1. Introduction
Coronavirus (CoV) belongs to the Coronaviridae family, which includes four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus [
1]. Betacoronavirus is important to humans because it includes severe acute respiratory syndrome-related coronavirus, Middle East respiratory syndrome-related coronavirus, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [
2]. Human Betacoronavirus HCoV-OC43 was first isolated from a patient with respiratory disease in the 1960s [
3], and BCoV was first described by Mebus et al. in the early 1970s as a cause of neonatal calf diarrhea [
4]. Bovine coronavirus (BCoV) is involved in the etiology of at least three distinct clinical syndromes: enteric disease with high mortality in neonatal calves, winter dysentery with hemorrhagic diarrhea in cattle, and respiratory tract infections in cattle of all ages [
5]. In 2007, Park et al. provided the first detailed report of dual enteric and respiratory tropisms of WD-BCoV in calves. Upon histopathological examination, BCoV-inoculated calves exhibited mild to severe villous atrophy, and the epithelia of the alveoli, bronchi, and bronchioles often appeared desquamated or necrotic [
6]. Statistical analysis of the presence of BCoV in the feces of healthy calves and those with diarrhea showed that the BCoV detection rate in healthy calves ranged from 0 to 46% and that diarrhea in calves ranged from 3.4 to 69.0% [
7,
8,
9]. The BCoV detection rate tended to be higher in calves with diarrhea than in healthy calves.
The virulence of BCoV related to calf diarrhea was clinically determined in Qinghai- Tibet Plateau, China, in 2019 [
10]. Many studies have revealed that BCoV may cause or exacerbate calf diarrhea by co-infection with other enteric viruses such as bovine rotavirus (BRV) [
11], bovine astrovirus (BoAstV), bovine torovirus (BToV), bovine viral diarrhea virus (BVDV), BCoV, and bovine kobuvirus (BKoV) (Cho et al., 2013). However, the prevalence of BCoV in diarrheal cases varies in these reports, at 69.05% in China (He et al., 2019; Keha et al., 2019), 68.6% in Brazil [
11], 57.7% in Japan [
12], 39.8% in South Korea [
13], 21.9% in Turkey [
14], and 7.2% in Iran [
15]. BCoV can infect and spread to many animals, including sheep, musk, oxen, elk, sambar, deer, goats, dromedaries, camels, alpacas, giraffes, and wisents [
2,
16].
BCoV serum antibody test results have revealed differences between countries. The positive rates have ranged from 19 to 86% in Sweden [
17,
18,
19,
20], 48% to 53% in the United States [
21], 30.0% in Belgium [
22], 72.6% in Poland [
23], 97.89% in Thailand [
24], 12.82% in Inner Mongolia, China [
25], and 30.8% in Campania, Italy [
26]. In summary, BCoV is widespread in all regions, and persistent low-dose detoxification occurs, even when herds show no clinical symptoms [
27].
At the molecular level, CoVs are positive-sense, single-stranded, enveloped RNA viruses [
28]. Their genomes start from a 5′ untranslated region (5′ UTR), followed by two open reading frames (ORFs) 1a and 1b, four or five structural proteins, i.e., spike protein (S), membrane protein (M), nucleocapsid protein (N), hemagglutinin-esterase protein (HE), and envelope protein (E), and a 3′ untranslated region (3′ UTR). According to the CoV classification criteria proposed by the International Committee on the Taxonomy of Viruses in 2012, the four genera are further classified into different genotypes based on the mean amino acid genetic distance.
Although BCoV is one of the earliest discovered CoVs, its genome remained uncharacterized until it was sequenced in the US in 2001 [
29]. To date, 98 genome sequences have been published in GenBank. However, few studies have addressed the prevalence and genetic diversity of BCoV and its association with ruminant diarrhea, likely owing to the difficulties in isolating this virus from cell and tissue cultures and the high proportion of co-infections in both diarrheal and asymptomatic animals. Fortunately, next-generation sequencing (NGS) [
30] has effectively replaced conventional cell cultures to identify BCoV and other co-infected viruses. Thus, we conducted this study to determine the correlation between BCoV and bovine diarrhea by using NGS to investigate the prevalence and genomic characteristics of BCoV in Chinese cattle with and without diarrhea using recent samples collected from various regions. We conducted molecular characterization of HE, S, and the whole genome in the identified BCoV strains. This genetic evolution analysis revealed the evolutionary direction of BCoV over the past 3 years and provides a reference for subsequent research.
