The Effects of Adding Hempseed Cake on Sperm Traits, Body Weight, Haematological and Biochemical Parameters in Rabbit Males
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals
2.3. Semen Collection and Handling
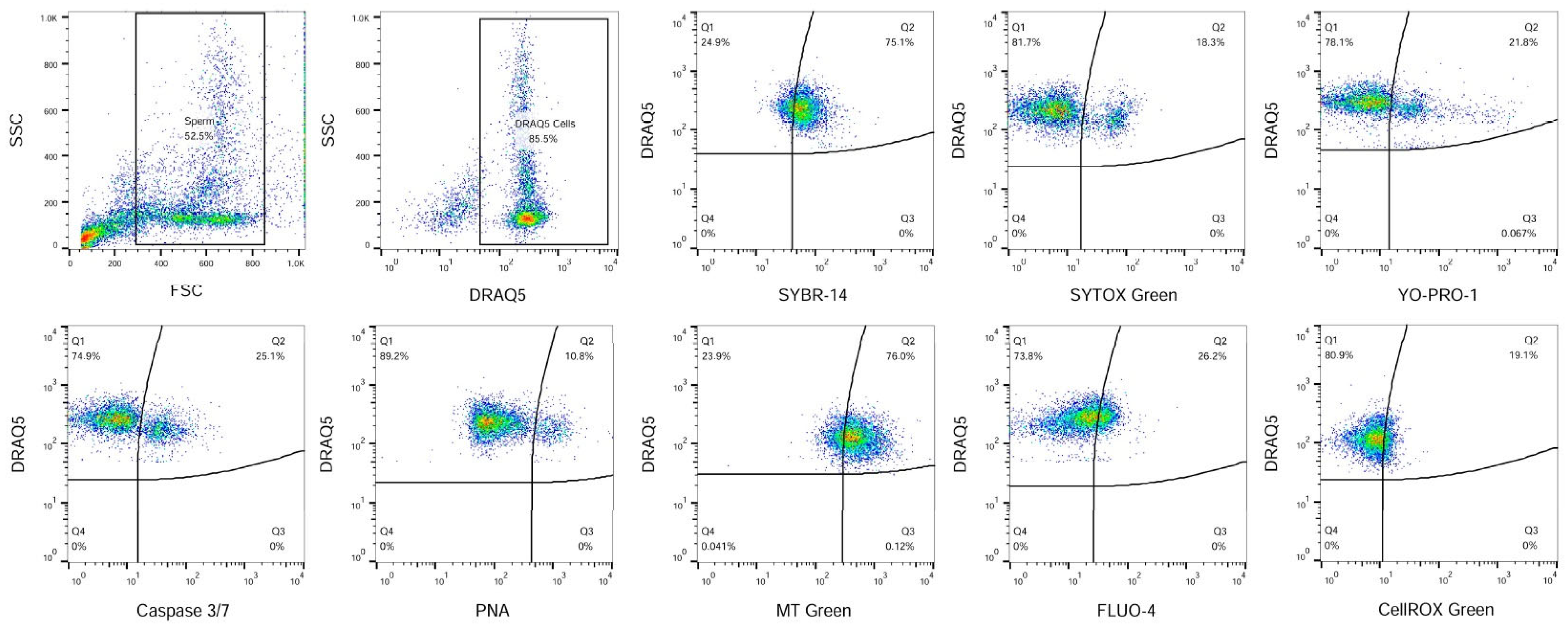
2.4. Measurement of Spermatozoa Characteristics Using Flow Cytometry
2.5. Measurement of Haematological and Biochemical Parameters
- Blood and serum sampling
- Haematology analysis
- Serum biochemistry analysis
2.6. Statistical Analysis
3. Results
3.1. Effect of Hempseed Cake on Weight Gains and Semen Quality
3.2. Haematology and Serum Biochemistry Observations
4. Discussion
4.1. Effect of Hempseed Cake on Weight Gain
4.2. Effect of Hempseed Cake on Semen Quality
4.3. Haematological and Biochemical Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinotti, L.; Manoni, M.; Fumagalli, F.; Rovere, N.; Luciano, A.; Ottoboni, M.; Ferrari, L.; Cheli, F.; Djuragic, O. Reduce, reuse, recycle for foodwaste: A second life for fresh-cut leafy salad crops in animal diets. Animals 2020, 10, 1082. [Google Scholar] [CrossRef] [PubMed]
- Vastolo, A.; Calabró, S.; Liotta, L.; Musco, N.; Di Rosa, A.R.; Cutrignelli, M.I.; Chiofalo, B. In vitro fermentation and chemical characteristics of mediterranean by-products for swine nutrition. Animals 2019, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Serrapica, F.; Masucci, F.; Raffrenato, E.; Sannino, M.; Vastolo, A.; Barone, C.M.A.; Di Francia, A. High fiber cakes from mediterranean multiporpuse oilseeds as protein sources for ruminant. Animals 2019, 9, 918. [Google Scholar] [CrossRef]
- Rakita, S.; Banjac, V.; Djuragic, O.; Cheli, F.; Pinotti, L. Soybean molasses in animal nutrition. Animals 2021, 11, 514. [Google Scholar] [CrossRef]
- Bailoni, L.; Bacchin, E.; Trocino, A.; Arango, S. Hemp (Cannabis sativa L.) seed and co-products inclusion in diets for dairy ruminants: A review. Animals 2021, 11, 856. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- The Commission of the European Communities. Council Regulation (EC) No 1124/2008. (2008) of 12 November 2008 amending Regulations (EC) No 795/2004, (EC) No 796/2004 and (EC) No (1973/2004, as regards the hemp varieties eligible for direct payments under Council Regulation (EC) No 1782/2003. Off. J. Eur. Union, 14.11.2008. L 303/7.
