Effect of Simultaneous Dietary Supplementation of Betaine, Selenomethionine, and Vitamins E and C under Summer Conditions in Growing–Finishing Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Setup and Animals
2.1.1. Housing
2.1.2. Climate Control
2.1.3. Temperature–Humidity Index
2.2. Measurements at Farm Level
2.2.1. Physiological Parameters and Animal Behaviour
2.2.2. Correlations
2.2.3. Performance Parameters
2.3. Measurements in the Slaughterhouse
2.4. Statistical Analysis
3. Results
3.1. Temperature Humidity Index
3.2. Measurements at Farm Level
3.2.1. Physiological Parameters and Animal Behaviour
3.2.2. Correlations
3.2.3. Performance Parameters
3.3. Measurements in the Slaughterhouse
3.3.1. Observations in the Lairage Area
3.3.2. Carcass Traits and Meat Quality
4. Discussion
4.1. Measurements at Farm Level
4.1.1. Physiological Parameters and Behaviour
4.1.2. Correlations
4.1.3. Performance Parameters
4.2. Measurements in the Slaughterhouse
4.2.1. Observations in the Lairage Area
4.2.2. Carcass Traits and Meat Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, P.H.R.F.; Le Floc’h, N.; Noblet, J.; Renaudeau, D. Physiological responses of growing pigs to high ambient temperature and/or inflammatory challenges. Rev. Bras. Zootec. 2017, 46, 537–544. [Google Scholar] [CrossRef]
- KMI. Klimaatverandering in België. 2022. Available online: https://www.meteo.be/nl/klimaat/klimaatverandering-in-belgie/klimaatstreepjes-voor-ukkel (accessed on 18 January 2024).
- KMI. De Klimaatvooruitzichten Voor 2100. KMI. 2020. Available online: https://www.meteo.be/nl/klimaat/klimaatverandering-in-belgie/de-klimaatvooruitzichten-voor-2100 (accessed on 8 December 2020).
- IPCC. The Intergovernmental Panel on Climate Change. Reports. 2020. Available online: https://www.ipcc.ch/ (accessed on 18 January 2024).
- Mount, L.E. Adaptation to Thermal Environment: Man and His Productive Animals; University Park Press: University Park, PA, USA, 1979; 333p. [Google Scholar]
- Collier, R.J.; Gebremedhin, K.G. Thermal biology of domestic animals. Annu. Rev. Anim. Biosci. 2015, 3, 513–532. [Google Scholar] [CrossRef]
- Renaudeau, D.; Anais, C.; Tel, L.; Gourdine, J.L. Effect of temperature on thermal acclimation in growing pigs estimated using a nonlinear function. J. Anim. Sci. 2010, 88, 3715–3724. [Google Scholar] [CrossRef]
- Huynh, T.T.T.; Aarnink, A.J.A.; Verstegen, M.W.A.; Gerrits, W.J.J.; Heetkamp, M.J.W.; Kemp, B.; Canh, T.T. Effects of increasing temperatures on physiological changes in pigs at different relative humidities. J. Anim. Sci. 2005, 83, 1385–1396. [Google Scholar] [CrossRef]
- Huynh, T.T.T.; Aarnink, A.J.A.; Gerrits, W.J.J.; Heetkamp, M.J.H.; Canh, T.T.; Spoolder, H.A.M.; Kemp, B.; Verstegen, M.W.A. Thermal behaviour of growing pigs in response to high temperature and humidity. Appl. Anim. Behav. Sci. 2005, 91, 1–16. [Google Scholar] [CrossRef]
- Collin, A.; Van Milgen, J.; Dubois, S.; Noblet, J. Effect of high temperature on feeding behaviour and heat production in group-housed young pigs. Br. J. Nutr. 2001, 86, 63–70. [Google Scholar] [CrossRef]
- Kemp, B.; Verstegen, M.W.A. The Influence of Climatic Environment on Sows. In Energy Metabolism in Farm Animals; Springer: Dordrecht, The Netherlands, 1987; pp. 115–132. [Google Scholar] [CrossRef]
- Quiniou, N.; Dubois, S.; Noblet, J. Voluntary feed intake and feeding behaviour of group-housed growing pigs are affected by ambient temperature and body weight. Livest. Prod. Sci. 2000, 63, 245–253. [Google Scholar] [CrossRef]
- Renaudeau, D.; Gourdine, J.L.; St-Pierre, N.R. Meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs. J. Anim. Sci. 2011, 89, 2220–2230. [Google Scholar] [CrossRef]
- Renaudeau, D.; Gilbert, H.; Noblet, J. Effect of climatic environment on feed efficiency in swine. In Feed Efficiency in Swine; Wageningen Academic Publishers: Wageningen, The Netherlands, 2012; pp. 183–210. [Google Scholar] [CrossRef]
