Taenia martis Cysticercosis in a Common Marmoset (Callithrix jacchus)
Simple Summary
Abstract
1. Introduction
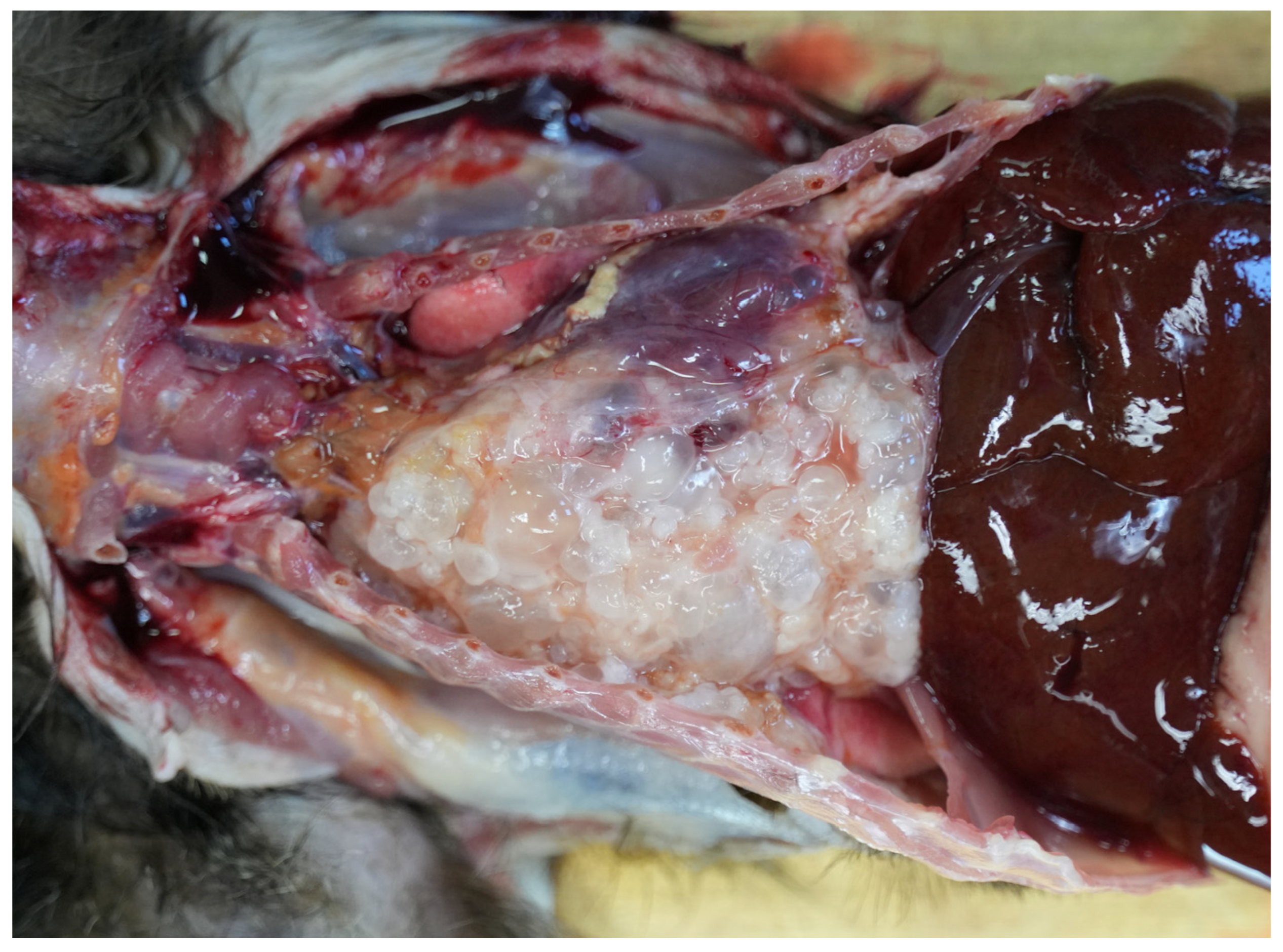
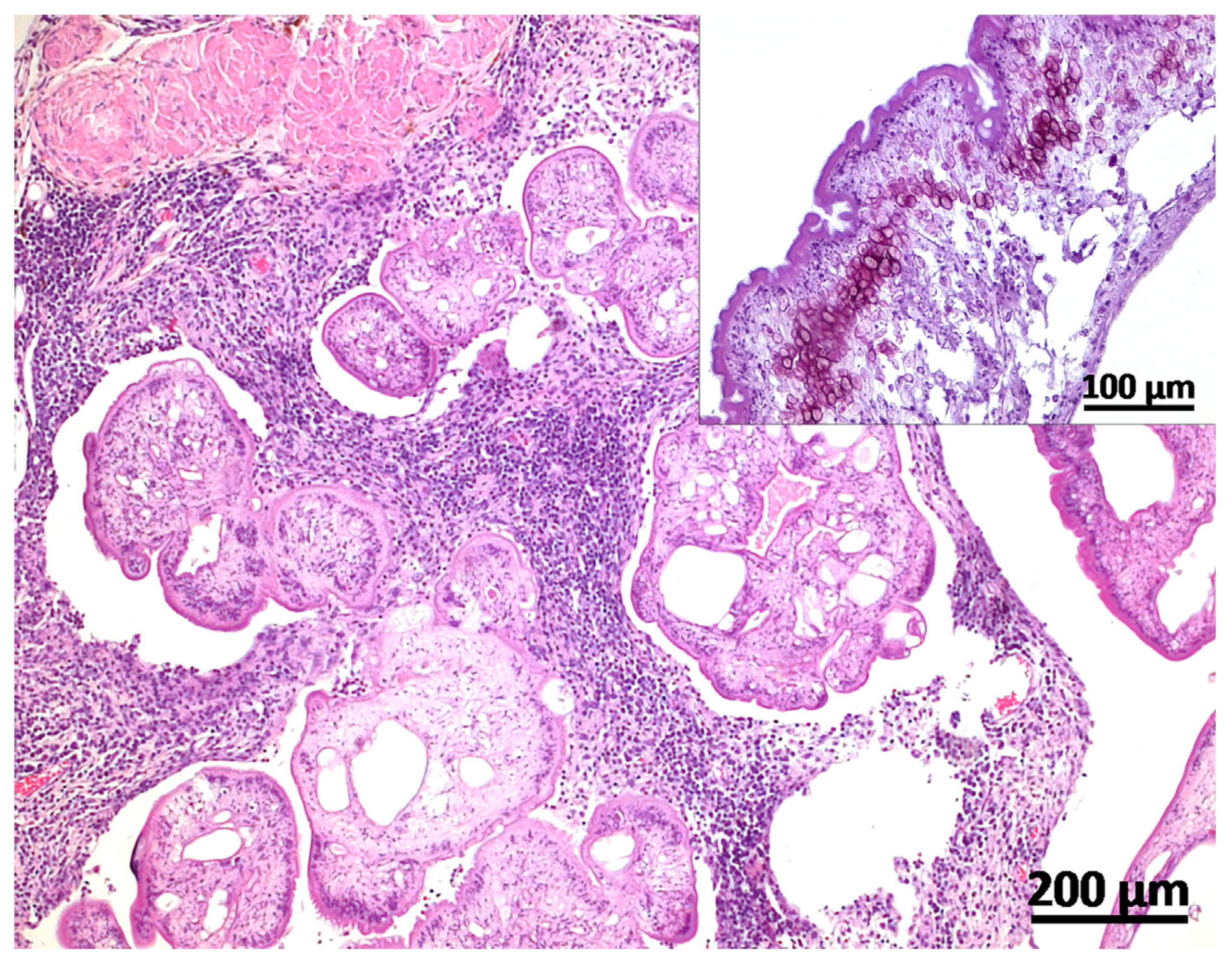
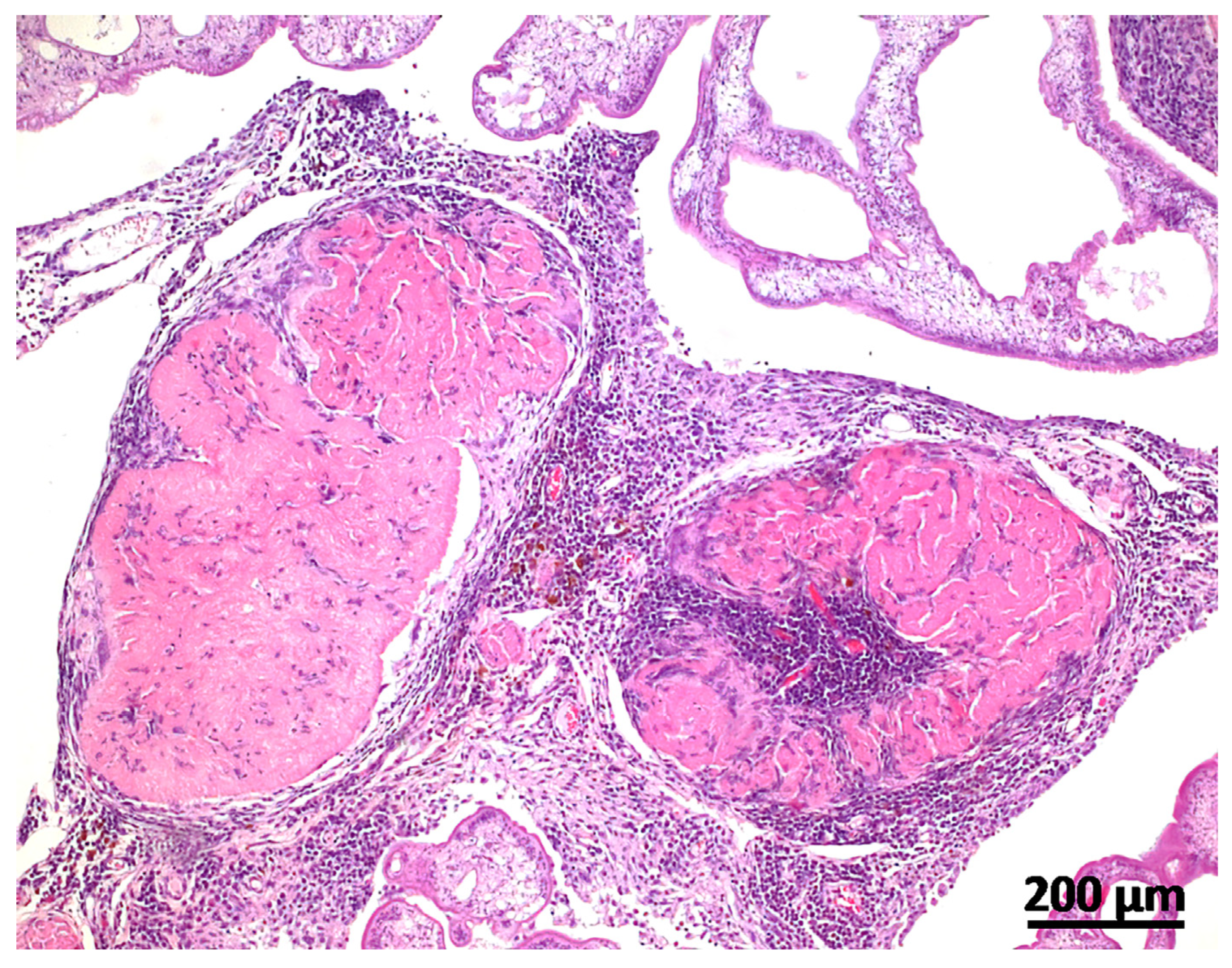
2. Case Description
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunet, J.; Pesson, B.; Chermette, R.; Regnard, P.; Grimm, F.; Deplazes, P.; Ferreira, X.; Sabou, M.; Pfaff, A.W.; Abou-Bacar, A.; et al. First case of peritoneal cysticercosis in a non-human primate host (Macaca tonkeana) due to Taenia martis. Parasit. Vectors 2014, 7, 422. [Google Scholar] [CrossRef]
- Danière, C.; Callait-Cardinal, M.P.; Grenouillet, F.; Lemberger, K.; Quintard, B. Taenia martis in an Alaotran gentle lemur (Hapale-mur alaotrensis): The importance of molecular identification. Vet. Rec. Case Rep. 2024, 12, e915. [Google Scholar] [CrossRef]
- Prokopic, J. Some notes on the distribution and life history of the cestode Taenia martis (Zeder, 1803). Helminthologia 1970, 11, 187–193. [Google Scholar]
- Loos-Frank, B.; Zeyhle, E. The intestinal helminths of the red fox and some other carnivores in southwest Germany. Z. Parasitenkd. 1982, 67, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Mormann, S.; Gies, N.; Rentería-Solís, Z. Taenia martis in a white-headed lemur (Eulemur albifrons) from a zoological park in North Rhine-Westphalia, Germany. Vet. Parasitol. Reg. Stud. Rep. 2023, 44, 100913. [Google Scholar] [CrossRef]
