An Outbreak of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) in a German Boar Stud: A Retrospective Analysis of PRRSV Shedding in Boar Semen
Simple Summary
Abstract
1. Introduction
2. Case Presentation
2.1. PRRSV Monitoring
2.2. Outbreak of PRRSV and Initial Diagnostic Measures
2.3. Laboratory Diagnostical Methods
2.4. Data Collection, Measurements and Analyses
3. Results
3.1. Testing of Semen Samples
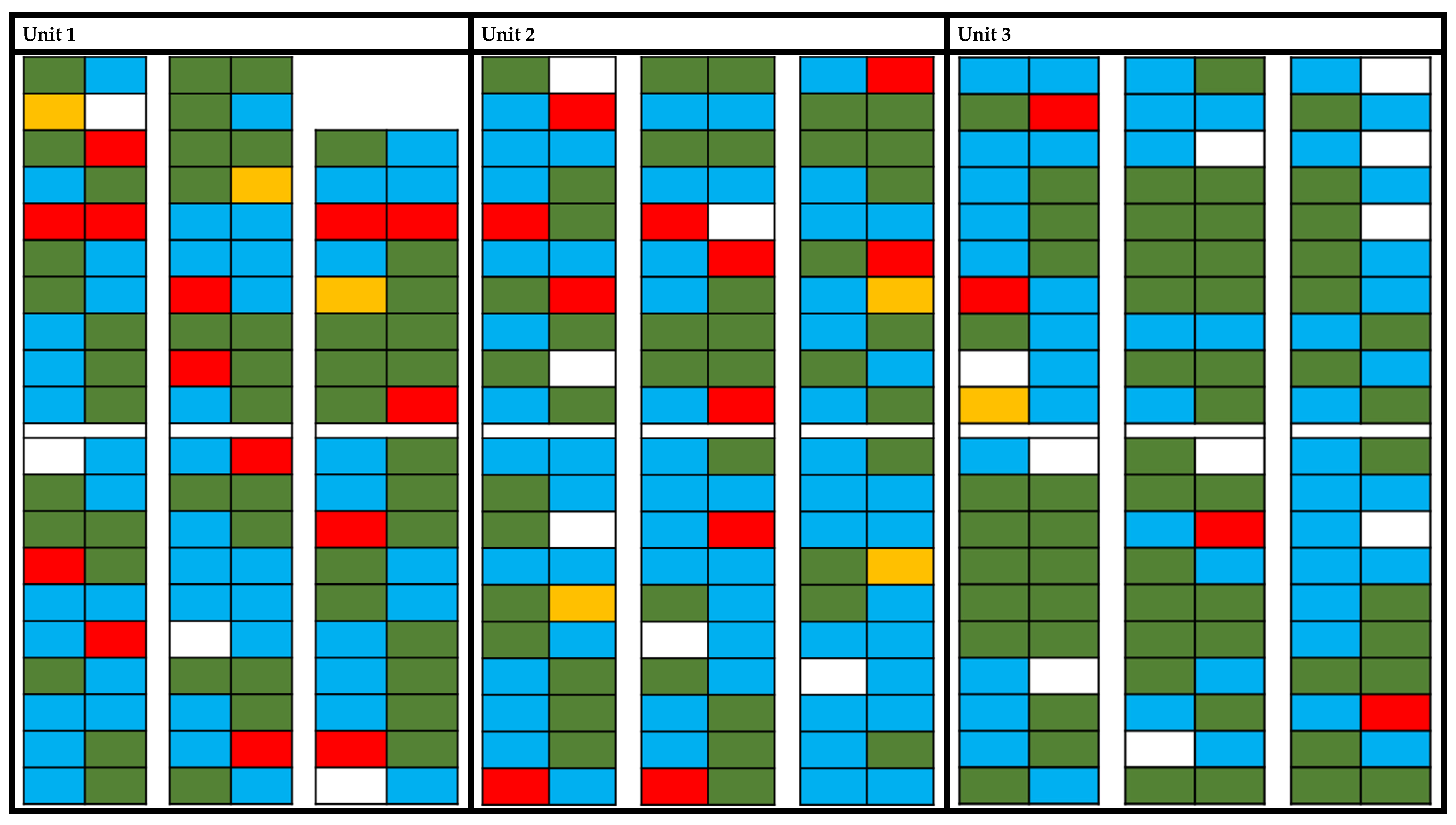
3.2. Profile of PRRSV Shedding in Semen
- Shedding of PRRSV (a): virus detection at least in one semen sample;
- Permanent (p): PRRSV was found in at least the last two semen samples without PRRSV-negative samples between (maximum until 83 DPO);
- No shedding (n): no virus detected in fresh semen samples;
- Intermittent shedding (i): intermittent detection of PRRSV in a minimum of four samples but with negative samples in between.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunney, J.K.; Benfield, D.A.; Rowland, R.R.R. Porcine reproductive and respiratory syndrome virus: An update on an emerging and re-emerging viral disease of swine. Virus Res. 2010, 154, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.J. Diseases of Swine. In Diseases of Swine, 11th ed.; Wiley-Blackwell: Chichester, UK, 2019; pp. 685–708. [Google Scholar]
- Corbellini, L.G.; Schwermer, H.; Presi, P.; Thür, B.; Stärk, K.D.C.; Reist, M. Analysis of national serological surveys for the documentation of freedom from porcine reproductive and respiratory syndrome in Switzerland. Vet. Microbiol. 2006, 118, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, U. Emergence of Porcine Reproductive and Respiratory Syndrome in Sweden: Detection, Response and Eradication. Transbound. Emerg. Dis. 2009, 56, 121–131. [Google Scholar] [CrossRef]
- Lium, B.; Zerihun, A.; Tarpai, A.; Hopp, P. The Surveillance and Control Programme for Specific Virus Infections in Swine Herds in Norway; Norwegian Veterinary Institute: Sentrum, Norway, 2011. [Google Scholar]
- Niederwerder, M.C.; Rowland, R.R.R. Is There a Risk for Introducing Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Through the Legal Importation of Pork? Food Environ. Virol. 2017, 9, 1–13. [Google Scholar] [CrossRef]