- Mayorga, E.J.; Renaudeau, D.; Ramirez, B.C.; Ross, J.W.; Baumgard, L.H. Heat stress adaptations in pigs. Anim. Front. 2019, 9, 54–61. [Google Scholar] [CrossRef]
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat Stress Reduces Intestinal Barrier Integrity and Favors Intestinal Glucose Transport in Growing Pigs. PLoS ONE 2013, 8, e70215. [Google Scholar] [CrossRef]
- Cottrell, J.J.; Liu, F.; Hung, A.T.; DiGiacomo, K.; Chauhan, S.S.; Leury, B.J.; Furness, J.B.; Celi, P.; Dunshea, F.R. Nutritional strategies to alleviate heat stress in pigs. Anim. Prod. Sci. 2015, 55, 1391–1402. [Google Scholar] [CrossRef]
- Spencer, J.D.; Gaines, A.M.; Berg, E.P.; Allee, G.L. Diet modifications to improve finishing pig growth performance and pork quality attributes during periods of heat stress. J. Anim. Sci. 2005, 83, 243–254. [Google Scholar] [CrossRef]
- Kerr, B.J.; Yen, J.T.; Nienaber, J.A.; Easter, R.A. Influences of dietary protein level, amino acid supplementation and environmental temperature on performance, body composition, organ weights and total heat production of growing pigs. J. Anim. Sci. 2003, 81, 1998–2007. [Google Scholar] [CrossRef]
- Ratriyanto, A.; Mosenthin, R.; Bauer, E.; Eklund, M. Metabolic, osmoregulatory and nutritional functions of betaine in monogastric animals. Asian-Australas. J. Anim. Sci. 2009, 22, 1461–1476. [Google Scholar] [CrossRef]
- Eklund, M.; Bauer, E.; Wamatu, J.; Mosenthin, R. Potential nutritional and physiological functions of betaine in livestock. Nutr. Res. Rev. 2005, 18, 31–48. [Google Scholar] [CrossRef]
- Lahučký, R.; Bahelka, I.; Novotná, K.; Vašíčková, K. Effects of dietary vitamin E and vitamin C supplementation on the level of α-tocopherol and L-ascorbic acid in muscle and on the antioxidative status and meat quality of pigs. Czech J. Anim. Sci. 2005, 50, 175–184. [Google Scholar] [CrossRef]
- Dugan, M.; Aalhus, J.; Uttaro, B. Nutritional manipulation of pork quality: Current opportunities. Adv. Pork Prod. 2004, 15, 237–243. [Google Scholar]
- Ellis, M.; McKeith, F. Nutritional Influences on Pork Quality. Pork Fact Sheets. 1999, pp. 1–8. Available online: https://porkgateway.org/resource/nutritional-influences-on-pork-quality-2/ (accessed on 4 January 2023).
- Ngapo, T.M.; Gariépy, C. Factors affecting the eating quality of pork. Crit. Rev. Food Sci. Nutr. 2008, 48, 599–633. [Google Scholar] [CrossRef]
- Rosenvold, K.; Andersen, H.J. Factors of significance for pork quality—A review. Meat Sci. 2003, 64, 219–237. [Google Scholar] [CrossRef]
- Habibian, M.; Sadeghi, G.; Ghazi, S.; Moeini, M.M. Selenium as a Feed Supplement for Heat-Stressed Poultry: A Review. Biol. Trace Elem. Res. 2015, 165, 183–193. [Google Scholar] [CrossRef]
- Stewart, K.R.; Cabezon, F.A.; Boyd, R.D. Betaine for Boars and Sows During Heat Stress. 2003, 765. Available online: https://www.researchgate.net/profile/R_Boyd3/publication/299375507_Effect_of_natural_betaine_on_estimates_of_semen_quality_in_mature_AI_boars_during_summer_heat_stress/links/56f5dc6008ae7c1fda2eec44/Effect-of-natural-betaine-on-estimates-of-semen-quality- (accessed on 2 December 2020).
- Tang, J.; Cao, L.; Jia, G.; Liu, G.; Chen, X.; Tian, G.; Cai, J.; Shang, H.; Zhao, H. The protective effect of selenium from heat stress-induced porcine small intestinal epithelial cell line (IPEC-J2) injury is associated with regulation expression of selenoproteins. Br. J. Nutr. 2019, 122, 1081–1090. [Google Scholar] [CrossRef]
- Liu, F.; Celi, P.; Cottrell, J.J.; Chauhan, S.S.; Leury, B.J.; Dunshea, F.R. Effects of a short-term supranutritional selenium supplementation on redox balance, physiology and insulin-related metabolism in heat-stressed pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, 276–285. [Google Scholar] [CrossRef]
- Liu, F.; Cottrell, J.J.; Collins, C.L.; Henman, D.J.; O’Halloran, K.S.B.; Dunshea, F.R. Supplementation of selenium, vitamin E, chromium and betaine above recommended levels improves lactating performance of sows over summer. Trop. Anim. Health Prod. 2017, 49, 1461–1469. [Google Scholar] [CrossRef]
