Identification of Sarcocystis spp. in Slaughtered Sheep from Spain and Evaluation of Bradyzoite Viability after Freezing
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
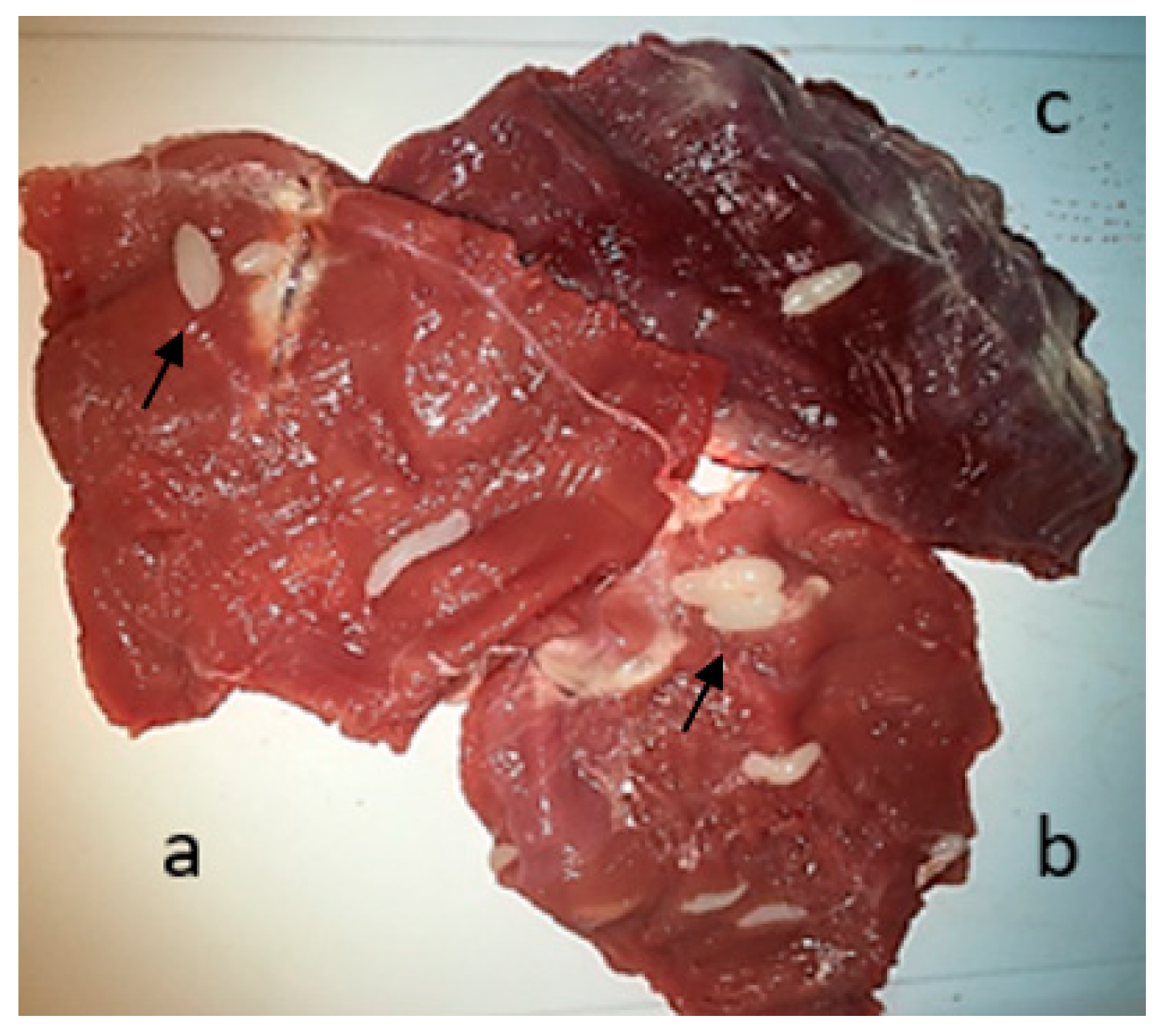
2.2. Macroscopic and Microscopic Examination of Fresh Tissues
2.3. Freezing Treatment
2.4. Pepsin Digestion
2.5. Molecular Identification
2.6. Evaluation of Bradyzoite Viability
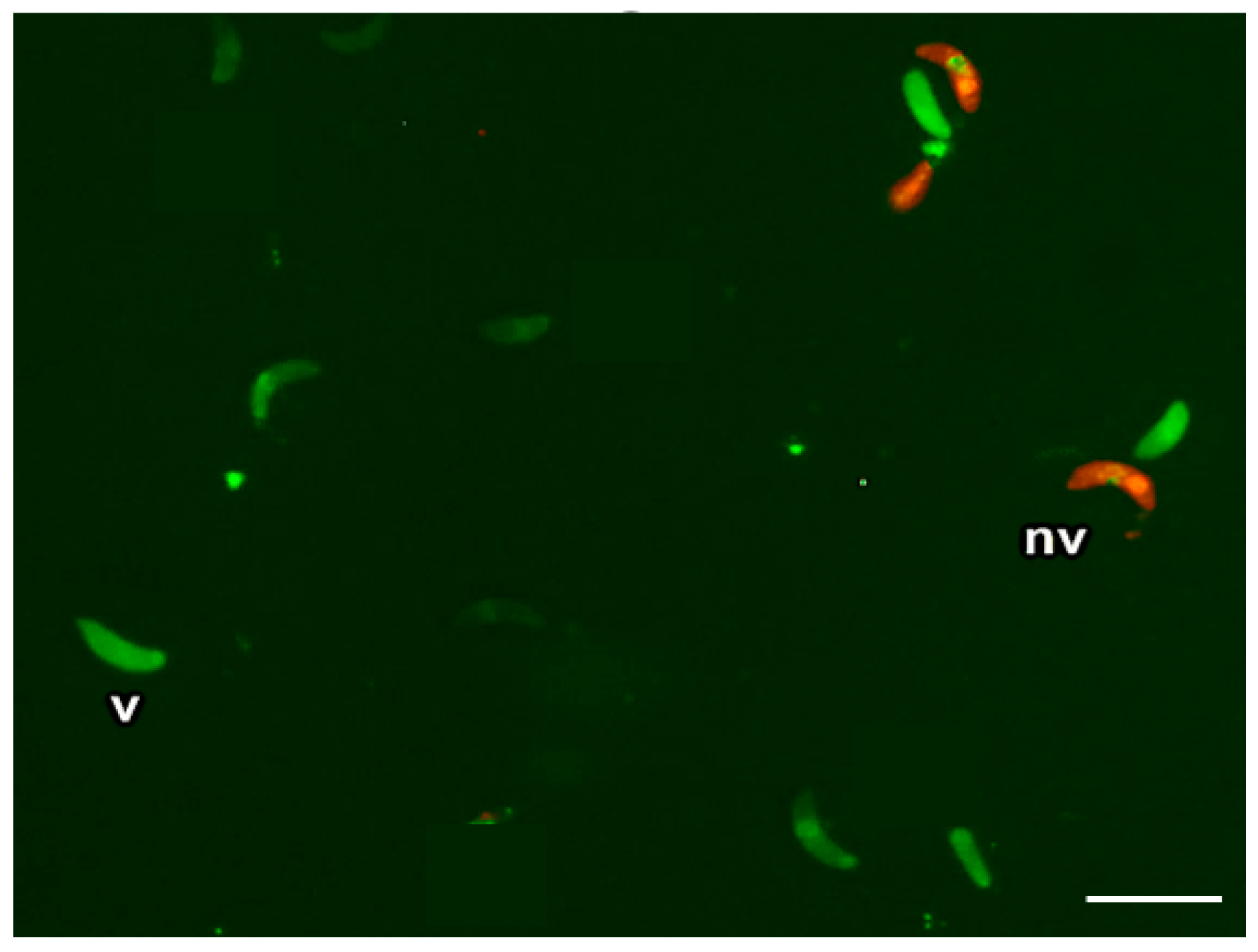
2.6.1. Trypan Blue Staining
2.6.2. Double Fluorescence Staining
2.7. Statistical Analysis
3. Results
3.1. Macroscopic and Microscopic Examination of Muscle
3.2. Molecular Analysis
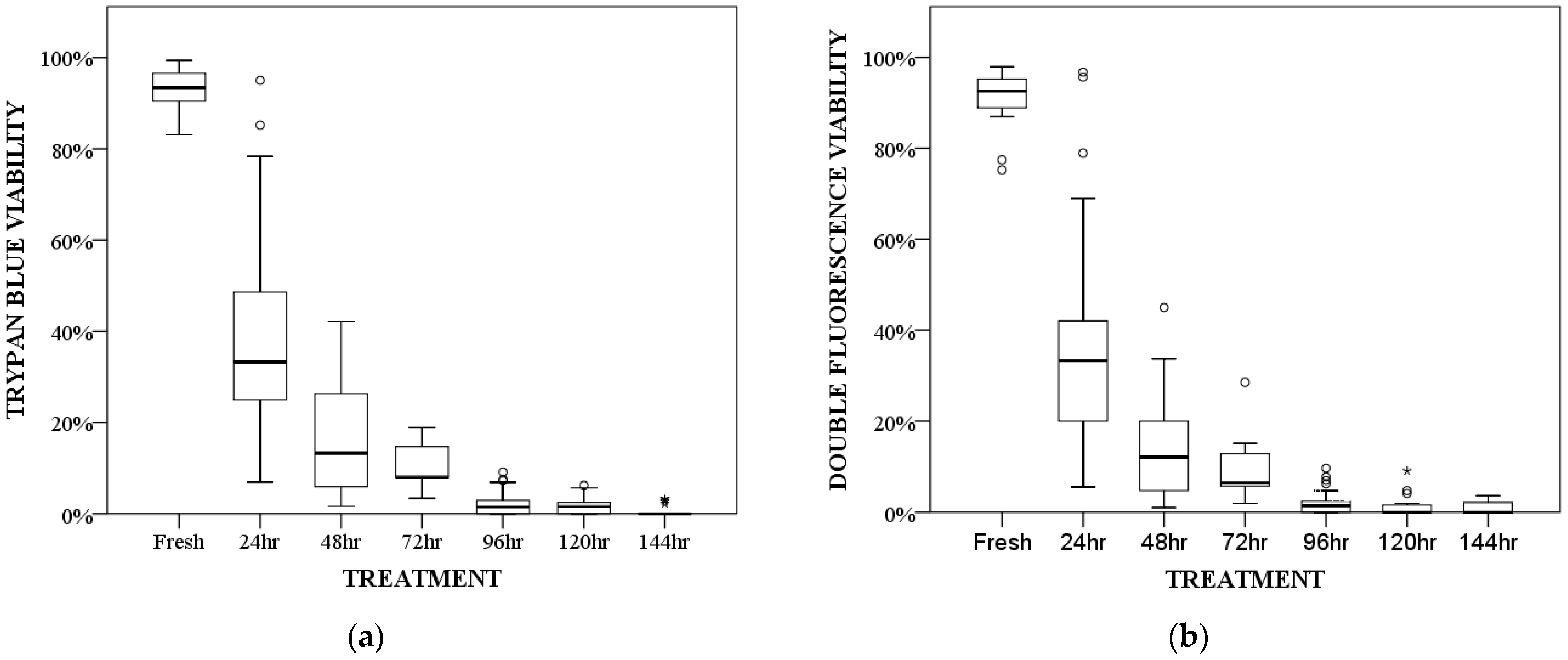
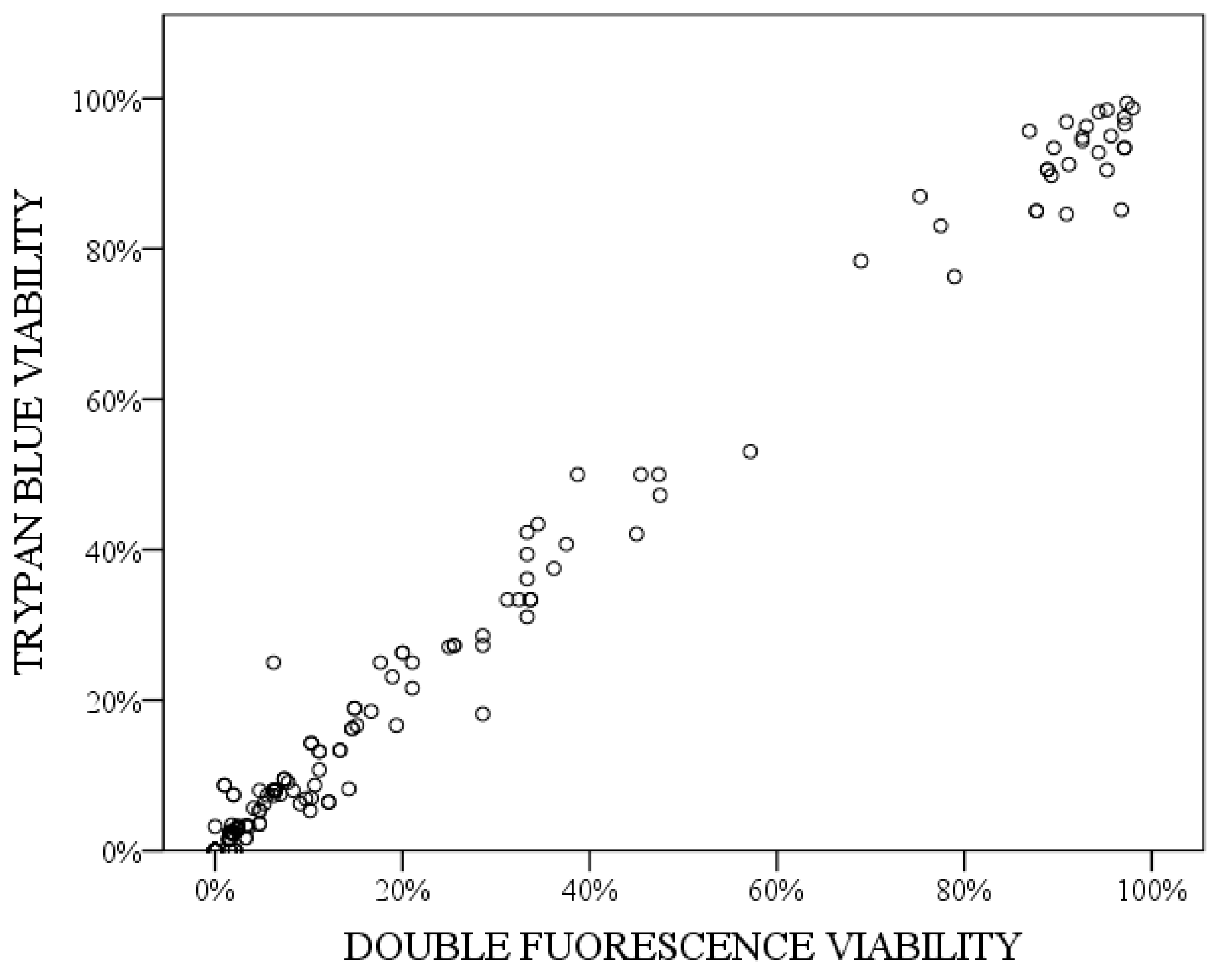
3.3. Viability Study after Freezing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, S.K.; Lindsay, D.S.; Grigg, M.E.; Dubey, J.P. Isolation, culture and cryopreservation of Sarcocystis species. Curr. Protoc. Microbiol. 2017, 45, 20D.1.1–20D.1.27. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Navalón, B.; Anastasio-Giner, B.; Cano-Fructuoso, M.; Sanchez-Martínez, P.; Llopis-Morant, A.; Perez-Castarlenas, B.; Goyena, E.; Berriatua, E. Sarcocystis infection: A major cause of carcass condemnation in adult sheep in Spain. Span. J. Agric. Res. 2012, 10, 388–392. [Google Scholar] [CrossRef]
- Marandykina-Prakienė, A.; Butkauskas, D.; Gudiškis, N.; Juozaitytė-Ngugu, E.; Bagdonaitė, D.L.; Kirjušina, M.; Calero-Bernal, R.; Prakas, P. Sarcocystis species richness in sheep and goats from Lithuania. Vet. Sci. 2023, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Spotin, A.; Rostamian, M.; Adami, M. Prevalence and molecular assessment of Sarcocystis infection in livestock in northeast Iran. Comp. Immunol. Microbiol. Infect. Dis. 2022, 80, 101738. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, S.; Gutiérrez-Palomino, P.N.; Martínez Cruz, S. Sarcocistosis. In Enfermedades Parasitarias del Ganado Ovino y Caprino; Sánchez Acedo, C., del Campillo, M.C., Eds.; GEA: Barcelona, Spain, 2003; pp. 74–79. [Google Scholar]