4. Discussion
Juvenile animals are usually more susceptible to diarrhea-causing viruses and present obvious symptoms, and our results were consistent with this. However, under the conditions of non-intensive management, infected older animals tend to have more fatalities even if they exhibit no signs of diarrhea. We found 351 cases of asymptomatic infections, primarily occurring in cattle older than 6 months. Conversely, most samples with diarrheal infections were from younger cattle, primarily under 6 months of age. Cattle without diarrhea symptoms posed a higher risk, particularly if they were older. Most of our samples were collected from non-intensively managed farms. Inadequate disinfection and protective measures allow diseases to spread much easier. The relatively poor environment and substandard prevention and control measures may have led to the BCoV epidemic among farms.
The high prevalence of BCoV may be similar to that of the porcine epidemic diarrhea virus, which caused a regional epidemic [
33,
35]. Previous reports stated that BCoV that caused diarrhea in cattle was often accompanied by co-infection with other pathogens [
11,
12,
13]. In this study, the infection rate of cattle with diarrhea and co-infected with four other viruses reached 7.96% (excluding other diarrhea-causing pathogens). Therefore, the co-infection rate was actually much higher. Whether BCoV infection causes diarrhea in cattle remains uncertain. Additionally, BCoV has great potential for cross-species transmission. Previous studies report that BCoV infects the respiratory tract, followed by the digestive tract, thus causing diarrhea [
35,
36]. Similar to SARS [
37,
38] and SARS-2 [
39], BCoV has two tissue tropisms. Szczepanski et al. indicated that BCoV can attach to HLA-I from humans, and HLA-I may be a candidate protein receptor [
40], the entry receptor of OC43 on some cells [
41,
42,
43], or the accessory receptor of HKU1 [
44]. Therefore, BCoV is a good model for exploring other betacoronaviruses.
This study yielded a high positive rate for BCoV. Importantly, infected asymptomatic cattle accounted for 51.39%, indicating that the infection rate of BCoV increased from 2020 to 2022 and is widely distributed in cattle. Infected cattle can discharge BCoV into the surrounding environment, and the virus can persist in low temperatures and high relative humidity for 120 h and in feces for ≥96 h [