- Liu, F.; Cottrell, J.J.; Furness, J.B.; Rivera, L.R.; Kelly, F.W.; Wijesiriwardana, U.; Pustovit, R.V.; Fothergill, L.J.; Bravo, D.M.; Celi, P.; et al. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp. Physiol. 2016, 101, 801–810. [Google Scholar] [CrossRef]
- Gan, F.; Chen, X.; Liao, S.F.; Lv, C.; Ren, F.; Ye, G.; Pan, C.; Huang, D.; Shi, J.; Shi, X.; et al. Selenium-enriched probiotics improve antioxidant status, immune function, and selenoprotein gene expression of piglets raised under high ambient temperature. J. Agric. Food Chem. 2014, 62, 4502–4508. [Google Scholar] [CrossRef]
- Lv, C.H.; Wang, T.; Regmi, N.; Chen, X.; Huang, K.; Liao, S.F. Effects of dietary supplementation of selenium-enriched probiotics on production performance and intestinal microbiota of weanling piglets raised under high ambient temperature. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1161–1171. [Google Scholar] [CrossRef]
- Lucas, E.M.; Randall, J.M.; Meneses, J.F. Potential for evaporative cooling during heat stress periods in pig production in Portugal (Alentejo). J. Agric. Eng. Res. 2000, 76, 363–371. [Google Scholar] [CrossRef]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003, 86 (Suppl. S1), E52–E77. [Google Scholar] [CrossRef]
- Vitt, R.; Weber, L.; Zollitsch, W.; Hörtenhuber, S.J.; Baumgartner, J.; Niebuhr, K.; Piringer, M.; Anders, I.; Andre, K.; Hennig-Pauka, I.; et al. Modelled performance of energy saving air treatment devices to mitigate heat stress for confined livestock buildings in Central Europe. Biosyst. Eng. 2017, 164, 85–97. [Google Scholar] [CrossRef]
- NWSCR. Livestock Hot Weather Stress; Regional Operations Manual Letter C-31–76; Department of Commerce, NOAA, National Weather Service Central Region: Kansas City, MO, USA, 1976. [Google Scholar]
- Brown-Brandl, T.M.; Eigenberg, R.A.; Purswell, J.L. Using thermal imaging as a method of investigating thermal thresholds in finishing pigs. Biosyst. Eng. 2013, 114, 327–333. [Google Scholar] [CrossRef]
- Petry, A.; McGilvray, W.; Rakhshandeh, A.R.; Rakhshandeh, A. Technical note: Assessment of an alternative technique for measuring body temperature in pigs. J. Anim. Sci. 2017, 95, 3270–3274. [Google Scholar] [CrossRef]
- Ekkel, E.D.; Spoolder, H.A.M.; Hulsegge, I.; Hopster, H. Lying characteristics as determinants for space requirements in pigs. Appl. Anim. Behav. Sci. 2003, 80, 19–30. [Google Scholar] [CrossRef]
- Maselyne, J.; Saeys, W.; de Ketelaere, B.; Mertens, K.; Vangeyte, J.; Hessel, E.F.; Millet, S.; van Nuffel, A. Validation of a High Frequency Radio Frequency Identification (HF RFID) system for registering feeding patterns of growing-finishing pigs. Comput. Electron. Agric. 2014, 102, 10–18. [Google Scholar] [CrossRef]
- Maselyne, J.; Adriaens, I.; Huybrechts, T.; de Ketelaere, B.; Millet, S.; Vangeyte, J.; van Nuffel, A.; Saeys, W. Measuring the drinking behaviour of individual pigs housed in group using radio frequency identification (RFID). Animal 2016, 10, 1557–1566. [Google Scholar] [CrossRef]
- Huynh, T.T.T. Heat Stress in Growing Pigs. 2005. Available online: https://library.wur.nl/WebQuery/wurpubs/fulltext/121639 (accessed on 2 December 2020).
- Gabler, N.K.; Frouel, S.; Awati, A.; Owusu-Asiedu, A.; Amerah, A.M.; Patridge, G.G.; Dunshea, F.R. Betaine Mitigates Intestinal Permeability in Growing Pigs Induced by Heat Stress. In Proceedings of the Manipulating Pig Production XIV, Melbourne, Australia, 24–27 November 2013; Pluske, J.R., Pluske, J.M., Eds.; p. 85. Available online: https://www.researchgate.net/profile/Frank-Dunshea/publication/280575793_Betaine_mitigates_heat_stress_induced_intestinal_permeability_in_growing_pigs/links/55bb716108ae9289a0954869/Betaine-mitigates-heat-stress-induced-intestinal-permeability-in-growing-pigs (accessed on 4 January 2023).
