A Retrospective Report on the Infestation and Distribution of Chiggers on an Endemic Rodent Species (Apodemus latronum) in Southwest China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
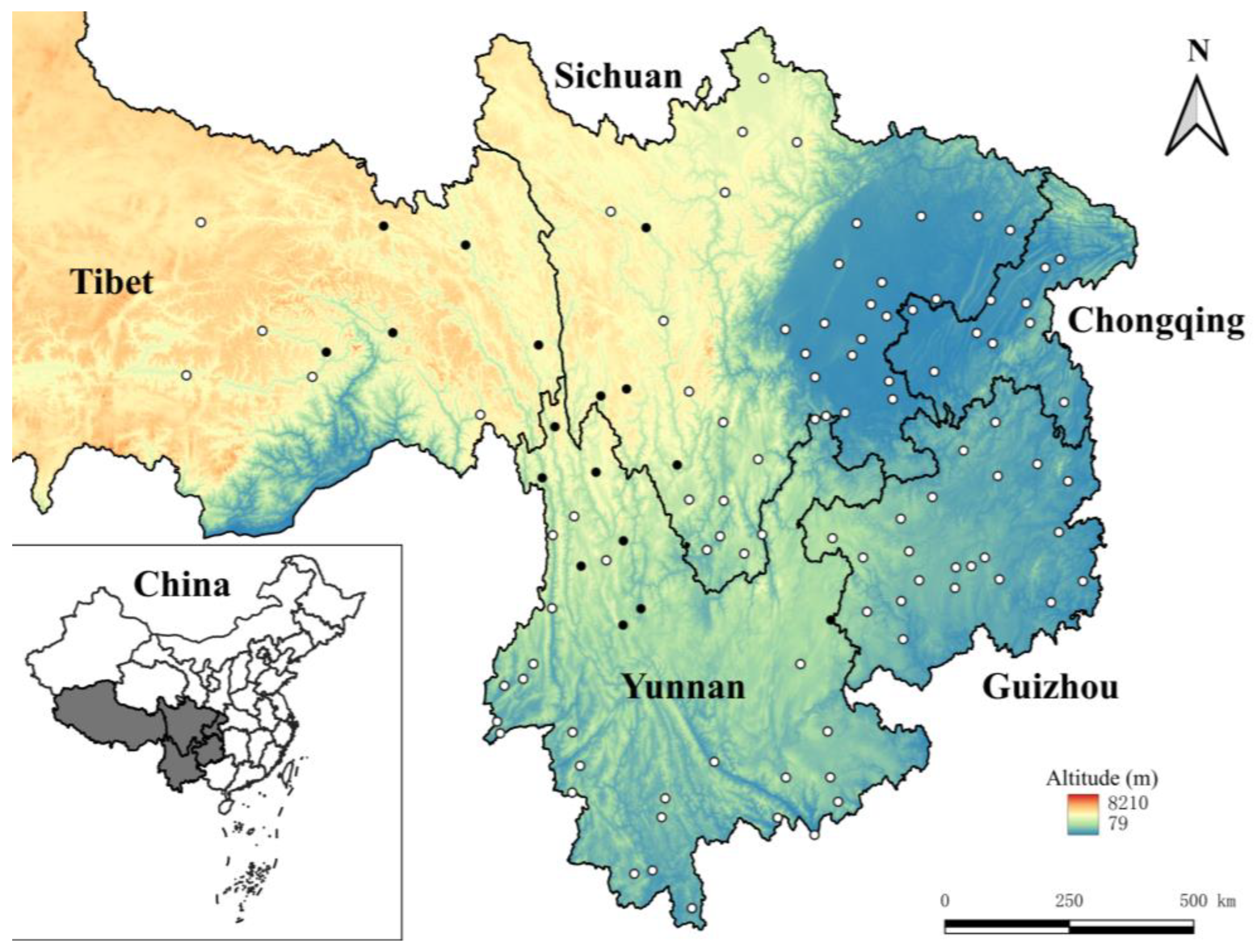
2.1. Collection and Identification of Rodent Hosts and Their Ectoparasitic Chiggers
2.2. Statistic Analysis
2.3. Calculation of Host Relative Fatness
2.4. Measurement of Species Spillover
2.5. Analysis on Risk Factors Influencing Chigger Abundance
3. Results
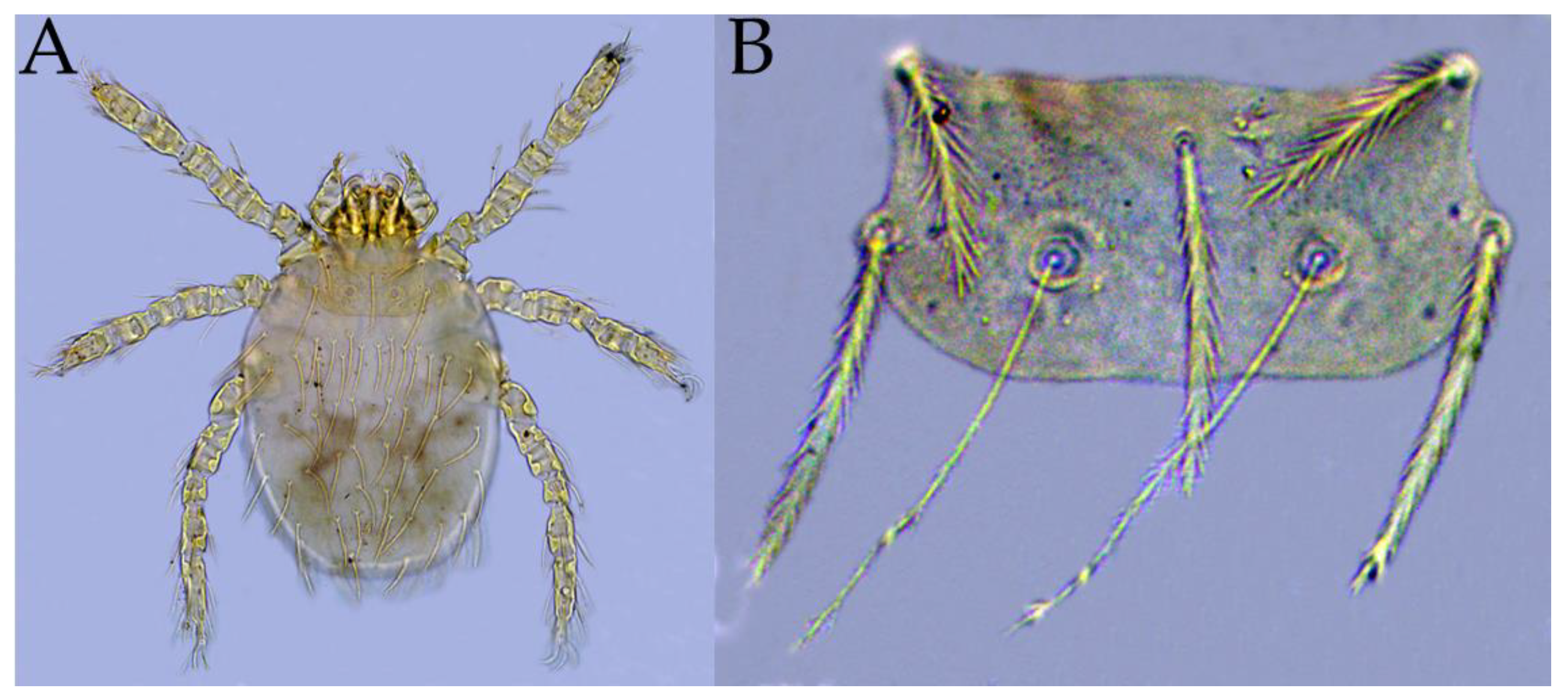
3.1. Species Diversity and Infestation of Chiggers on A. latronum
3.2. Variations of Infestation and Community Indices of Chiggers Along Different Altitude Gradients
3.3. Variations of Infestation and Community Indices of Chiggers with Different Host Statuses
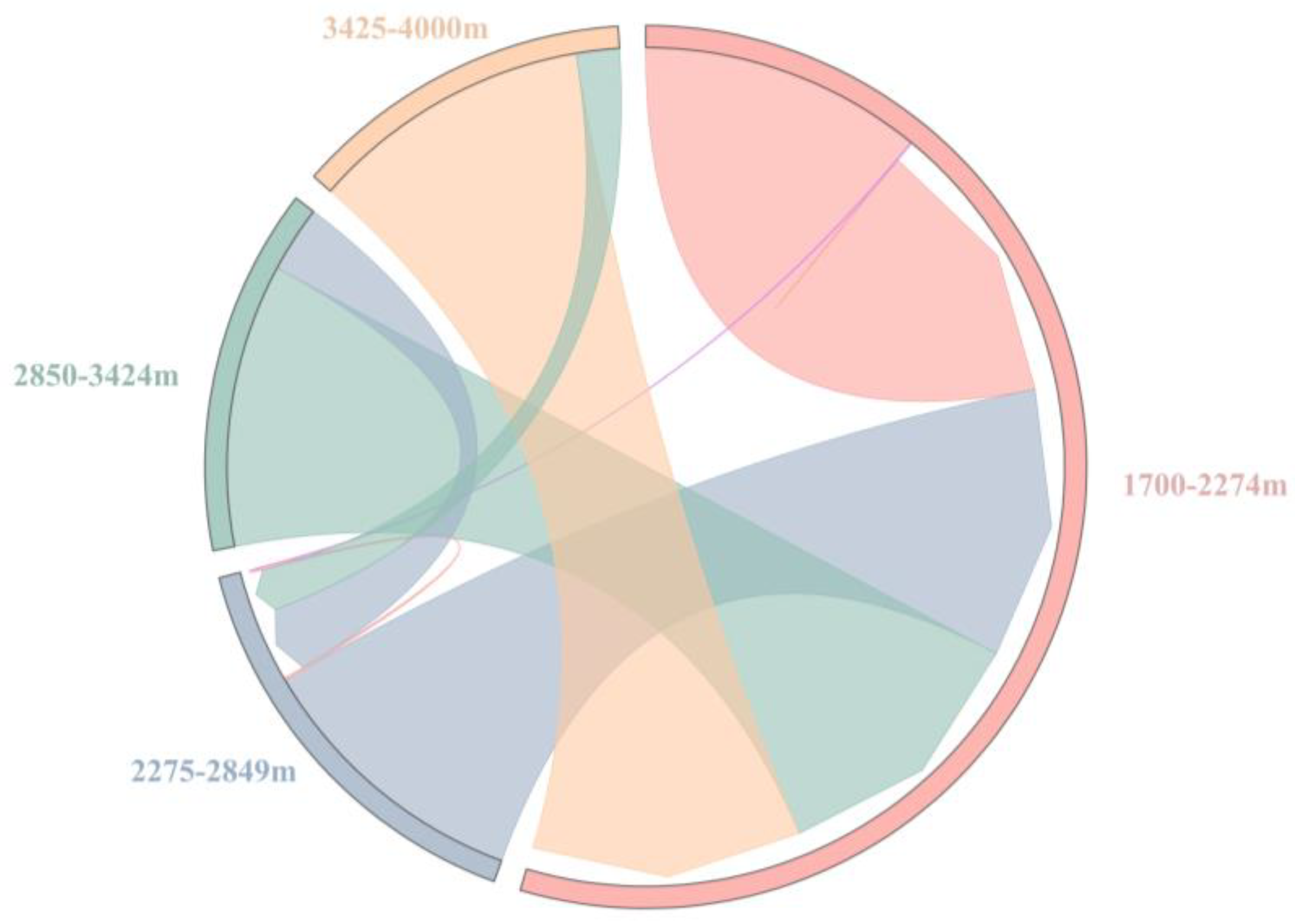
3.4. Species Spillover of Dominant Chiggers
3.5. Risk Factors Influencing Chigger Abundance
4. Discussion
4.1. Geographical Distribution of A. latronum
4.2. Infestation of Chiggers on A. latronum and Medical Significance
4.3. Influence Factors on Chigger Infestation
4.4. Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elliott, I.; Pearson, I.; Dahal, P.; Thomas, N.V.; Roberts, T.; Newton, P.N. Scrub typhus ecology: A systematic review of Orientia in vectors and hosts. Parasit Vectors 2019, 12, 513. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C. Trombiculid Mites of China Studies on Vector and Pathogen of Tsutsugamushi Disease; Guangzhou Technology Press: Guangzhou, China, 1997; pp. 1–570. (In Chinese) [Google Scholar]
- Wu, G.H.; Jiang, Z.K.; Wang, L.; Ding, L.Y.; Mao, C.Q.; Ma, B.Y. Accordance and identification of vector chigger mites of tsutsugamushi disease in China. Chin. J. Hyg. Insect. Equip. 2013, 19, 286–292. (In Chinese) [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, L.; Zhang, Z.; Liu, M.; Xue, Z.; Ma, D.; Sun, X.; Sun, Y.; Zhou, C.; Qin, X.; et al. Detection of a Novel Rickettsia From Leptotrombidium scutellare Mites (Acari: Trombiculidae) From Shandong of China. J. Med. Entomol. 2017, 54, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Ganjeer, T.; Patyal, A.; Shakya, S.; Parkar, S.S.; Shukla, A.; Chandrakar, C.; Naik, V. Rodent borne zoonoses: A brief review. Pharma Innov. 2021, 10, 721–725. [Google Scholar] [CrossRef]