- Tenter, A.M. Current research on Sarcocystis species of domestic animals. Int. J. Parasitol. 1995, 25, 1311–1330. [Google Scholar] [CrossRef]
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Unique Multiple-Host Sarcocystis Species. In Sarcocystosis of Animals and Humans, 2nd ed.; CRC Press—Taylor & Francis Group: Boca Ratón, FL, USA, 2016; pp. 125–168. [Google Scholar]
- Gjerde, B.; de la Fuente, C.; Alunda, J.M.; Luzón, M. Molecular characterisation of five Sarcocystis species in domestic sheep (Ovis aries) from Spain. Parasitol. Res. 2020, 119, 215–231. [Google Scholar] [CrossRef]
- Saleque, A.; Juyal, P.D.; Bhatia, B.B. Effect of temperature on the infectivity of Sarcocystis miescheriana cysts in pork. Vet. Parasitol. 1990, 36, 343–346. [Google Scholar] [CrossRef]
- Koudela, B.; Steinhauser, L. Evaluation of vitality of Sarcocystis in beef by the DAPI fluorescence test. Acta Vet. Brno 1984, 53, 193–197. [Google Scholar] [CrossRef]
- Gorman, T.R.; Alcaíno, H.A.; Muñuz, H.; Cunazza, C. Sarcocystis sp. in guanaco (Lama guanicoe) and effect of temperature on its viability. Vet. Parasitol. 1984, 15, 95–101. [Google Scholar] [CrossRef]
- Srivastava, P.S.; Saha, A.K.; Sinha, S.R.P. Effects of heating and freezing on the viability of sarcocysts of Sarcocystis levinei from cardiac tissues of buffaloes. Vet. Parasitol. 1986, 19, 329–332. [Google Scholar] [CrossRef]
- Singh, K.P.; Shah, H.L. Viability and infectivity of Sarcocysts of Sarcocystis capracanis of the goat after maintaining them at different temperatures. Indian J. Anim. Sci. 1990, 60, 429–430. [Google Scholar]
- Harada, S.; Furukawa, M.; Tokuoka, E.; Matsumoto, K.; Yahiro, S.; Miyasaka, J.; Saito, M.; Kamata, Y.; Watanabe, M.; Irikura, D.; et al. Control of toxicity of Sarcocystis fayeri in horsemeat by freezing treatment and prevention of food poisoning caused by raw consumption of horsemeat. J. Food Hyg. Soc. 2013, 54, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Godoy, R.; Vilca, M.; Gonzáles, A.; Leyva, V.; Sam, R. Saneamiento y detoxificación de carne de llama (Lama glama) indectada con Sarcocystis aucheniae mediante coccion, horneado, fritura y congelado. Rev. Investig. Vet. Peru 2007, 18, 51–56. [Google Scholar]