- Brunet, J.; Benoilid, A.; Kremer, S.; Dalvit, C.; Lefebvre, N.; Hansmann, Y.; Chenard, M.P.; Mathieu, B.; Grimm, F.; Deplazes, P.; et al. First case of human cerebral Taenia martis cysticercosis. J. Clin. Microbiol. 2015, 53, 2756–2759. [Google Scholar] [CrossRef]
- Eberwein, P.; Haeupler, A.; Kuepper, F.; Wagner, D.; Kern, W.V.; Muntau, B.; Racz, P.; Agostini, H.; Poppert, S. Human infection with marten tapeworm. Emerg. Infect. Dis. 2013, 19, 1152–1154. [Google Scholar] [CrossRef]
- Koch, T.; Schoen, C.; Muntau, B.; Addo, M.; Ostertag, H.; Wiechens, B.; Tappe, D. Molecular Diagnosis of Human Taenia martis eye infection. Am. J. Trop. Med. Hyg. 2016, 94, 1055–1057. [Google Scholar] [CrossRef]
- Mueller, A.; Förch, G.; Zustin, J.; Muntau, B.; Schuldt, G.; Tappe, D. Case Report: Molecular identification of larval Taenia martis infection in the Pouch of Douglas. Am. J. Trop. Med. Hyg. 2020, 103, 2315–2317. [Google Scholar] [CrossRef]
- Rudelius, M.; Brehm, K.; Poelcher, M.; Spinner, C.; Rosenwald, A.; da Costa, C.P. First case of human peritoneal cysticercosis mimicking peritoneal carcinosis: Necessity of laparoscopy and histologic assessment for the correct diagnosis. JMM Case Rep. 2017, 4, e005097. [Google Scholar] [CrossRef]