- Blasdell, K.R.; Morand, S.; Laurance, S.G.W.; Doggett, S.L.; Hahs, A.; Trinh, K.; Perera, D.; Firth, C. Rats and the city: Implications of urbanization on zoonoticdisease risk in Southeast Asia. PNAS 2022, 119, e2112341119. [Google Scholar] [CrossRef]
- Moniuszko, H.; Makol, J. Chigger mites (Actinotrichida: Parasitengona, Trombiculidae) of Poland. An updated distribution and hosts. Ann. Parasitol. 2014, 60, 103–117. [Google Scholar]
- Vercammen-Grandjean, P.H.; Benoit, P.L.; Van Mol, J.J. Terrestrial snail a new host for trombiculid larvae. Acta Trop. 1970, 27, 177. [Google Scholar]
- Devasagayam, E.; Dayanand, D.; Kundu, D.; Kamath, M.S.; Kirubakaran, R.; Varghese, G.M. The burden of scrub typhus in India: A systematic review. PLoS Negl. Trop. Dis. 2021, 15, e0009619. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Walker, D.H.; Jupiter, D.; Melby, P.C.; Arcari, C.M. A review of the global epidemiology of scrub typhus. PLoS Negl. Trop. Dis. 2017, 11, e0006062. [Google Scholar] [CrossRef]
- Xin, H.; Sun, J.; Yu, J.; Huang, J.; Chen, Q.; Wang, L.; Lai, S.; Clements, A.C.A.; Hu, W.; Li, Z. Spatiotemporal and demographic characteristics of scrub typhus in Southwest China, 2006-2017: An analysis of population-based surveillance data. Transbound. Emerg. Dis. 2020, 67, 1585–1594. [Google Scholar] [CrossRef]
- Yue, Y.; Ren, D.; Liu, X.; Wang, Y.; Liu, Q.; Li, G. Spatio-temporal patterns of scrub typhus in mainland China, 2006-2017. PLoS Negl. Trop. Dis. 2019, 13, e0007916. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.J.; Wang, Y.J.; Li, G.C.; Li, X.Z.; Wang, J.; Liu, Q.Y. Epidemiological characteristics of scrub typhus in high-incidence areas in the mainland of China, 2006–2018. Dis. Surveill. 2020, 35, 301–306. (In Chinese) [Google Scholar]
- Yue, H.; Liu, S.; Liu, Y.; Zhang, X.; Fan, Z. Mitochondrial genome of the Sichuan field mouse (Apodemus latronum). Mitochondrial DNA A DNA Mapp Seq. Anal. 2016, 27, 1088–1089. [Google Scholar] [CrossRef]
- Zhang, Z.B. Chinese Encyclopedia of Plant Protection Rodent; China Forestry Publishing House: Beijing, China, 2022; pp. 1–508. (In Chinese) [Google Scholar]