- Chauhan, S.S.; Celi, P.; Fahri, F.T.; Celi, P.; Dunshea, F.R. Dietary antioxidants at supranutritional doses modulate skeletal muscle heat shock protein and inflammatory gene expression in sheep exposed to heat stress. J. Anim. Sci. 2014, 92, 4897–4908. [Google Scholar] [CrossRef]
- Attia, Y.A.; Hassan, R.A.; Qota, E.M.A. Recovery from adverse effects of heat stress on slow-growing chicks in the tropics: Effect of ascorbic acid and different levels of betaine. Trop. Anim. Health Prod. 2009, 41, 807–818. [Google Scholar] [CrossRef]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Ringuet, M.; Furness, J.B.; Dunshea, F.R. Betaine and antioxidants improve growth performance, breast muscle development and ameliorate thermoregulatory responses to cyclic heat exposure in broiler chickens. Animals 2018, 8, 162. [Google Scholar] [CrossRef]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Le, H.H.; Suleria, H.A.R.; Warner, R.D.; Dunshea, F.R. Growth performance and characterization of meat quality of broiler chickens supplemented with betaine and antioxidants under cyclic heat stress. Antioxidants 2019, 8, 336. [Google Scholar] [CrossRef]
- Schmidt, M.; Lahrmann, K.H.; Ammon, C.; Berg, W.; Schön, P.; Hoffmann, G. Assessment of body temperature in sows by two infrared thermography methods at various body surface locations. J. Swine Health Prod. 2013, 21, 203–209. [Google Scholar]
- Dewulf, J.; Koenen, F.; Laevens, H.; de Kruif, A. The use of infra-red thermometry for the detection of fever in pigs. In Proceedings of the 10th International Symposium on Veterinary Epidemiology and Economics, Santiago, Chile, 17–21 November 2003; Volume 72, pp. 9–11. [Google Scholar]
- Lawrence, B.V.; Schinckel, A.P.; Adeola, O.; Cera, K. Impact of betaine on pig finishing performance and carcass composition. J. Anim. Sci. 2002, 80, 475–482. [Google Scholar] [CrossRef]
- Matthews, J.O.; Southern, L.L.; Higbie, A.D.; Persica, M.A.; Bidner, T.D. Effects of betaine on growth, carcass characteristics, pork quality, and plasma metabolites of finishing pigs. J. Anim. Sci. 2001, 79, 722–728. [Google Scholar] [CrossRef]
- Mendoza, S.M.; Boyd, R.D.; Zier-Rush, C.E.; Ferket, P.R.; Haydon, K.D.; van Heugten, E. Effect of natural betaine and ractopamine HCl on whole-body and carcass growth in pigs housed under high ambient temperatures. J. Anim. Sci. 2017, 95, 3047–3056. [Google Scholar] [CrossRef]
- Lan, R.; Kim, I. Effects of feeding diets containing essential oils and betaine to heat-stressed growing-finishing pigs. Arch. Anim. Nutr. 2018, 72, 368–378. [Google Scholar] [CrossRef]
- Quisirumbay-Gaibor, J.; Patiño-Patroni, D.M.; Perales, C.V. Effect of Selenium Supplementation on Productive Performance in Pigs: Meta-Analysis. Revista Chapingo, Serie Horticultura 2020, 26. Available online: https://www.webofscience.com/wos/woscc/summary/d214cad2-b3a5-45bb-98e2-a980b4158f8e-61fb4c32/relevance/1 (accessed on 29 November 2022).
- Hyun, Y.; Ellis, M. Effect of group size and feeder type on growth performance and feeding patterns in growing pigs. J. Anim. Sci. 2001, 79, 803–810. [Google Scholar] [CrossRef]
- Hyun, Y.; Ellis, M. Effect of group size and feeder type on growth performance and feeding patterns in finishing pigs. J. Anim. Sci. 2002, 80, 568–574. [Google Scholar] [CrossRef]
- Prandini, A.; Sigolo, S.; Morlacchini, M.; Grilli, E.; Fiorentini, L. Microencapsulated lysine and low-protein diets: Effects on performance, carcass characteristics and nitrogen excretion in heavy growing–finishing pigs. J. Anim. Sci. 2013, 91, 4226–4234. [Google Scholar] [CrossRef]
- Kyriazakis, I.; Emmans, G.C. The voluntary feed intake of pigs given feeds based on wheat bran, dried citrus pulp and grass meal, in relation to measurements of feed bulk. Br. J. Nutr. 1995, 73, 191–207. [Google Scholar] [CrossRef]
- Zhang, W.; Li, D.; Liu, L.; Zang, J.; Duan, Q.; Yang, W.; Zhang, L. The effects of dietary fiber level on nutrient digestibility in growing pigs. J. Anim. Sci. Biotechnol. 2013, 4, 17. [Google Scholar] [CrossRef]
- Landfald, B.; Strom, A.R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J. Bacteriol. 1986, 165, 849–855. [Google Scholar] [CrossRef]
- Kettunen, H.; Peuranen, S.; Tiihonen, K. Betaine aids in the osmoregulation of duodenal epithelium of broiler chicks, and affects the movement of water across the small intestinal epithelium in vitro. Comp. Biochem. A Physiol. Mol. Integr. Physiol. 2001, 129, 595–603. [Google Scholar] [CrossRef]
- Kempf, B.; Bremer, E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 1998, 170, 319–330. [Google Scholar] [CrossRef]
- Labroue, F.; Guéblez, R.; Sellier, P.; Meunier-Salaün, M.C. Feeding behaviour of group-housed large white and landrace pigs in french central test stations. Livest. Prod. Sci. 1994, 40, 303–312. [Google Scholar] [CrossRef]
- Kanis, E.; Koops, W.J. Daily gain, food intake and food efficiency in pigs during the growing period. Anim. Prod. 1990, 50, 353–364. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, J.; Nie, X.; Wu, Q.; Wang, L.; Jiang, Z. Influences of Dietary Vitamin E, Selenium-Enriched Yeast, and Soy Isoflavone Supplementation on Growth Performance, Antioxidant Capacity, Carcass Traits, Meat Quality and Gut Microbiota in Finishing Pigs. Antioxidants 2022, 11, 1510. [Google Scholar] [CrossRef]
 = days where physiological parameters and animal behaviour were observed between 13:00 and 17:00;
= days where physiological parameters and animal behaviour were observed between 13:00 and 17:00;  = a THI of 75 indicates a warning for heat stress).