- Honda, M.; Sawaya, M.; Taira, K.; Yamazaki, A.; Kamata, Y.; Shimizu, H.; Kobayashi, N.; Sakata, R.; Asakura, H.; Sugita-Konishi, Y. Effects of temperature, pH and curing on the viability of Sarcocystis, a Japanese sika deer (Cervus nippon centralis) parasite, and the inactivation of their diarrheal toxin. J. Vet. Med. Sci. 2018, 80, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.H.; Charleston, W.A.G. Studies on Sarcocystis species VII: The effect of temperature on the viability of macrocysts (Sarcocystis gigantea) of sheep. N. Z. Vet. J. 1980, 28, 189–191. [Google Scholar] [CrossRef]
- Borges, I.P.; Castanheira, L.E.; Barbosa, B.F.; Naves De Souza, D.L.; Da Silva, R.J.; Mineo, J.R.; Tudini, K.A.Y.; Rodrigues, R.S.; Ferro, E.A.V.; De Melo Rodrigues, V. Anti-parasitic effect on Toxoplasma gondii induced by BnSP-7, a Lys49-phospholipase A 2 homologue from Bothrops pauloensis venom. Toxicon 2016, 119, 84–91. [Google Scholar] [CrossRef]
- Ahmadpour, E.; Ebrahimzadeh, M.A.; Sharif, M.; Edalatian, S.; Sarvi, S.; Montazeri, M.; Mehrzadi, S.; Akbari, M.; Rahimi, M.T.; Daryani, A. Anti-Toxoplasma activities of Zea mays and Eryngium caucasicum extracts, in vitro and in vivo. J. Pharmacopunct. 2019, 22, 154–159. [Google Scholar] [CrossRef]
- Shojaee, S.; Firouzeh, N.; Keshavarz, H.; Jafar-Pour Azami, S.; Salimi, M.; Mohebali, M. Nanosilver Colloid inhibits Toxoplasma gondii tachyzoites and bradyzoites in vitro. Iran. J. Parasitol. 2019, 14, 362–367. [Google Scholar] [CrossRef]
- Tasca, T.; Borges, F.P.; Bonan, C.D.; De Carli, G.A.; Battastini, A.M.O.; Sarkis, J.J.F. Effects of metronidazole and tinidazole on NTPDase1 and ecto-5′-nucleotidase from intact cells of Trichomonas vaginalis. FEMS Microbiol. Lett. 2003, 226, 379–384. [Google Scholar] [CrossRef]