- Ge, D.; Feijó, A.; Cheng, J.; Lu, L.; Liu, R.; Abramov, A.V.; Xia, L.; Wen, Z.; Zhang, W.; Shi, L.; et al. Evolutionary history of field mice (Murinae: Apodemus), with emphasis on morphological variation among species in China and description of a new species. Zool. J. Linn. Soc. 2019, 187, 518–534. [Google Scholar] [CrossRef]
- Chen, Z.P.; Liu, R.Q.; Li, C.Y.; Wang, Y.X. Studies on the Chromosomes of three species of wood mice. Zool. Sci. 1996, 17, 347–352. (In Chinese) [Google Scholar]
- Luo, Y.Y.; Liu, S.T.; He, Q.N.; Hong, R.D.; Zhu, J.J.; Ai, Z.Q.; Yin, J.X. Orientia tsutsugamushi Infection in Wild Small Mammals in Western Yunnan Province, China. Pathogens 2023, 12, 128. [Google Scholar] [CrossRef]
- Kaneko, Y. Taxonomic status of Apodemus semotus in Taiwan by morphometrical comparison with A. draco, A. peninsulae and A. latronum in China, Korea and Myanmar. Mamm. Study 2011, 36, 11–22. [Google Scholar] [CrossRef]
- Wang, B.; Yang, X.D. Seed Predation of Apodemus latronum on 18 Plant Species in Northwest Yunnan. Zool. Res. 2007, 28, 389–394. (In Chinese) [Google Scholar]
- Motokawa, M.; Wu, Y.; Harada, M.; Shintaku, Y.; Jiang, X.L.; Li, Y.C. Karyotypes of field mice of the genus Apodemus (Mammalia: Rodentia) from China. Zool. Res. 2018, 39, 348–355. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, X.G.; Song, W.Y.; Lv, Y.; Yin, P.W.; Jin, D.C. Comparison of Chiggers (Acari: Trombiculidae, Leeuwenhoekiidae) on Two Sibling Mouse Species, Apodemus draco and A. ilex (Rodentia: Muridae), in Southwest China. Animals 2023, 13, 1480. [Google Scholar] [CrossRef]
- Fan, Z.X.; Liu, S.Y.; Liu, Y.; Zhang, X.Y.; Yue, B.S. How Quaternary geologic and climatic events in the southeastern margin of the Tibetan Plateau influence the genetic structure of small mammals: Inferences from phylogeography of two rodents, Neodon irene and Apodemus latronum. Genetica 2011, 139, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Veitch, C.R.; Craig, J.L. An evaluation of the efficiency of rodent trapping methods the effect of trap arrangement, cover type, and bait. N. Z. J. Ecol. 1999, 23, 45–51. [Google Scholar]
- Liu, Q.Y.; Fan, R.; Song, W.Y.; Peng, P.Y.; Zhao, Y.F.; Jin, D.C.; Guo, X.G. The Distribution and Host-Association of the Vector Chigger Species Leptotrombidium imphalum in Southwest China. Insects 2024, 15, 504. [Google Scholar] [CrossRef]