45]. During this study, cattle were infected, and some exhibited diarrhea, while others did not. However, calves were more susceptible to developing diarrhea as a result of the infection. Furthermore, freezing temperatures can aggravate diarrhea, easily causing winter dysentery. However, other studies have shown that BCoV-induced diarrhea was not correlated with low temperatures [
30,
33,
46].
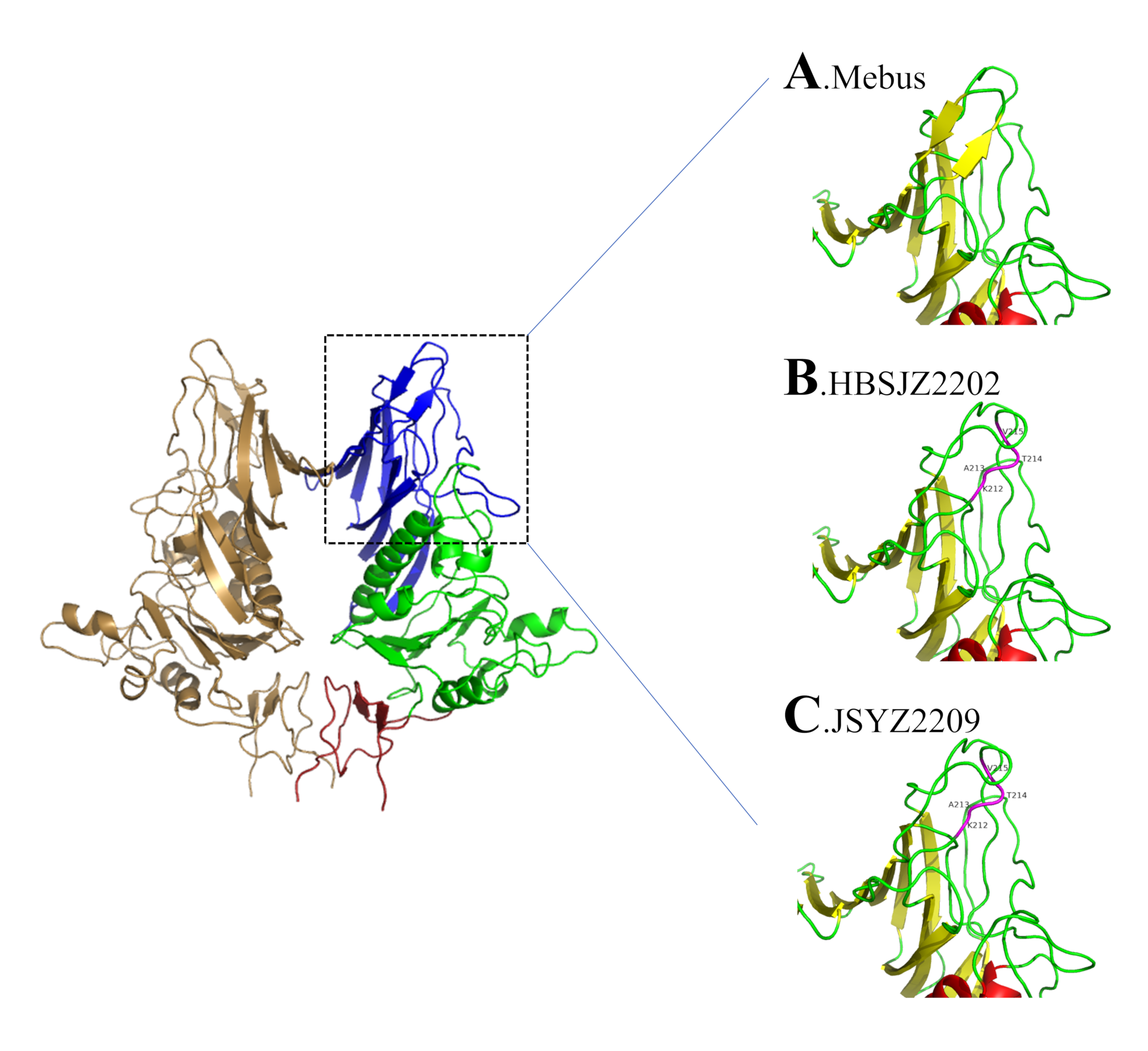
HE and S are critical for BCoV invasion and cellular release and are critical antibody-neutralization epitopes. Zeng et al. [
34] showed that BCoV HE was similar to the HA of IV but was more flexible than HA and was limited to S. S and HE can attach to the same sialid acid, 9-O-acetylated sialoglycan [
47,
48,
49]. Lang et al. indicated that HE and S are whole, and when HE incurs a critical mutation, it induces S mutations and strengthens its affinity [
50]. Interestingly, HE forms large branches in the phylogenetic tree and insertions from American strains independent of those from China and different G-type strains. HE sequences are distributed between the two branches, but S is clearly separated. On S, S1 is a highly mutated region, and S2 is relatively conserved. Previous reports revealed co-evolution between the paired amino acids [
35,
51], and the appearance of GIIa subgroups may be associated with co-evolution between amino acids of the same protein. S structural changes are induced by host-receptor binding. The key to this transformation depends on their stability, and S1 shedding is considered a prerequisite [
52]. According to Guan et al., BCoV may use the two-receptor binding motif, in which S1-NTD attaches to 9-O-acetylated sialoglycans, and S1-RBD may function as the protein receptor [
53]. Thus, mutations at receptor-binding positions on S1 are particularly critical and will impact the affinity and modify and alter the types of binding receptors.
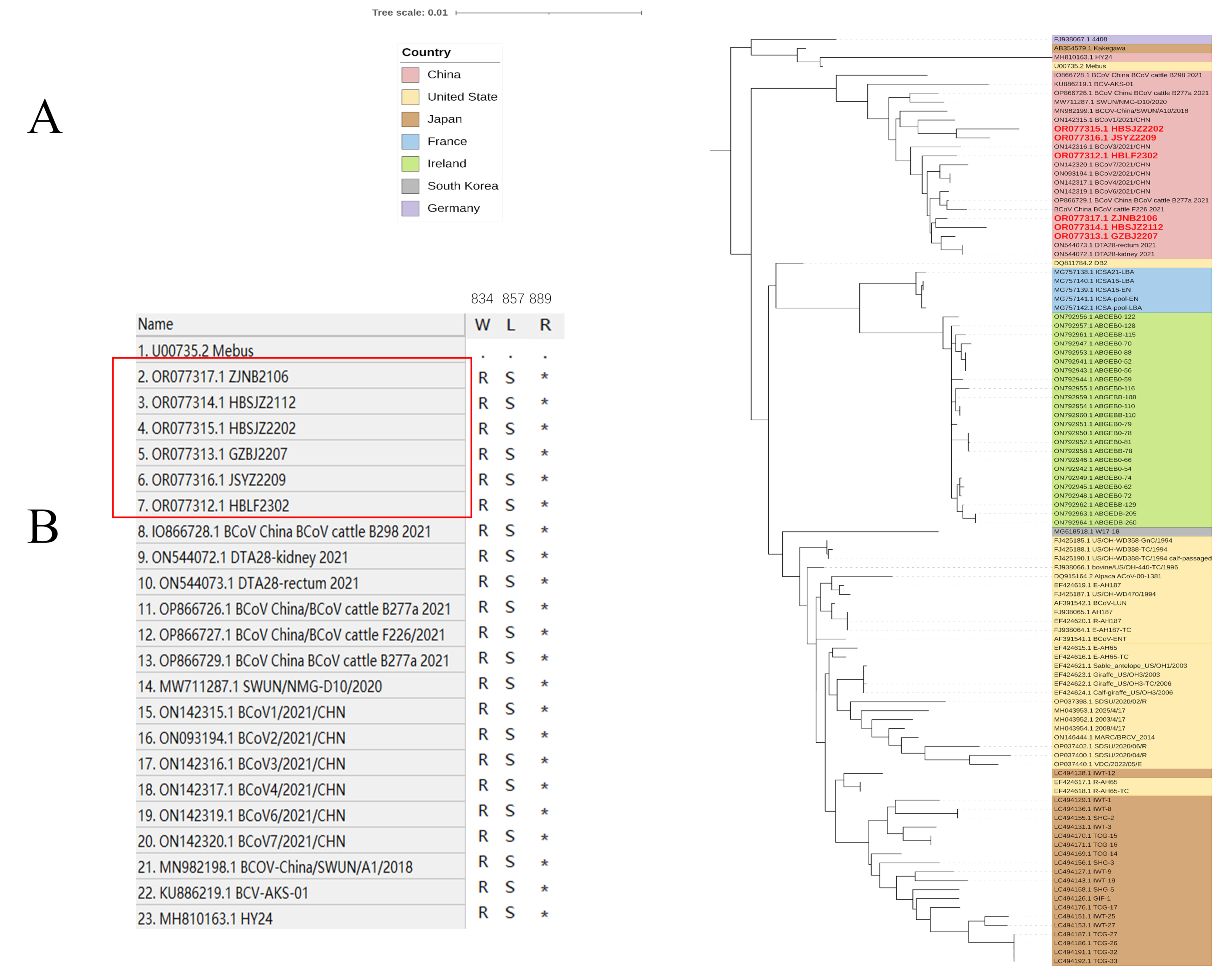
Insertions and deletions in HE sequences have been reported from both China and the United States [
5,
10,
54,
55]. Samples isolated from Liaoning, China, revealed four amino acid insertions (GenBank accession number MK095142). We previously detected no HE insertions until 2022, when HBSJZ2202 and JSYZ2209 were collected from Hebei and Jiangsu, respectively. A report from 4 years ago revealed that HE insertions were detected in various regions, which may have attributed to the recombination of strains. These mutations occurred in a critical position on the glycan receptor and changed its structure [