- Prieto, C.; Castro, J.M. Porcine reproductive and respiratory syndrome virus infection in the boar: A review. Theriogenology 2005, 63, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Revilla-Fernández, S.; Schmoll, F.; Grossfeld, R.; Griessler, A. Effects on boar semen quality after infection with porcine reproductive and respiratory syndrome virus: A case report. Acta Vet. Scand. 2013, 55, 16. [Google Scholar] [CrossRef]
- Brinton, M.A. ICTV Virus Taxonomy Profile: Arteriviridae 2021. J. Gen. Virol. 2021, 102, 001632. [Google Scholar] [CrossRef] [PubMed]
- Montaner-Tarbes, S.; Del Portillo, H.A.; Montoya, M.; Fraile, L. Key Gaps in the Knowledge of the Porcine Respiratory Reproductive Syndrome Virus (PRRSV). Front. Vet. Sci. 2019, 6, 38. [Google Scholar] [CrossRef]
- Prieto, C.; Garcı, C.; Simarro, I.; Castro, J.M. Temporal localization of porcine reproductive and respiratory syndrome virus in reproductive tissues of experimentally infected boars. Theriogenology 2003, 60, 1505–1514. [Google Scholar] [CrossRef]
- Christopher-Hennings, J.; Holler, L.D.; Benfield, D.A.; Nelson, E.A. Detection and Duration of Porcine Reproductive and Respiratory Syndrome Virus in Semen, Serum, Peripheral Blood Mononuclear Cells, and Tissues from Yorkshire, Hampshire, and Landrace Boars. J. Vet. Diagn. Investig. 2001, 13, 133–142. [Google Scholar] [CrossRef]
- Arruda, A.G. Aerosol Detection and Transmission of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): What Is the Evidence, and What Are the Knowledge Gaps? Viruses 2019, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Nathues, C. An Outbreak of Porcine Reproductive and Respiratory Syndrome Virus in Switzerland Following Import of Boar Semen. Transbound. Emerg. Dis. 2016, 63, e251–e261. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, M.J. Evidence for the transmission of porcine reproductive and respiratory syndrome (PRRS) virus in boar semen. J. Swine Health Prod. 1993, 1, 7–9. [Google Scholar]
- Gradil, C.; Dubuc, C.; Eaglesome, M.D. Porcine reproductive and respiratory syndrome virus: Seminal transmission. Vet. Rec. 1996, 138, 521–522. [Google Scholar] [CrossRef]
- Torremorell, M.; Conroy, P. Case Study: Application of Sequencing to Investigate an Infection in a Boar Stud and the Herds That Received Semen from It. Available online: https://conservancy.umn.edu/bitstream/handle/11299/146010/Torremorell.pdf?sequence=1 (accessed on 1 September 2024).