- Wixson, S.K.; Smiler, K.L. Chapter 9—Anesthesia and Analgesia in Rodents. In Anesthesia and Analgesia in Laboratory Animals; Kohn, D.F., Wixson, S.K., White, W.J., John Benson, G., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 165–203. [Google Scholar]
- Underwood, W.; Anthony, R. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2020; Volume 2013, pp. 1–121. [Google Scholar] [CrossRef]
- Li, Z.P.; Zhou, H.F.; Yang, Q.G. Medical Acarology; People’s Military Medical Press: Beijing, China, 2006; pp. 1–406. (In Chinese) [Google Scholar]
- Gu, Y.M.; Wang, J.S. Gamasid Mites and Chigger Mites in Guizhou; Guizhou Science and Technology Press: Guiyang, China, 1999; pp. 1–344. (In Chinese) [Google Scholar]
- Gu, Y.M.; Wang, J.S. Chinese Rodents; Fudan University Press: Shanghai, China, 1995. (In Chinese) [Google Scholar]
- Ding, F.; Jiang, W.L.; Guo, X.G.; Fan, R.; Zhao, C.F.; Zhang, Z.W.; Mao, K.Y.; Xiang, R. Infestation and Related Ecology of Chigger Mites on the Asian House Rat (Rattus tanezumi) in Yunnan Province, Southwest China. Korean J. Parasitol. 2021, 59, 377–392. [Google Scholar] [CrossRef]
- Peng, P.Y.; Guo, X.G.; Ren, T.G.; Song, W.Y. Faunal analysis of chigger mites (Acari: Prostigmata) on small mammals in Yunnan province, southwest China. Parasitol. Res. 2015, 114, 2815–2833. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.Y.; Guo, X.G.; Ren, T.G.; Song, W.Y.; Dong, W.G.; Fan, R. Species diversity of ectoparasitic chigger mites (Acari: Prostigmata) on small mammals in Yunnan Province, China. Parasitol. Res. 2016, 115, 3605–3618. [Google Scholar] [CrossRef]
- Vercammen-Grandjean, P.H.; Langston, R.L. The Chigger Mites of the World (Acarina: Trombiculidae & Leeuwenhoekiidae). III. Leptotrombidium Complex; George Williams Hooper Foundation, University of California: San Francisco, CA, USA, 1975; pp. 1–1061. [Google Scholar]
- Liu, Z.; Guo, X.G.; Fan, R.; Zhao, C.F.; Mao, K.Y.; Zhang, Z.W.; Zhao, Y. Ecological analysis of gamasid mites on the body surface of Norway rats (Rattus norvegicus) in Yunnan Province, Southwest China. Biologia 2020, 75, 1325–1336. [Google Scholar] [CrossRef]
- Margolis, L.; Esch, G.; Holmes, J.; Kuris, A.; Schad, G. The use of ecological terms in parasitology (report of an ad hoc committee of the American Society of Parasitologists). J. Parasitol. 1982, 68, 131–133. [Google Scholar] [CrossRef]