- Dong, X.; Abdelnabi, G.H.; Lee, S.H.; Li, G.; Jin, H.; Lillehoj, H.S.; Suo, X. Enhanced egress of intracellular Eimeria tenella sporozoites by splenic lymphocytes from coccidian-infected chickens. Infect. Immun. 2011, 79, 3465–3470. [Google Scholar] [CrossRef]
- Eligio-García, L.; Pontifez-Pablo, E.; Pérez-Gutiérrez, S.; Jiménez-Cardoso, E. Antigiardial effect of kramecyne in experimental giardiasis. J. Evid. Based Complement. Altern. Med. 2017, 2017, 6832789. [Google Scholar] [CrossRef]
- Forson, P.O.; Tetteh-Quarcoo, P.B.; Ahenkorah, J.; Aryee, R.; Okine, E.N.; Afutu, E.; Djameh, G.I.; Agyapong, J.; Anang, A.K.; Ayeh-Kumi, P.F. Ability of vital and fluorescent staining in the differentiation of Schistosoma haematobium live and dead eggs. Med. Sci. 2019, 7, 64. [Google Scholar] [CrossRef]
- Toral-Bastida, E.; Garza-Rodriguez, A.; Jimenez-Gonzalez, D.E.; Garcia-Cortes, R.; Avila-Ramirez, G.; Maravilla, P.; Flisser, A. Development of Taenia pisiformis in golden hamster (Mesocricetus auratus). Parasit. Vectors 2011, 4, 147. [Google Scholar] [CrossRef]
- Dubey, J.P.; Moré, G.; Van Wilpe, E.; Calero-Bernal, R.; Verma, S.K.; Schares, G. Sarcocystis rommeli, n. sp. (Apicomplexa: Sarcocystidae) from cattle (Bos taurus) and its differentiation from Sarcocystis hominis. J. Eukaryot. Microbiol. 2016, 63, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Murata, R.; Suzuki, J.; Hyuga, A.; Shinkai, T.; Sadamasu, K. Molecular identification and characterization of Sarcocystis spp. in horsemeat and beef marketed in Japan. Parasite 2018, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Korich, D.G.; Mead, J.R.; Madore, M.S.; Sinclair, N.A.; Sterling, C.R. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 1990, 56, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Shamseddin, J.; Akhlaghi, L.; Razmjou, E.; Shojaee, S.; Monavari, S.H.R.; Tajik, N.; Ebrahimi, S.A.; Meamar, A.R. Conjugated linoleic acid stimulates apoptosis in RH and tehran strains of Toxoplasma gondii, in vitro. Iran. J. Parasitol. 2015, 10, 238–244. [Google Scholar]
