via the VEGF signaling pathway. Int. J. Oncol. 2019, 55, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Yang, G.M.; Dayem, A.A.; Saha, S.K.; Kim, K.; Yoo, Y.; Hong, K.; Kim, J.H.; Yee, C.; Lee, K.M.; et al. Hydrodynamic shear stress promotes epithelial-mesenchymal transition by downregulating ERK and GSK3β activities. Breast Cancer Res. 2019, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, T.; Kondo, J.; Endo, H.; Ohue, M.; Sakai, Y.; Inoue, M. Hydrodynamic stress stimulates growth of cell clusters via the ANXA1/PI3K/AKT axis in colorectal cancer. Sci. Rep. 2019, 9, 20027. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Liu, B.; Yao, Y.; Qu, S.; Luo, W.; Tan, W.; Liu, Q.; Yao, H.; Zou, L.; Su, F.; et al. Potentiated DNA Damage Response in Circulating Breast Tumor Cells Confers Resistance to Chemotherapy. J. Biol. Chem. 2015, 290, 14811–14825. [Google Scholar] [CrossRef] [PubMed]
- Jordan, N.V.; Bardia, A.; Wittner, B.S.; Benes, C.; Ligorio, M.; Zheng, Y.; Yu, M.; Sundaresan, T.K.; Licausi, J.A.; Desai, R.; et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature 2016, 537, 102–106. [Google Scholar] [CrossRef]
- Lin, S.; Huang, C.; Gunda, V.; Sun, J.; Chellappan, S.P.; Li, Z.; Izumi, V.; Fang, B.; Koomen, J.; Singh, P.K.; et al. Fascin Controls Metastatic Colonization and Mitochondrial Oxidative Phosphorylation by Remodeling Mitochondrial Actin Filaments. Cell Rep. 2019, 28, 2824–2836. [Google Scholar] [CrossRef]


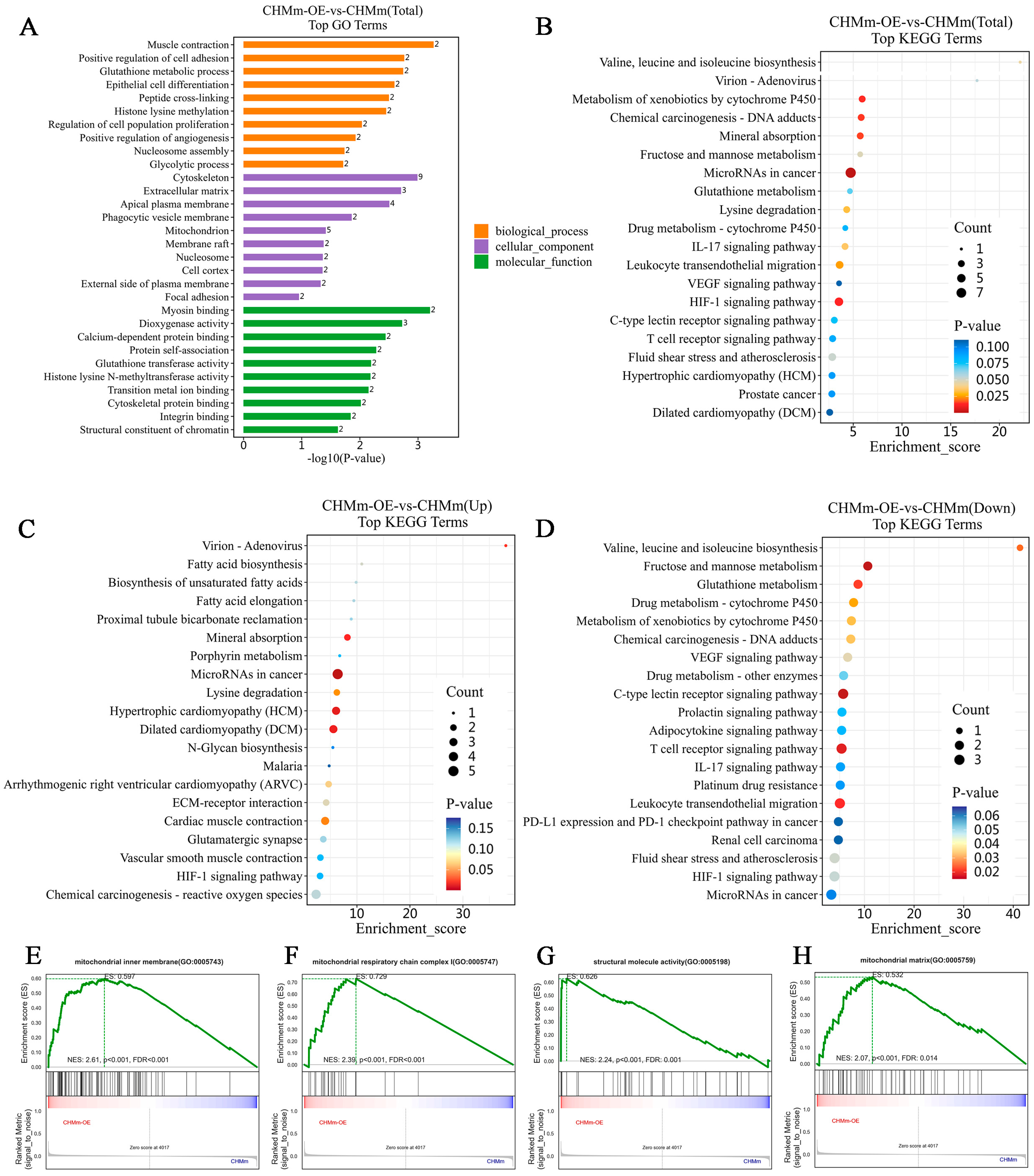


| Accession | Protein Name | Gene Name | Fold Change | p-Value |
|---|---|---|---|---|
| Upregulated proteins | ||||
| A0A8C0T2U6 | Metalloproteinase inhibitor 1 | TIMP1 | 2.1842 | 0.0028 |
| A0A8P0T8T4 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | UCHL1 | 1.8738 | 0.0021 |
| A0A8I3MRJ3 | Actin like 8 | ACTL8 | 1.8603 | 0.0003 |
| A0A0H5AU05 | Matrix metalloproteinase-3 | MMP3 | 1.5845 | 0.0437 |
| A0A8I3PYT6 | Thymopoietin | TMPO | 1.5054 | 0.0429 |
| A0A8I3NNH3 | Cadherin-2 | CDH2 | 1.4293 | 0.0074 |
| A0A8C0PBF7 | SH3 domain-containing protein | SH3KBP1 | 1.4239 | 0.0242 |
| A0A8C0TCB7 | 10 kDa heat shock protein, mitochondrial | LOC609748 | 1.4066 | 0.0117 |
| A0A8P0TUR9 | RRM domain-containing protein | - | 1.4024 | 0.0101 |
| A0A8C0S9A5 | IF rod domain-containing protein | KRT13 | 1.3998 | 0.0025 |
| A0A8C0TG32 | Tetraspanin | TSPAN4 | 1.3953 | 0.0384 |
| A0A8C0RGJ6 | RBR-type E3 ubiquitin transferase | Pdr-1 | 1.3395 | 0.0345 |
| E2QXB4 | Large neutral amino acids transporter small subunit 3 | SLC43A1 | 1.3269 | 0.0125 |
| A0A8I3NPV3 | Tetraspanin | UPK1B | 1.3228 | 0.0134 |