- Simpson, E. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Legendre, P. Studying beta diversity: Ecological variation partitioning by multiple regression and canonical analysis. J. Plant Ecol. 2007, 1, 3–8. [Google Scholar] [CrossRef]
- Zhou, J.X.; Guo, X.G.; Song, W.Y.; Zhao, C.F.; Zhang, Z.W.; Fan, R.; Chen, T.; Lv, Y.; Yin, P.W.; Jin, D.C. Preliminary study on species diversity and community characteristics of gamasid mites on small mammals in three parallel rivers area of China. Animals 2022, 12, 3217. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Comunity Ecology Package (Version 2.6-4). Available online: https://github.com/vegandevs/vegan (accessed on 20 September 2022).
- Rogers, P.; Webb, G.P. Estimation of body fat in normal and obese mice. Br. J. Nutr. 1980, 43, 83–86. [Google Scholar] [CrossRef]
- Caldwell, A.E.; Sayer, R.D. Evolutionary considerations on social status, eating behavior, and obesity. Appetite 2019, 132, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.X.; Long, G.; Jin, X.; Guo, Y.W.; Liu, J. Relative Fatness Variation of Anourosorex squamipes of Different Genders, Ages and Seasons. Guizhou Agric. Sci. 2013, 41, 92–94. (In Chinese) [Google Scholar]
- Fang, J.M.; Wang, H.M.; Yu, X.D. Analysis of relative fatness indices of rodents. Acta Ecol. Sin. 1995, 15, 221–222. (In Chinese) [Google Scholar]
- Morris, R.J.; Lewis, O.T.; Godfray, H.C. Experimental evidence for apparent competition in a tropical forest food web. Nature 2004, 428, 310–313. [Google Scholar] [CrossRef]
- Morris, R.J.; Lewis, O.T.; Godfray, H.C. Apparent competition and insect community structure towards a spatial perspective. Ann. Zool. Fenn. 2005, 42, 449–462. [Google Scholar]
- Cappellari, A.; Santoiemma, G.; Sanna, F.; D’Ascenzo, D.; Mori, N.; Lami, F.; Marini, L.J.E.G. Spatio-temporal dynamics of vectors of Xylella fastidiosa subsp. pauca across heterogeneous landscapes. Entomol. Gen. 2022, 42, 515–521. [Google Scholar] [CrossRef]
- Nardi, D.; Marini, L. Role of abandoned grasslands in the conservation of spider communities across heterogeneous mountain landscapes. Agr. Ecosyst. Env. 2021, 319, 107526. [Google Scholar] [CrossRef]
- Dormann, C.F.; Fruend, J.; Gruber, B.; Dormann, M.C.F. Package ‘Bipartite’. Available online: https://github.com/biometry/bipartite (accessed on 20 September 2022).