- Steinsiepe, V.K.; Ruf, M.T.; Rossi, M.; Fricker-Feer, C.; Kolenc, D.; Buser, B.S.; Concu, M.; Neumayr, A.; Schneider, U.C. Human Taenia martis neurocysticercosis, Switzerland. Emerg. Infect. Dis. 2023, 29, 2569–2572. [Google Scholar] [CrossRef] [PubMed]
- Eggink, H.; Maas, M.; van den Brand, J.M.A.; Dekker, J.; Franssen, F.; Hoving, E.W.; Kortbeek, L.M.; Kranendonk, M.E.G.; Meiners, L.C.; Rittscher, A.E.; et al. Taenia martis neurocysticercosis-like lesion in child, associated with local source, the Netherlands. Emerg. Infect. Dis. 2024, 30, 555–559. [Google Scholar] [CrossRef] [PubMed]
- De Liberato, C.; Berrilli, F.; Meoli, R.; Friedrich, K.G.; Di Cerbo, P.; Cocumelli, C.; Eleni, C. Fatal infection with Taenia martis metacestodes in a ring-tailed lemur (Lemur catta) living in an Italian zoological garden. Parasitol. Int. 2014, 63, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Fashing, P.J.; Boyd, D.A.; Barry, T.S.; Burke, R.J.; Goodale, C.B.; Jones, S.C.; Kerby, J.T.; Kellogg, B.S.; Lee, L.M.; et al. Fitness impacts of tapeworm parasitism on wild gelada monkeys at Guassa, Ethiopia. Am. J. Primatol. 2015, 77, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef]