- Pedersen, K. Virological and Histopathological Findings in Boars Naturally Infected With Porcine Reproductive and Respiratory Syndrome Virus Type 1. Front. Microbiol. 2022, 13, 874498. [Google Scholar] [CrossRef]
- Prieto, C. Semen changes in boars after experimental infection with porcine reproductive and respiratory syndrome (PRRS) virus. Theriogenology 1996, 45, 383–395. [Google Scholar] [CrossRef]
- Nienhoff, H.; Baier, S.; Wettlaufer-Zimmer, U. Certification of a PRRS-unsuspicious status of pig farms. Prakt. Tierarzt 2010, 91, 513–519. [Google Scholar]
- Kittawornrat, A. Porcine reproductive and respiratory syndrome virus (PRRSV) in serum and oral fluid samples from individual boars: Will oral fluid replace serum for PRRSV surveillance? Virus Res. 2010, 154, 170–176. [Google Scholar] [CrossRef]
- Christopher-Hennings, J. Detection of porcine reproductive and respiratory syndrome virus in boar semen by PCR. J. Clin. Microbiol. 1995, 33, 1730–1734. [Google Scholar] [CrossRef]
- Christopher-Hennings, J. Persistence of Porcine Reproductive and Respiratory Syndrome Virus in Serum and Semen of Adult Boars. J. Vet. Diagn. Investig. 1995, 7, 456–464. [Google Scholar] [CrossRef]
- Shin, J.; Torrison, J.; Choi, C.S.; Gonzalez, S.M.; Crabo, B.G.; Molitor, T.W. Monitoring of porcine reproductive and respiratory syndrome virus infection in boars. Vet. Microbiol. 1997, 55, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.L.; Nielsen, J.; Have, P.; Bækbo, P.; Hoff-Jørgensen, R.; Bøtner, A. Examination of virus shedding in semen from vaccinated and from previously infected boars after experimental challenge with porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 1997, 54, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Düngelhoef, A.; Lösken, S.; Beilage, E.G. Antibody reaction in immunologically naïve replacement gilts vaccinated with an attenuated PRRSV live vaccine. Tierärztl. Prax. Ausg. G Großtiere Nutztiere 2014, 42, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Dortmans, J.C.F.M.; Buter, G.J.; Dijkman, R.; Houben, M.; Duinhof, T.F. Molecular characterization of type 1 porcine reproductive and respiratory syndrome viruses (PRRSV) isolated in the Netherlands from 2014 to 2016. PLoS ONE 2019, 14, e0218481. [Google Scholar] [CrossRef] [PubMed]
- Mardassi, H.; Wilson, L.; Mounir, S.; Dea, S. Detection of porcine reproductive and respiratory syndrome virus and efficient differentiation between Canadian and European strains by reverse transcription and PCR amplification. J. Clin. Microbiol. 1994, 32, 2197–2203. [Google Scholar] [CrossRef]
- Schneider-Buehl, L.; Hiller, E.; Akimkin, V.; Hoferer, M.; Sting, R. Comparative ORF and whole genome sequencing analysis of the porcine reproductive and respiratory syndrome virus (PRRSV) in routine samples reveal a recombinant virus strain. Berl. Münch. Tierärztl. Wochenschr. 2020, 133. [Google Scholar] [CrossRef]
- Pesch, S. Etablierung einer Nachweismethode für die Zwei Genotypen von dem Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) und ein Beitrag zu Seiner Molekularen Epidemiologie; Universität Leipzig: Leipzig, Germany, 2003. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rovira, A.; Reicks, D.; Muñoz-Zanzi, C. Evaluation of Surveillance Protocols for Detecting Porcine Reproductive and Respiratory Syndrome Virus Infection in Boar Studs by Simulation Modeling. J. Vet. Diagn. Investig. 2007, 19, 492–501. [Google Scholar] [CrossRef]
- Reicks, D.L.; Munoz-Zanzi, C.; Rossow, K. Sampling of adult boars during early infection with porcine reproductive and respiratory syndrome virus for testing by polymerase chain reaction using a new blood collection technique (blood-swab method). J. Swine Health Prod. 2006, 14, 258–264. [Google Scholar] [CrossRef]
- Christopher-Hennings, J.; Nelson, E.A.; Nelson, J.K.; Benfield, D.A. Effects of a modified-live virus vaccine against porcine reproductive and respiratory syndrome in boars. Am. J. Vet. Res. 1997, 58, 40–45. [Google Scholar] [CrossRef]