- Cutler, D.R.; Edwards, T.C., Jr.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random forests for classification in ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Rigatti, S.J. Random forest. J. Insur. Med. 2017, 47, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X.; Zhou, Y.; Liu, D.; Wang, H. The dominant factors and influence of urban characteristics on land surface temperature using random forest algorithm. Sustain. Cities Soc. 2022, 79, 103722. [Google Scholar] [CrossRef]
- Correa Ayram, C.A.; Mendoza, M.E.; Etter, A.; Pérez Salicrup, D.R. Anthropogenic impact on habitat connectivity: A multidimensional human footprint index evaluated in a highly biodiverse landscape of Mexico. Ecol. Indic. 2017, 72, 895–909. [Google Scholar] [CrossRef]
- Sanderson, E.W.; Jaiteh, M.; Levy, M.A.; Redford, K.H.; Wannebo, A.V.; Woolmer, G. The Human Footprint and the Last of the Wild: The human footprint is a global map of human influence on the land surface, which suggests that human beings are stewards of nature, whether we like it or not. BioScience 2002, 52, 891–904. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, W.; Li, J.; Wang, W. Evaluation of the Vegetation Coverage Resilience in Areas Damaged by the Wenchuan Earthquake Based on MODIS-EVI Data. Sensors 2017, 17, 259. [Google Scholar] [CrossRef] [PubMed]
- Yan, E.; Wang, G.; Lin, H.; Xia, C.; Sun, H. Phenology-based classification of vegetation cover types in Northeast China using MODIS NDVI and EVI time series. Int. J. Remote Sens. 2015, 36, 489–512. [Google Scholar] [CrossRef]
- Mu, H.; Li, X.; Wen, Y.; Huang, J.; Du, P.; Su, W.; Miao, S.; Geng, M. A global record of annual terrestrial Human Footprint dataset from 2000 to 2018. Sci. Data 2022, 9, 176. [Google Scholar] [CrossRef]
- Myatt, M.; Guevarra, E. Zscorer: Child Anthropometry Z-Score Calculator; J R Foundation: Vienna, Austria, 2019; p. 1. [Google Scholar] [CrossRef]
- Archer, E. rfPermute: Estimate Permutation p-Values for Random Forest Importance Metrics (2013). J. R Package Version 2018, 2. [Google Scholar] [CrossRef]
- Revelle, W.; Revelle, M.W. Package ‘psych’. J Compr. R Arch. Netw. 2015, 337, 161–165. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Traub, R.; Wisseman, C.L., Jr. The ecology of chigger-borne rickettsiosis (scrub typhus). J. Med. Entomol. 1974, 11, 237–303. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.Y.; Walker, D.H.; Yu, S.R.; Liu, Q.H. Epidemiology and ecology of rickettsial diseases in the People’s Republic of China. Rev. Infect Dis. 1987, 9, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Guo, X.G.; Ren, T.G.; Zhang, L.; Fan, R.; Zhao, C.F.; Zhang, Z.W.; Mao, K.Y.; Huang, X.B.; Qian, T.J. Infestation and distribution of chigger mites on Chevrieri’s field mouse (Apodemus chevrieri) in Southwest China. Int. J. Parasitol. Parasites Wildl. 2022, 17, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Guo, X.G.; Ren, T.G.; Zhang, L.; Fan, R.; Zhao, C.F.; Zhang, Z.W.; Mao, K.Y.; Huang, X.B.; Qian, T.J. A Report of Chigger Mites on the Striped Field Mouse, Apodemus agrarius, in Southwest China. Korean J. Parasitol. 2021, 59, 625–634. [Google Scholar] [CrossRef]
- Fielding, E.; Isacks, B.; Barazangi, M.; Duncan, C. How flat is Tibet? Geology 1994, 22, 163–167. [Google Scholar] [CrossRef]
- Li, W.J.; Peng, M.C.; Higa, M.; Tanaka, N.; Matsui, T.; Tang, C.Q.; Ou, X.K.; Zhou, R.W.; Wang, C.Y.; Yan, H.Z. Effects of climate change on potential habitats of the cold temperate coniferous forest in Yunnan province, southwestern China. J. Mountain Sci. 2016, 13, 1411–1422. [Google Scholar] [CrossRef]