- Boissière, A.; Arnathau, C.; Duperray, C.; Berry, L.; Lachaud, L.; Renaud, F.; Durand, P.; Prugnolle, F. Isolation of Plasmodium falciparum by flow-cytometry: Implications for single-trophozoite genotyping and parasite DNA purification for whole-genome high-throughput sequencing of archival samples. Malar. J. 2012, 11, 163. [Google Scholar] [CrossRef]
- Rashidi, S.; Kalantar, K.; Rostamzadeh, D.; Hatam, G. The importance of checking Leishmania promastigotes viability in the proteomics analysis of secretions. Türk. Parazitol. Derg. 2018, 42, 245–248. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Saeed, M.A.; Fitzgerald, S.D.; Murphy, A.J.; Mansfield, L.S. Effects of and host cell type on the in vitro growth and development of Sarcocystis falcatula. Parasitol. Res. 2003, 91, 22–26. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Pratsinis, H.; Nebe-von-Caron, G.; Kletsas, D.; Tsakalidou, E. Rapid assessment of the physiological status of Streptococcus macedonicus by flow cytometry and fluorescence probes. Int. J. Food Microbiol. 2006, 111, 197–205. [Google Scholar] [CrossRef]
- Bunthof, C.J.; Bloemen, K.; Breeuwer, P.; Rombouts, F.M.; Abee, T. Flow cytometric assessment of viability of lactic acid bacteria. Appl. Environ. Microbiol. 2001, 67, 2326–2335. [Google Scholar] [CrossRef]
- Dubey, J.P. Refinement of pepsin digestion method for isolation of Toxoplasma gondii from infected tissues. Vet. Parasitol. 1998, 74, 75–77. [Google Scholar] [CrossRef]
- Bayarri, S.; Gracia, M.J.; Lázaro, R.; Pérez-Arquillué, C.; Barberán, M.; Herrera, A. Determination of the viability of Toxoplasma gondii in cured ham using bioassay: Influence of technological processing and Food safety implications. J. Food Prot. 2010, 73, 2239–2243. [Google Scholar] [CrossRef]