= a THI of 75 indicates a warning for heat stress).
 = days where physiological parameters and animal behaviour were observed between 13:00 and 17:00;
= days where physiological parameters and animal behaviour were observed between 13:00 and 17:00;  = a THI of 75 indicates a warning for heat stress).
= a THI of 75 indicates a warning for heat stress).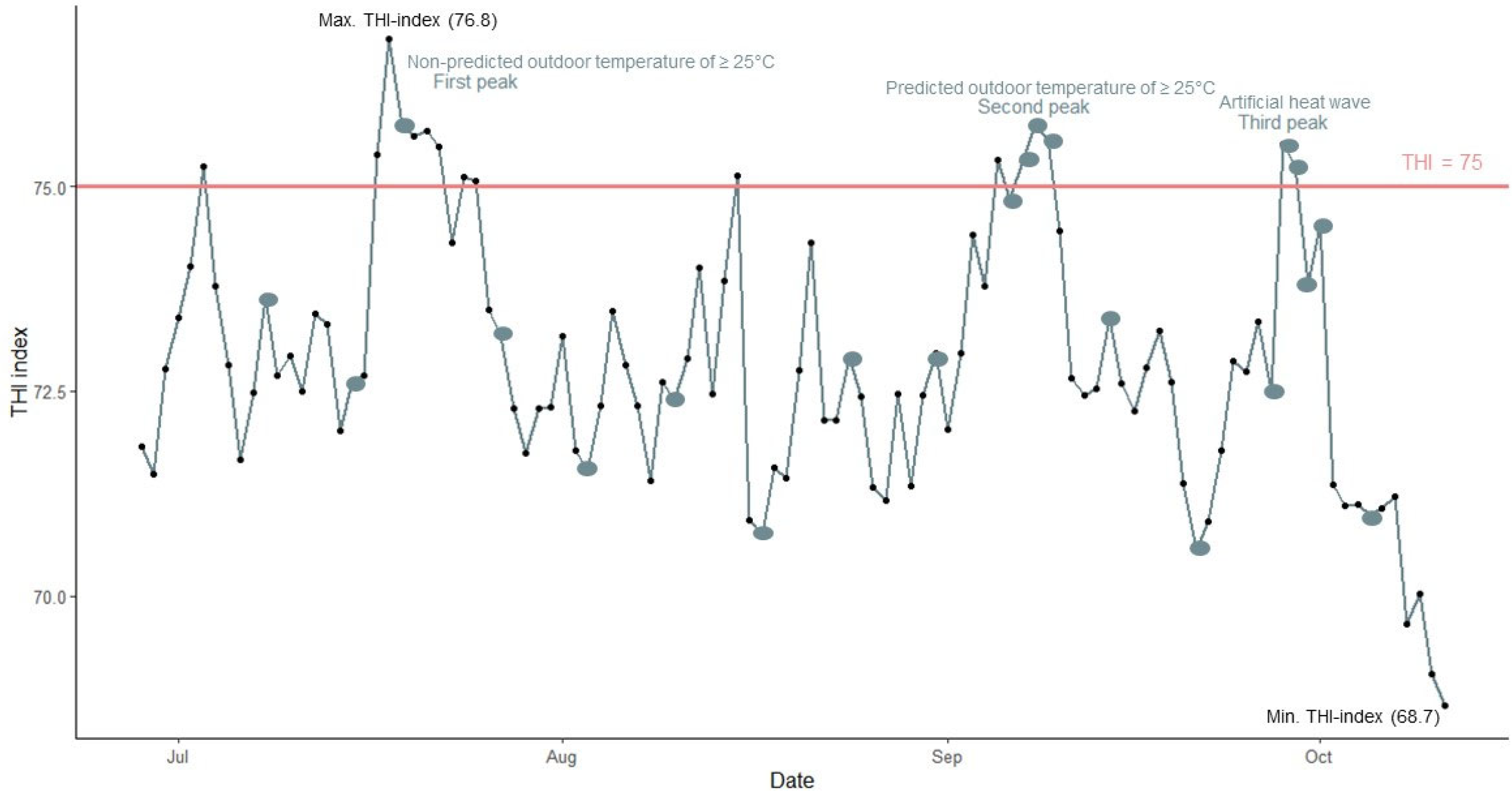

 = mean of the parameter of the diet group.)
= mean of the parameter of the diet group.)
 = mean of the parameter of the diet group.)