- Xu, S.; Cheng, B.; Huang, Z.F.; Shen, C.Y. An Investigation on the Thermal Environment of Residential Courtyards in the Cold Area of Western Sichuan Plateau. Buildings 2022, 12, 49. [Google Scholar] [CrossRef]
- Chaisiri, K.; Gill, A.C.; Stekolnikov, A.A.; Hinjoy, S.; McGarry, J.W.; Darby, A.C.; Morand, S.; Makepeace, B.L. Ecological and microbiological diversity of chigger mites, including vectors of scrub typhus, on small mammals across stratified habitats in Thailand. Anim Microbiome 2019, 1, 18. [Google Scholar] [CrossRef]
- Alkathiry, H.; Al-Rofaai, A.; Ya’cob, Z.; Cutmore, T.S.; Mohd-Azami, S.N.I.; Husin, N.A.; Lim, F.S.; Koosakulnirand, S.; Mahfodz, N.H.; Ishak, S.N.; et al. Habitat and Season Drive Chigger Mite Diversity and Abundance on Small Mammals in Peninsular Malaysia. Pathogens 2022, 11, 1087. [Google Scholar] [CrossRef]
- Morand, S. (macro-) Evolutionary ecology of parasite diversity: From determinants of parasite species richness to host diversification. Int. J. Parasitol. Parasites Wildl. 2015, 4, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Wulandhari, S.A.; Paladsing, Y.; Saesim, W.; Charoennitiwat, V.; Sonthayanon, P.; Kumlert, R.; Morand, S.; Sumruayphol, S.; Chaisiri, K. High prevalence and low diversity of chigger infestation in small mammals found in Bangkok Metropolitan parks. Med. Vet. Entomol. 2021, 35, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Xiang, R.; Guo, X.G.; Ren, T.G.; Zhao, C.F.; Fan, R.; Zhang, Z.W.; Mao, K.Y.; Peng, P.Y.; Huang, X.B.; Qian, T.J. Infestation and distribution of mites on the Yunnan red-backed vole (Eothenomys miletus) in Yunnan Province of southwest China between 2001 and 2015. Biologia 2021, 77, 61–68. [Google Scholar] [CrossRef]
- Kataranovski, M.; Mirkov I Fau—Belij, S.; Belij S Fau—Popov, A.; Popov A Fau—Petrovic, Z.; Petrovic Z Fau—Gaci, Z.; Gaci Z Fau—Kataranovski, D.; Kataranovski, D. Intestinal helminths infection of rats (Ratus norvegicus) in the Belgrade area (Serbia): The effect of sex, age and habitat. Parasite J. La Société Française Parasitol. 2011, 18, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Poulin, R. Species richness of parasite assemblages: Evolution and patterns. Annu. Rev. Ecol. Evol. S 1997, 28, 341–358. [Google Scholar] [CrossRef]
- Huang, Z.C. Pollen nutrition affects honey bee stress resistance. Terrestrial. Arthropod Rev. 2012, 5, 175–189. [Google Scholar] [CrossRef]
- PG, G.; Parry, H. Discussion on nutrition and resistance to infection. P Roy Soc. Med. 1948, 41, 323–328. [Google Scholar] [CrossRef]
- Qing, Y.; Zhu-Guo, M.; Liang, C. A Preliminary Analysis of the Relationship between Precipitation Variation Trends and Altitude in China. Atmos. Ocean. Sci. Lett. 2015, 4, 41–46. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, X.; Smettem, K.; Wang, T. Climate and land use influences on changing spatiotemporal patterns of mountain vegetation cover in southwest China. Ecol. Indic. 2021, 121, 107193. [Google Scholar] [CrossRef]
- Dirk Van Peenen, P.F.; Lien, J.-C.; Santana, F.J.; Richard, S. Correlation of chigger abundance with temperature at a hyperendemic focus of scrub typhus. J. Parasitol. 1976, 62, 653–654. [Google Scholar] [CrossRef]
- Sasa, M. Biology of chiggers. Annu. Rev. Entomol. 1961, 6, 221–244. [Google Scholar] [CrossRef]