- Hoeve-Bakker, B.J.A.; van der Giessen, J.W.B.; Franssen, F.F.J. Molecular identification targeting cox1 and 18S genes confirms the high prevalence of Sarcocystis spp. in cattle in the Netherlands. Int. J. Parasitol. 2019, 49, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Moré, G.; Schares, S.; Maksimov, A.; Conraths, F.J.; Venturini, M.C.; Schares, G. Development of a multiplex real time PCR to differentiate Sarcocystis spp. affecting cattle. Vet. Parasitol. 2013, 197, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, B. Molecular characterisation of Sarcocystis bovifelis, Sarcocystis bovini n. sp.; Sarcocystis hirsuta and Sarcocystis cruzi from cattle (Bos taurus) and Sarcocystis sinensis from water buffaloes (Bubalus bubalis). Parasitol. Res. 2016, 115, 1473–1492. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Harrison, R.A.P.; Vickers, S.E. Use of fluorescent probes to assess membrane integrity in mammalian spermatozoa. J. Reprod. Fertil. 1990, 88, 343–352. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Farhang-Pajuh, F.; Yakhchali, M.; Mardani, K. Molecular determination of abundance of infection with Sarcocystis species in slaughtered sheep of Urmia, Iran. Vet. Res. Forum 2014, 5, 181–186. [Google Scholar] [PubMed]
- Pipia, A.P.; Varcasia, A.; Zidda, A.; Dessì, G.; Panzalis, R.; Tamponi, C.; Marrosu, R.; Tosciri, G.; Sanna, G.; Dore, F.; et al. Cross sectional investigation on sheep sarcosporidiosis in Sardinia, Italy. Vet. Parasitol. Reg. Stud. Rep. 2016, 3–4, 13–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, Y.; Guo, R.; Sang, X.; Zhang, X.; Li, M.; Li, X.; Yang, N.; Jiang, T. A systematic meta-analysis of global Sarcocystis infection in sheep and goats. Pathogens 2023, 12, 902. [Google Scholar] [CrossRef]
- Marandykina-Prakienė, A.; Butkauskas, D.; Gudiškis, N.; Juozaitytė-Ngugu, E.; Januškevičius, V.; Rudaitytė-Lukošienė, E.; Prakas, P. Molecular identification of Sarcocystis species in sheep from Lithuania. Animals 2022, 12, 2048. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, B.; Yang, Z.; Li, C.; Attwood, S.W.; Wang, W.; Lei, L.; Sun, X.; Zhang, Z. Effects of frozen storage on the structure of sarcocysts in pig muscle and implications in taxonomic studies. Exp. Parasitol. 2007, 115, 393–398. [Google Scholar] [CrossRef]