- Christopher-Hennings, J.; Dammen, M.; Nelson, E.; Rowland, R.; Oberst, R. Comparison of RNA extraction methods for the detection of porcine reproductive and respiratory syndrome virus from boar semen. J. Virol. Methods 2006, 136, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Wasilk, A. Detection of U.S., Lelystad, and European-Like Porcine Reproductive and Respiratory Syndrome Viruses and Relative Quantitation in Boar Semen and Serum Samples by Real-Time PCR. J. Clin. Microbiol. 2004, 42, 4453–4461. [Google Scholar] [CrossRef]
- Pepin, B.J. Comparison of Specimens for Detection of Porcine Reproductive and Respiratory Syndrome Virus Infection in Boar Studs. Transbound. Emerg. Dis. 2015, 62, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, C.S.; Christiansen, M.G.; Pedersen, K.; Larsen, L.E. Production losses five months after outbreak with a recombinant of two PRRSV vaccine strains in 13 Danish sow herds. Porc. Health Manag. 2020, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Guérin, B.; Pozzi, N. Viruses in boar semen: Detection and clinical as well as epidemiological consequences regarding disease transmission by artificial insemination. Theriogenology 2005, 63, 556–572. [Google Scholar] [CrossRef] [PubMed]
- Rossow, K.D. Porcine Reproductive and Respiratory Syndrome. Vet. Pathol. 1998, 35, 1–20. [Google Scholar] [CrossRef]
- Han, K. Effect of the Modified Live Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Vaccine on European and North American PRRSV Shedding in Semen from Infected Boars. Clin. Vaccine Immunol. 2011, 18, 1600–1607. [Google Scholar] [CrossRef]


| Unit | Pens in Unit | Number of Boars in Individual Pens | Breed (410/DU/PI/TN) | Age 1 (Days) |
|---|---|---|---|---|
| 1 | 116 | 116 | 6/17/81/12 | 660 (226;2059) |
| 2 | 120 | 120 | 3/15/94/8 | 720 (223;2528) |
| 3 | 120 | 118 | 8/8/89/13 | 710 (228;2356) |
| total | 356 | 354 | 17/40/264/33 | 703.5 (223;2528) |
| Profile | Boars/Number of Tests | Tests per Boar 1 |
|---|---|---|
| A | 157/900 | 5.73 (1;17) |
| P | 28/166 | 5.93 (3;9) |
| N | 144/1067 | 7.41 (2;14) |
| I | 7/51 | 7.29 (5;9) |
| total | 336/2184 | 6.50 (1;17) |
| Unit | Profile of PRRSV Shedding in Semen | Total | ||
|---|---|---|---|---|
| A (Shedder) | P (Permanent) | N (No) | I (Intermittent) | |
| 1 | 48 (42.9) | 13 (11.6) | 48 (42.8) | 3 (2.7) |
| 2 | 62 (54.4) | 11 (9.7) | 38 (33.3) | 3 (2.6) |
| 3 | 47 (42.8) | 4 (3.6) | 58 (52.7) | 1 (0.9) |
| 157 (46.8) | 28 (8.4) | 144 (42.8) | 7 (2.0) | |
| Breed | Profile of PRRSV Shedding in Semen | Total | |||
|---|---|---|---|---|---|
| A | P | N | I | ||
| PI | 120 (47.6) | 27 (10.7) | 99 (39.3) | 6 (2.4) | 252 |
| Du | 18 (51.4) | 0 | 17 (48.6) | 0 | 35 |
| 410 | 6 (35.3) | 1 (5.9) | 10 (58.8) | 0 | 17 |
| TN | 13 (40.5) | 0 | 18 (56.2) | 1 (0,3) | 32 |
| total | 157 (46.7) | 28 (8.3) | 144 (44.9) | 7 (2.1) | 336 |
| Breed | Number of Boars | Estimation of Duration of Shedding (Days 1) |
|---|---|---|
| PI | 146 | 37 (2;83) |
| Du | 12 | 30 (9;65) |
| 410 | 6 | 37 (16;71) |
| TN | 14 | 20 (8;49) |
| Total | 178 | 35 (2;83) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aundrup, J.; Lüken, C.; Heenemann, K.; Vahlenkamp, T.W.; Hennig-Pauka, I. An Outbreak of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) in a German Boar Stud: A Retrospective Analysis of PRRSV Shedding in Boar Semen. Vet. Sci. 2024, 11, 557. https://doi.org/10.3390/vetsci11110557
Aundrup J, Lüken C, Heenemann K, Vahlenkamp TW, Hennig-Pauka I. An Outbreak of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) in a German Boar Stud: A Retrospective Analysis of PRRSV Shedding in Boar Semen. Veterinary Sciences. 2024; 11(11):557. https://doi.org/10.3390/vetsci11110557
Chicago/Turabian StyleAundrup, Jakob, Caroline Lüken, Kristin Heenemann, Thomas W. Vahlenkamp, and Isabel Hennig-Pauka. 2024. "An Outbreak of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) in a German Boar Stud: A Retrospective Analysis of PRRSV Shedding in Boar Semen" Veterinary Sciences 11, no. 11: 557. https://doi.org/10.3390/vetsci11110557
APA StyleAundrup, J., Lüken, C., Heenemann, K., Vahlenkamp, T. W., & Hennig-Pauka, I. (2024). An Outbreak of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) in a German Boar Stud: A Retrospective Analysis of PRRSV Shedding in Boar Semen. Veterinary Sciences, 11(11), 557. https://doi.org/10.3390/vetsci11110557