- Segovia, J.M.; Torres, J.; Miquel, J.; Sospedra, E.; Guerrero, R.; Feliu, C. Analysis of helminth communities of the pine marten, Martes martes, in Spain: Mainland and insular data. Acta Parasit. 2007, 52, 156–164. [Google Scholar] [CrossRef]
- Millán, J.; Ferroglio, E. Helminth parasites in stone martens (Martes foina) from Italy. Eur. J. Wildl. Res. 2001, 47, 229–231. [Google Scholar] [CrossRef]
- Deplazes, P.; Eichenberger, R.M.; Grimm, F. Wildlife-transmitted Taenia and Versteria cysticercosis and coenurosis in humans and other primates. Int. J. Parasitol. Parasites Wildl. 2019, 9, 342–358. [Google Scholar] [CrossRef]
- Chervy, L. The terminology of larval cestodes or metacestodes. Syst. Parasitol. 2002, 52, 1–33. [Google Scholar] [CrossRef]
- Bleyer, M.; Risch, T.; Roos, C.; Kaup, F.J.; Mätz-Rensing, K. Taenia crassiceps cysticercosis in a Nilgiri langur (Semnopithecus johnii). J. Zoo Wildl. Med. 2018, 49, 501–504. [Google Scholar] [CrossRef]
- Yamano, K.; Kouguchi, H.; Uraguchi, K.; Mukai, T.; Shibata, C.; Yamamoto, H.; Takaesu, N.; Ito, M.; Makino, Y.; Takiguchi, M.; et al. First detection of Echinococcus multilocularis infection in two species of nonhuman primates raised in a zoo: A fatal case in Cercopithecus