- Costa, A.F.P.; de Brito, R.C.F.; Carvalho, L.M.; Cardoso, J.M.O.; Vieira, P.M.A.; Reis, A.B.; Aguiar-Soares, R.D.O.; Roatt, B.M. Liver infusion tryptose (LIT): The best choice for growth, viability, and infectivity of Leishmania infantum parasites. Parasitol. Res. 2020, 119, 4185–4195. [Google Scholar] [CrossRef]
- Sikorski, P.M.; Grigg, M.E. A flow cytometry-based assay to measure C3B deposition on protozoan parasites such as Toxoplasma gondii. Curr. Protoc. 2022, 2, e593. [Google Scholar] [CrossRef]
- Mirzaei, M.; Rezaei, H. The role of sheep in the epidemiology of Sarcocystis spp. in Tabriz area northwest of Iran. J. Parasit. Dis. 2016, 40, 285–288. [Google Scholar] [CrossRef][Green Version]


| 18S Gene | Cox-1 Gene | ||||||
|---|---|---|---|---|---|---|---|
| Sarcocystis spp. | % | ID | Length | Sarcocystis spp. | % | ID | Length |
| S. tenella | 100% | KP263759.1 | 1804 | S. tenella | 99% | MK420001.1 | 1038 |
| S. medusiformis | 100% | MK420021.1 | 1928 | S. medusiformis | 92% | MK420014.1 | 1038 |
| S. gigantea | 99% | MK420020.1 | 1910 | S. gigantea | 100% | MK420011.1 | 1038 |
| S. gigantea | 98% | MK420020.1 | 1910 | S. gigantea | 99% | MK420013.1 | 1038 |
| NV | NV | NV | NV | S. gigantea | 99% | MK420013.1 | 1038 |
| S. tenella | 98% | MF401626.1 | 837 | S. tenella | 99% | MK419987.1 | 1038 |
| S. gigantea | 98% | MK420020.1 | 1910 | S. gigantea | (99%) | MK420011.1 | 1038 |
| S. tenella | 99% | KP263759.1 | 1804 | S. tenella | (99%) | MK420001.1 | 1038 |
| S. gigantea | 90% | MK420020.1 | 1910 | S. gigantea | (99%) | MK420011.1 | 1038 |
| NV | NV | NV | NV | S. medusiformis | 99% | MK420014.1 | 1038 |
| S. gigantea | 99% | MK420020.1 | 1910 | S. gigantea | 99% | MK420011.1 | 1038 |
| S. medusiformis | 100% | MK420021.1 | 1928 | NV | NV | NV | NV |
| S. medusiformis | 99% | MK420020.1 | 1910 | S. medusiformis | 90% | MK420014.1 | 1038 |