= mean of the parameter of the diet group.)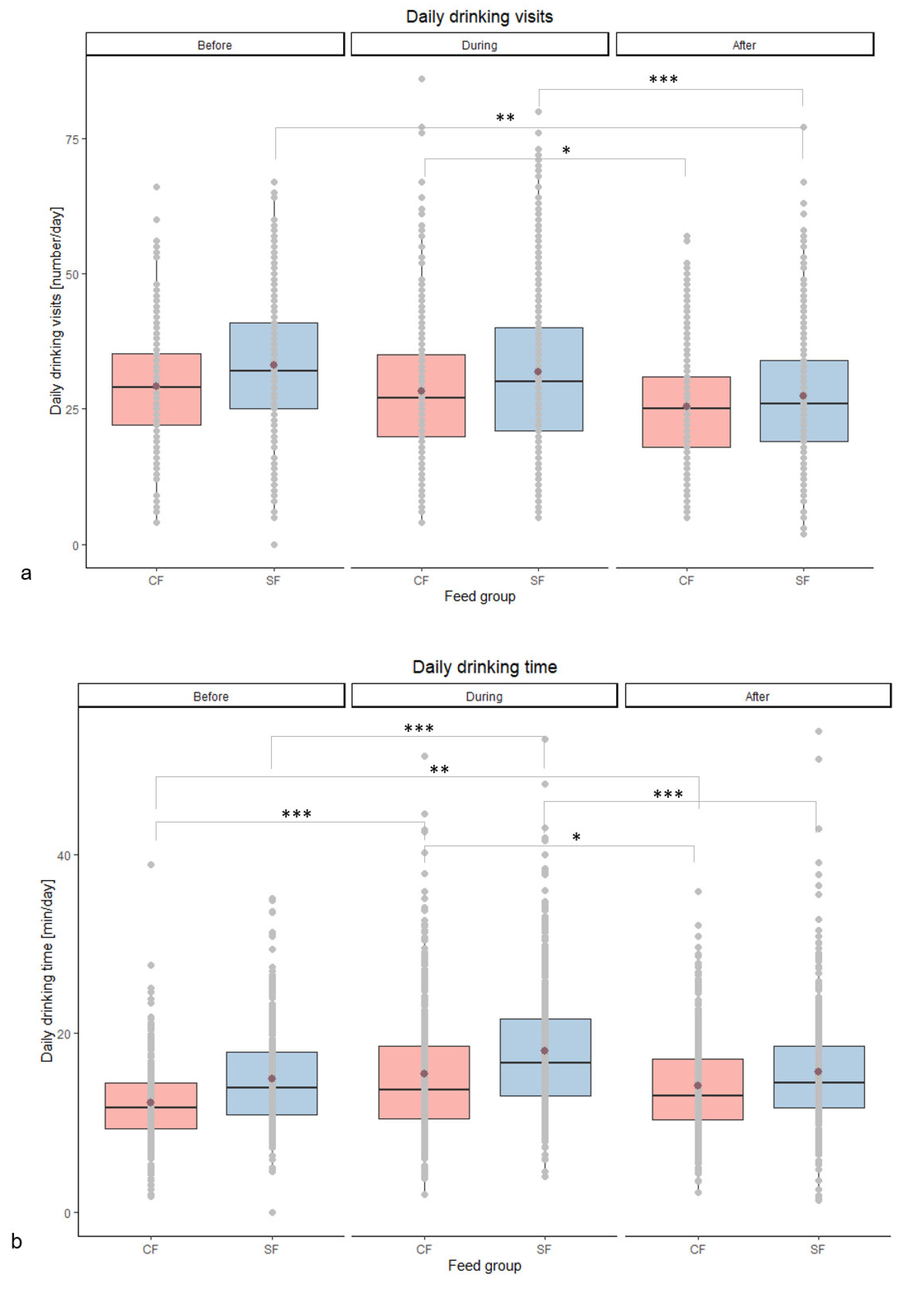
| Diet | ||||
|---|---|---|---|---|
| Starter Phase (10–15 Weeks of Age) | Grower Phase (15–25 Weeks of Age) | |||
| Ingredients and Composition | CF | SF | CF | SF |
| Ingredients | ||||
| Wheat [%] | 31.094 | 31.365 | 36.902 | 36.386 |
| Barley [%] | 20.000 | 20.000 | 20.000 | 20.000 |
| Maize [%] | 5.000 | 5.000 | - | - |
| Soybean meal (48% crude protein) [%] | 13.096 | 13.339 | 6.500 | 6.500 |
| Biscuits [%] | 8.000 | 8.000 | 7.500 | 7.500 |
| Corn flakes [%] | 3.000 | 3.000 | 3.000 | 3.000 |
| Wheat middlings [%] | 0.000 | 0.000 | 3.700 | 3.606 |
| Wheat gluten [%] | 8.590 | 5.317 | 13.000 | 13.000 |
| Palm oil [%] | 1.636 | 1.831 | 0.900 | 0.900 |
| Beet molasses [%] | 2.000 | 2.000 | 2.700 | 2.641 |
| Palm kernels [%] | 2.000 | 2.000 | 0.835 | 0.835 |
| Beet pulp [%] | 1.500 | 3.085 | 1.500 | 1.500 |
| Feed chalk [%] | 1.472 | 1.392 | 1.474 | 1.403 |
| Table salt [%] 1 | 0.473 | 0.165 | 0.543 | 0.167 |
| DL-Methionine [%] | 0.203 | 0.208 | 0.131 | 0.130 |
| L-Valine [%] | 0.052 | 0.055 | 0.005 | 0.000 |
| L-Lysine (50%) [%] | 0.799 | 0.792 | 0.715 | 0.700 |
| Tryptophan (25%) [%] | 0.167 | 0.172 | 0.046 | 0.038 |
| vL-Threonine [%] | 0.202 | 0.198 | 0.200 | 0.160 |
| Betaine [%] 2 | 0.000 | 0.667 | 0.000 | 0.667 |
| Vitamin E [%] 3 | 0.020 | 0.030 | 0.020 | 0.030 |
| Vitamin C [%] 4 | 0.000 | 0.057 | 0.000 | 0.057 |
| Sodium bicarbonate [%] 1 | 0.000 | 0.450 | 0.000 | 0.400 |
| Magnesium oxide [%] | 0.121 | 0.128 | 0.109 | 0.110 |
| Monocalcium phosphate [%] | 0.119 | 0.246 | 0.067 | 0.067 |
| Organic acid mix | 0.300 | 0.300 | - | - |
| Premix CF [%] 5 | 0.150 | 0.000 | 0.150 | 0.000 |
| Premix SF [%] 6 | 0.000 | 0.200 | 0.000 | 0.200 |
| Phytase [%] | 0.006 | 0.003 | 0.003 | 0.003 |
| Analysed chemical composition | ||||
| Crude protein (N × 6.25) [%V] | 16.4 | 16.0 | 15.3 | 15.7 |
| Crude fat [%V] | 4.9 | 5.1 | 4.6 | 4.6 |
| Crude ash [%V] | 5.2 | 5.2 | 5.5 | 5.0 |
| Crude fibre [%V] | 4.5 | 4.6 | 4.5 | 4.8 |