diana and a strongly suspected case of spontaneous recovery in Macaca nigra. Parasitol. Int. 2014, 63, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Lampe, K. Untersuchungen zur Diagnostik und Prophylaxe der alveolären Echinokokkose bei Makaken (German); Optimus: Göttingen, Germany, 2013; Available online: https://nbn-resolving.org/urn:nbn:de:gbv:95-104408 (accessed on 15 November 2013).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bleyer, M.; Erffmeier, L.; Batura, O.; Roos, C. Taenia martis Cysticercosis in a Common Marmoset (Callithrix jacchus). Vet. Sci. 2024, 11, 623. https://doi.org/10.3390/vetsci11120623
Bleyer M, Erffmeier L, Batura O, Roos C. Taenia martis Cysticercosis in a Common Marmoset (Callithrix jacchus). Veterinary Sciences. 2024; 11(12):623. https://doi.org/10.3390/vetsci11120623
Chicago/Turabian StyleBleyer, Martina, Lena Erffmeier, Olga Batura, and Christian Roos. 2024. "Taenia martis Cysticercosis in a Common Marmoset (Callithrix jacchus)" Veterinary Sciences 11, no. 12: 623. https://doi.org/10.3390/vetsci11120623
APA StyleBleyer, M., Erffmeier, L., Batura, O., & Roos, C. (2024). Taenia martis Cysticercosis in a Common Marmoset (Callithrix jacchus). Veterinary Sciences, 11(12), 623. https://doi.org/10.3390/vetsci11120623