- Stanko, M.; Fricova, J.; Miklisova, D.; Khokhlova, I.S.; Krasnov, B.R. Environment-related and host-related factors affecting the occurrence of lice on rodents in Central Europe. Parasitology 2015, 142, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Lareschi, M.; Krasnov, B.R. Determinants of ectoparasite assemblage structure on rodent hosts from South American marshlands: The effect of host species, locality and season. Med. Vet. Entomol. 2010, 24, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K.; Eichert, U.; Bogdziewicz, M.; Rychlik, L. Differentiation of flea communities infesting small mammals across selected habitats of the Baltic coast, central lowlands, and southern mountains of Poland. Parasitol. Res. 2014, 113, 1725–1734. [Google Scholar] [CrossRef]
- Zajac, Z.; Kulisz, J.; Wozniak, A. Flea Communities on Small Rodents in Eastern Poland. Insects 2020, 11, 894. [Google Scholar] [CrossRef]
- Krawczyk, A.I.; van Duijvendijk, G.L.A.; Swart, A.; Heylen, D.; Jaarsma, R.I.; Jacobs, F.H.H.; Fonville, M.; Sprong, H.; Takken, W. Effect of rodent density on tick and tick-borne pathogen populations: Consequences for infectious disease risk. Parasit. Vectors 2020, 13, 34. [Google Scholar] [CrossRef]




| Dominant Chigger Species | Constituent Ratio (Cr)% | Prevalence (PM)% | Mean Abundance (MA) | Mean Intensity (MI) |
|---|---|---|---|---|
| Leptotrombidium bayanense | 29.69 | 2.40 | 0.55 | 23.08 |
| Neotrombicula tongtianhensis | 8.15 | 1.00 | 0.15 | 15.20 |
| Leptotrombidium rupestre | 6.86 | 1.40 | 0.13 | 9.14 |
| Leptotrombidium yongshengense | 5.47 | 1.00 | 0.10 | 10.20 |
| Altitude Gradients (m) | Community Indices of Chigger Community | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. Hosts | PM (%) | MA | MI | S | H′ | D | E | Cody Indices | |
| 1700–2274 | 30 | 33.33 | 4.83 | 14.50 | 3 | 0.50 | 0.75 | 0.45 | 4.5 |
| 2275–2849 | 100 | 1.61 | 0.08 | 5.00 | 6 | 1.61 | 0.24 | 0.90 | 18 |
| 2850–3424 | 200 | 27.50 | 2.23 | 8.09 | 32 | 2.76 | 0.09 | 0.80 | 22 |
| 3425–4000 | 171 | 22.81 | 2.63 | 11.51 | 31 | 1.72 | 0.39 | 0.50 | |
| Different Statuses of the Host A. latronum | Community Indices | Infestation Indices | ||||||
|---|---|---|---|---|---|---|---|---|
| No. Hosts | S | H′ | E | D | PM (%) | MA | MI | |
| Female | 182 | 26 | 2.82 | 0.86 | 0.09 | 18.68 | 1.11 | 5.94 |
| Male | 316 | 55 | 2.62 | 0.65 | 0.09 | 20.25 | 2.31 | 11.42 |
| Adult | 330 | 35 | 2.26 | 0.63 | 0.13 | 38.28 ** | 5.11 ** | 13.35 |
| Juvenile | 172 | 19 | 1.94 | 0.66 | 0.17 | 12.63 ** | 0.97 ** | 7.67 |
| Poor nutrition | 128 | 21 | 2.01 | 0.66 | 0.26 | 37.80 ** | 5.13 ** | 13.58 |
| Good nutrition | 94 | 34 | 2.24 | 0.64 | 0.22 | 13.68 ** | 0.99 ** | 7.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.-Y.; Guo, X.-G.; Fan, R.; Song, W.-Y.; Peng, P.-Y.; Zhao, Y.-F.; Jin, D.-C. A Retrospective Report on the Infestation and Distribution of Chiggers on an Endemic Rodent Species (Apodemus latronum) in Southwest China. Vet. Sci. 2024, 11, 547. https://doi.org/10.3390/vetsci11110547
Liu Q-Y, Guo X-G, Fan R, Song W-Y, Peng P-Y, Zhao Y-F, Jin D-C. A Retrospective Report on the Infestation and Distribution of Chiggers on an Endemic Rodent Species (Apodemus latronum) in Southwest China. Veterinary Sciences. 2024; 11(11):547. https://doi.org/10.3390/vetsci11110547
Chicago/Turabian StyleLiu, Qiao-Yi, Xian-Guo Guo, Rong Fan, Wen-Yu Song, Pei-Ying Peng, Ya-Fei Zhao, and Dao-Chao Jin. 2024. "A Retrospective Report on the Infestation and Distribution of Chiggers on an Endemic Rodent Species (Apodemus latronum) in Southwest China" Veterinary Sciences 11, no. 11: 547. https://doi.org/10.3390/vetsci11110547
APA StyleLiu, Q.-Y., Guo, X.-G., Fan, R., Song, W.-Y., Peng, P.-Y., Zhao, Y.-F., & Jin, D.-C. (2024). A Retrospective Report on the Infestation and Distribution of Chiggers on an Endemic Rodent Species (Apodemus latronum) in Southwest China. Veterinary Sciences, 11(11), 547. https://doi.org/10.3390/vetsci11110547