| S. medusiformis | 87% | MK420021.1 | 1928 | S. medusiformis | 99% | MK420015.1 | 1038 |
| Hours at −20 °C | Trypan Blue 1 | Double Fluorescence 1 |
|---|---|---|
| Fresh | 93.42 (83.05–99.38) a | 92.59 (75.25–97.96) a |
| 24 | 33.33 (6.98–95) a,b,c | 33.33 (5.56–96.77) a,b,c |
| 48 | 13.33 (1.67–42.11) b,c | 12.12 (0.99–45) b,c |
| 72 | 8 (3.33–18.92) c | 6.45 (1.96–28.57) c |
| 96 | 1.47 (0–9.09) d | 1.42 (0–9.68) d |
| 120 | 1.58 (0–6.25) d | 0 (0–9.09) d |
| 144 | 0 (0–3.23) d | 0 (0–3.61) d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peris, M.P.; Gracia, M.J.; Moreno, B.; Juan-Puente, P.; Morales, M.; Serrano, M.; Manzano, M.D.; Halaihel, N.; Badiola, J.; Castillo, J.A. Identification of Sarcocystis spp. in Slaughtered Sheep from Spain and Evaluation of Bradyzoite Viability after Freezing. Vet. Sci. 2024, 11, 103. https://doi.org/10.3390/vetsci11030103
Peris MP, Gracia MJ, Moreno B, Juan-Puente P, Morales M, Serrano M, Manzano MD, Halaihel N, Badiola J, Castillo JA. Identification of Sarcocystis spp. in Slaughtered Sheep from Spain and Evaluation of Bradyzoite Viability after Freezing. Veterinary Sciences. 2024; 11(3):103. https://doi.org/10.3390/vetsci11030103
Chicago/Turabian StylePeris, María Paz, María Jesús Gracia, Bernardino Moreno, Paula Juan-Puente, Mariano Morales, María Serrano, María Dolores Manzano, Nabil Halaihel, Juan Badiola, and Juan Antonio Castillo. 2024. "Identification of Sarcocystis spp. in Slaughtered Sheep from Spain and Evaluation of Bradyzoite Viability after Freezing" Veterinary Sciences 11, no. 3: 103. https://doi.org/10.3390/vetsci11030103
APA StylePeris, M. P., Gracia, M. J., Moreno, B., Juan-Puente, P., Morales, M., Serrano, M., Manzano, M. D., Halaihel, N., Badiola, J., & Castillo, J. A. (2024). Identification of Sarcocystis spp. in Slaughtered Sheep from Spain and Evaluation of Bradyzoite Viability after Freezing. Veterinary Sciences, 11(3), 103. https://doi.org/10.3390/vetsci11030103