| Water [%V] | 10.4 | 10.9 | 10.1 | 10.9 |
| Lysine [g/kg] 7 | 10.6 | 10.5 | 9.0 | 8.9 |
| NE [MJ/kg] 7 | 9.6 | 9.6 | 9.4 | 9.3 |
| Animal Behaviour | ||
|---|---|---|
| Active 1 | Standing | Body supported by three or more legs and with head raised |
| Moving | Walking or running, body supported by three or more legs, position change possible and head held high. | |
| Exploring | Sniffing the floor and feeder, interacting with materials or pen mates | |
| Sitting | One or two front legs support the body, with hindquarters touching the ground. | |
| Inactive | Sternal lying | The pig lies on its sternum with its head high or down and 0 or 2 legs are extended |
| Semi-sternal lying | The pig lies on its sternum with its head high or down and 2 legs extended, or the pig lies (half) on its side with only two legs extended. | |
| Lateral lying | The pig lies entirely on its side with four legs extended. | |
| Diet | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | Baseline at THI = 75 | Slope 1 per ∆THI or THI 2 | p-Value | ||||
| CF | SF | CF | SF | Diet | ∆THI | Diet × ∆THI | |
| Physiological parameters | |||||||
| Respiration rate [breaths/min] | 46.3 | 45.7 | 34.5 | 34.5 | 0.904 | <0.001 | n.s. |
| Rectal temperature [°C] | 39.6 | 39.6 | 0.129 | 0.129 | 0.869 | 0.216 | n.s. |
| Skin temperature 2 [°C] | 35.1 | 35.2 | 0.294 | 0.294 | 0.875 | <0.001 | n.s. |
| Animal behaviour [%] | |||||||
| Active behaviour | 43 | 43 | −14 | −14 | 1.000 | 0.079 | n.s. |
| Standing | 11 | 11 | −5 | −5 | 0.975 | 0.186 | n.s. |
| Exploring | 25 | 23 | −5 | −5 | 0.669 | 0.108 | n.s. |
| Sitting | 7 | 8 | −4 | −4 | 0.204 | 0.084 | n.s. |
| Inactive behaviour [%] | 47 | 49 | 15 | 15 | 0.649 | 0.056 | n.s. |
| Sternal lying | 37 | 35 | 0 | 0 | 0.659 | 0.965 | n.s. |
| Semi-sternal lying | 3 | 5 | 4 | 4 | 0.334 | 0.281 | n.s. |
| Lateral lying | 8 | 8 | 7 | 17 | 0.971 | 0.298 | 0.086 |
| Parameter | Period of Heat Load | Diet | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|