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A Review of Hemp as Food and Nutritional Supplement. Cannabis Cannabinoid Res. 2021, 6, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The Seed of Industrial Hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Zhao, J.; Wang, W.; Griffin, J.; Li, Y.; Bean, S.; Tilley, M.; Wang, D. Hempseed as a nutritious and healthy human food or animal feed source: A review. Int. J. Food Sci. Technol. 2021, 56, 530–543. [Google Scholar] [CrossRef]
- Matthäus, B.; Brühl, L. Virgin hemp seed oil: An interesting niche product. Eur. J. Lipid Sci. Technol. Stud. 2008, 110, 655–661. [Google Scholar] [CrossRef]
- Lebas, F.; Coudert, P.; de Rochambeau, H.; Thébault, R.G. The Rabbit Husbandry, Health and Reproduction; FAO Animal Pruduction and Health Series; FAO: Rome, Italy, 1997; Volume 21, 205p, ISSN 1010-9021. [Google Scholar]
- Davies Rees, R.; Davies Rees, J.A.E. Rabbit gastrointestinal physiology. Vet. Clin. Exot. Anim. Pract. 2003, 6, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Foote, R.H.; Carney, E.W. The rabbit as a model for reproductive and developmental toxicity studies. Reprod. Toxicol. 2000, 14, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Alvariño, J.M.R. Reproductive performance of male rabbits. In Proceedings of the 7th World Rabbit Congress, Valencia, Spain, 4–7 July 2000; Blasco, A., Ed.; World Rabbit Science, 2000; Volume 8a, pp. 13–35. [Google Scholar]
- Pollesel, M.; Tassinari, M.; Frabetti, A.; Fornasini, D.; Cavallini, D. Effect of does parity order on litter homogeneity parameters. Ital. J. Anim. Sci. 2020, 19, 1188–1194. [Google Scholar] [CrossRef]
- Mocé, E.; Vicente, J.S.; Lavara, R.; Viudes-de-Castro, M.P.; López, M.; Bolet, G. Characteristics of fresh semen from eight rabbit breeds. In Proceedings of the 9th Annual Conference ESDAR, Belgrade, Serbia, 12–14 September 2024; Vázquez, J.M., Ed.; Reproduction in Domestic Animals, 2005; Volume 40, p. 191. [Google Scholar]
- Castellini, C.; Cardinali, R.; Dal Bosco, A.; Minelli, A.; Camici, O. Lipid composition of the main fractions of rabbit semen. Theriogenology 2006, 65, 703–712. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Wiseman, J.; Villamide, M.J.; De Blas, C.; Carabano, M.J.; Carabano, R.M. Prediction of the digestible energy and digestibility of gross energy of feed for rabbits. 1. Individual classes of feeds. Anim. Feed. Sci. Technol. 1992, 39, 27–83. [Google Scholar] [CrossRef]
- Baláži, A.; Sirotkin, A.V.; Makovický, P.; Chrastinová, Ľ.; Makarevich, A.; Chrenek, P. Effect of Green Tea on Weight Gain and Semen Quality of Rabbit Males. Vet. Sci. 2022, 9, 321. [Google Scholar] [CrossRef]
- Stastnik, O.; Pavlata, L.; Mrkvicova, E. The Milk Thistle Seed Cakes and Hempseed Cakes are Potential Feed for Poultry. Animals 2020, 10, 1384. [Google Scholar] [CrossRef]
- Jacobson, K.J.; Verret, E.G.; Runyan, C.L.; Kinman, L.A.; Owsley, W.F.; Muir, J.P.; Smith, W.B. Hempseed meal as a feedstuff in the diet of growing meat rabbits. Appl. Anim. Sci. 2023, 39, 125–132. [Google Scholar] [CrossRef]