| A0A8C0N1I7 | DNA helicase | DNA helicase | 1.3120 | 0.0245 |
| Downregulated proteins | ||||
| A0A8C0Q0V4 | Importin 11 | IPO11 | 0.8327 | 0.0047 |
| A0A8C0PMC0 | Poly(A) binding protein interacting protein 2B | PAIP2B | 0.8326 | 0.0479 |
| A0A8P0TJG8 | Rap1 GTPase-GDP dissociation stimulator 1 | RAP1GDS1 | 0.8319 | 0.0121 |
| A0A8I3P5A5 | 1,4-alpha-glucan branching enzyme | GBE1 | 0.8296 | 0.0214 |
| A0A8I3P398 | GB1/RHD3-type G domain-containing protein | GBP6 | 0.8290 | 0.0333 |
| A0A8C0PKV5 | RNA pseudouridine synthase domain containing 2 | RPUSD2 | 0.8286 | 0.0014 |
| A0A8P0SE37 | protein-tyrosine-phosphatase | PTPN11 | 0.8282 | 0.0012 |
| A0A8C0SLE6 | DNA repair protein SWI5 homolog | Swi5 | 0.8262 | 0.0304 |
| A0A8C0QEC9 | HECT domain and ankyrin repeat-containing E3 ubiquitin protein ligase 1 | HACE1 | 0.8257 | 0.0053 |
| A0A8I3PZ15 | Transglutaminase 2 | TGM2 | 0.8236 | 0.0230 |
| A0A8C0Q3M0 | 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 | PFKFB3 | 0.8235 | 0.0045 |
| A0A8I3P7C2 | TRAF-type zinc finger domain containing 1 | TRAFD1 | 0.8215 | 0.0048 |
| A0A8I3MJM1 | Protein S100 | S100A2 | 0.8215 | 0.0298 |
| A0A8C0SW41 | CKLF like MARVEL transmembrane domain containing 7 | CMTM7 | 0.8198 | 0.0012 |
| A0A8C0PU63 | CobW C-terminal domain-containing protein | CBWD1 | 0.8195 | 0.0114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhou, Y.; Wang, L.; Haseeb, A.; Li, H.; Zheng, X.; Guo, J.; Cheng, X.; Yin, W.; Sun, N.; et al. Fascin-1 Promotes Cell Metastasis through Epithelial–Mesenchymal Transition in Canine Mammary Tumor Cell Lines. Vet. Sci. 2024, 11, 238. https://doi.org/10.3390/vetsci11060238
Wang X, Zhou Y, Wang L, Haseeb A, Li H, Zheng X, Guo J, Cheng X, Yin W, Sun N, et al. Fascin-1 Promotes Cell Metastasis through Epithelial–Mesenchymal Transition in Canine Mammary Tumor Cell Lines. Veterinary Sciences. 2024; 11(6):238. https://doi.org/10.3390/vetsci11060238
Chicago/Turabian StyleWang, Xin, Ye Zhou, Linhao Wang, Abdul Haseeb, Hongquan Li, Xiaozhong Zheng, Jianhua Guo, Xiaoliang Cheng, Wei Yin, Na Sun, and et al. 2024. "Fascin-1 Promotes Cell Metastasis through Epithelial–Mesenchymal Transition in Canine Mammary Tumor Cell Lines" Veterinary Sciences 11, no. 6: 238. https://doi.org/10.3390/vetsci11060238
APA StyleWang, X., Zhou, Y., Wang, L., Haseeb, A., Li, H., Zheng, X., Guo, J., Cheng, X., Yin, W., Sun, N., Sun, P., Zhang, Z., Yang, H., & Fan, K. (2024). Fascin-1 Promotes Cell Metastasis through Epithelial–Mesenchymal Transition in Canine Mammary Tumor Cell Lines. Veterinary Sciences, 11(6), 238. https://doi.org/10.3390/vetsci11060238