| CF | SF | Diet | Period | Diet × Period | |||
| Daily drinking visits [number/day] | Before | 29 abcd | 33 bd | 0.43 | 0.150 | 0.008 | 0.013 |
| During | 28 cd | 32 bd | 0.45 | ||||
| After | 26 ab | 27 ac | 0.37 | ||||
| Daily drinking time [min/day] | Before | 12 ab | 15 ace | 0.20 | 0.099 | <0.001 | 0.036 |
| During | 15 ef | 18 bdf | 0.24 | ||||
| After | 14 cd | 16 ace | 0.20 | ||||
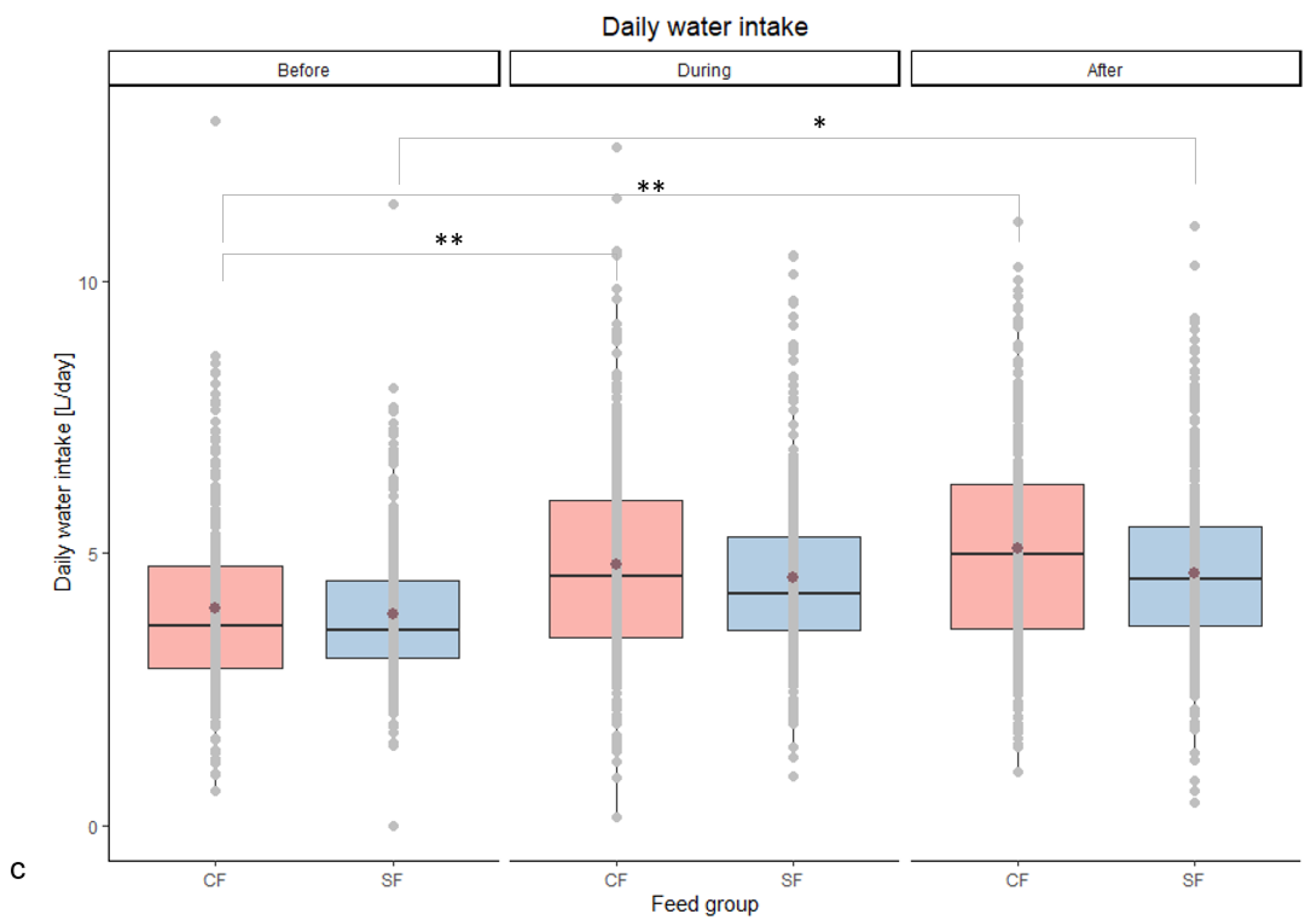
| Daily water intake [L/day] | Before | 4.0 ac | 3.9 ab | 0.06 | 0.730 | <0.001 | 0.013 |
| During | 4.8 bd | 4.6 abcd | 0.05 | ||||
| After | 5.1 bd | 4.6 cd | 0.06 | ||||
| Daily feed intake [g/day] | Before | 2088 ac | 2007 ab | 25.7 | 0.503 | <0.001 | n.s. |
| During | 2144 abcd | 2085 abcd | 18.5 | ||||
| After | 2296 bd | 2234 cd | 19.8 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Prekel, L.; Maes, D.; Van den Broeke, A.; Ampe, B.; Aluwé, M. Effect of Simultaneous Dietary Supplementation of Betaine, Selenomethionine, and Vitamins E and C under Summer Conditions in Growing–Finishing Pigs. Vet. Sci. 2024, 11, 110. https://doi.org/10.3390/vetsci11030110
De Prekel L, Maes D, Van den Broeke A, Ampe B, Aluwé M. Effect of Simultaneous Dietary Supplementation of Betaine, Selenomethionine, and Vitamins E and C under Summer Conditions in Growing–Finishing Pigs. Veterinary Sciences. 2024; 11(3):110. https://doi.org/10.3390/vetsci11030110
Chicago/Turabian StyleDe Prekel, Lotte, Dominiek Maes, Alice Van den Broeke, Bart Ampe, and Marijke Aluwé. 2024. "Effect of Simultaneous Dietary Supplementation of Betaine, Selenomethionine, and Vitamins E and C under Summer Conditions in Growing–Finishing Pigs" Veterinary Sciences 11, no. 3: 110. https://doi.org/10.3390/vetsci11030110
APA StyleDe Prekel, L., Maes, D., Van den Broeke, A., Ampe, B., & Aluwé, M. (2024). Effect of Simultaneous Dietary Supplementation of Betaine, Selenomethionine, and Vitamins E and C under Summer Conditions in Growing–Finishing Pigs. Veterinary Sciences, 11(3), 110. https://doi.org/10.3390/vetsci11030110