34]. Workman et al. suggested that insertions and deletions do not abolish binding receptors [
5]. Insertions and deletions are the most important mutations of BCoV HE. Notably, the ability of binding receptor 9-O-acetylated sialoglycans is stronger than that of insertions and deletions. However, Langereis et al. showed that moderately changing the structure of MHV HE changed the glycan tropism. Therefore, BCoV-HE mutations may be similar to those of MHV [
56]. The sequences with HE mutations on the phylogenetic trees were on a small branch, and S was on a closer branch. This is consistent with the idea of co-evolution. Whether the relationship between HE mutations and S is associated with BCoV prevalence is unknown; however, BCoV is widely distributed in parts of China.
Alignment and the phylogenetic tree revealed that the genetic diversity of BCoV was reflected in HE and S, as well as in other structural and non-structural proteins. A previous report found that truncation of a large-scale genome encoding 4.8-kDa non-structural and 4.9-kDa non-structural proteins was associated with adaptation to human hosts [
57]. Although this truncation differs from that of CoV-OC43, truncation of the 4.8-kDa non-structural and 4.9-kDa non-structural proteins may have been associated with adaptation to bovines or other hosts.
Our research revealed the prevalence and detailed molecular characterization of BCoV and indicated the increasing infection rate of asymptomatic cattle. The occurrence of diarrhea following BCoV infection is significantly correlated with herd age and may be influenced by breed. HE mutations detected from the same area can emerge from nothing and may reflect this pattern in the evolution of BCoV. We analyzed the ORF1a gene. Despite belonging to the same genotype, the Chinese strain was identified in a separate branch along with other GIIb genotypes on ORF1a, suggesting differences in the non-structural proteins. Our results offer insight into diarrhea-associated BCoV, which demonstrates higher susceptibility among calves. Notably, the Chinese strain of the GIIb genotype exhibited distinct differences within the ORF1a region compared with that of other regions, providing important information for future research.