- Kanbur, G.; Göçmen, R.A. Comparative study on the effects of use hemp seed oil substitute to soybean oil in growing quail diets. Turk. J. Agric. Food Sci. Technol. 2021, 9, 1390–1394. [Google Scholar]
- Konca, Y.; Cimen, B.; Yalcin, H.; Kaliber, M.; Beyzi, S.B. Effect of Hempseed (Cannabis sativa sp.) Inclusion to the Diet on Performance, Carcass and Antioxidative Activity in Japanese Quail (Coturnix coturnix japonica). Korean J. Food Sci. Anim. Resour. 2014, 34, 141–150. [Google Scholar] [CrossRef]
- Formelová, Z.; Chrastinová, Ľ.; Chrenková, M.; Polačiková, M.; Rajský, M.; Bučko, O.; Kalafová, A.; Kováčik, A.; Baxa, S.; Mlyneková, Z.; et al. Hempseed cake in rabbit nutrition: Livestock performances, quality of meat, digestibility of nutrients and animal health status. Slovak J. Anim. Sci. 2023, 56, 3–15. [Google Scholar] [CrossRef]
- Hazekamp, A.; Fischedick, J.T.; Díez, M.L.; Lubbe, A.; Ruhaak, L.R. Chemistry of Cannabis. In Comprehensive Natural Products II Chemistry Biology; Mander, L., Lui, H.W., Eds.; Elsevier: Oxford, UK, 2010; Volume 3, pp. 1033–1084. [Google Scholar]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Gibb, D.J.; Shah, M.A.; Mir, P.S.; McAllister, T.A. Effect of full-fat hemp seed on performance and tissue fatty acids of feedlot cattle. Can. J. Anim. Sci. 2005, 85, 223–230. [Google Scholar] [CrossRef]
- Hessle, A.; Eriksson, M.; Nadeau, E.; Turner, T.; Johansson, B. Cold-pressed hempseed cake as a protein feed for growing cattle. Acta Agric. Scand Sect. A 2008, 58, 136–145. [Google Scholar] [CrossRef]
- Karlsson, L.; Finell, M.; Martinsson, K. Effects of increasing amounts of hempseed cake in the diet of dairy cows on the production and composition of milk. Animal 2010, 4, 1854–1860. [Google Scholar] [CrossRef]
- Mourot, J.; Guillevic, M. Effect of introducing hemp oil into feed on the nutritional quality of pig meat. OCL 2015, 22, D612. [Google Scholar] [CrossRef]
- Cozma, A.; Andrei, S.; Pintea, A.; Miere, D.; Filip, L.; Loghin, F.; Ferlay, A. Effect of hemp seed oil supplementation on plasma lipid profile, liver function, milk fatty acid, cholesterol, and vitamin A concentrations in Carpathian goats. Czech J. Anim. Sci. 2015, 60, 289–301. [Google Scholar] [CrossRef]
- Ran, T.; Xu, Z.; Yang, W.; Liu, D.; Wu, D. Partially substituting alfalfa hay with hemp forage in the diet of goats improved feed efficiency, ruminal fermentation pattern and microbial profiles. Anim. Nutr. 2024, 17, 49–60. [Google Scholar] [CrossRef]
- Mierlita, D. Fatty acid profile and health lipid indices in the raw milk of ewes grazing part-time and hemp seed supplemen-tation of lactating ewes. S. Afr. J. Anim. Sci. 2016, 46, 237–246. [Google Scholar] [CrossRef]
- Mierlița, D. Effects of diets containing hemp seeds or hemp cake on fatty acid composition and oxidative stability of sheep milk. S. Afr. J. Anim. Sci. 2018, 48, 504. [Google Scholar] [CrossRef]
- Skřivan, M.; Englmaierová, M.; Taubner, T.; Skřivanová, E. Effects of Dietary Hemp Seed and Flaxseed on Growth Performance, Meat Fatty Acid Compositions, Liver Tocopherol Concentration and Bone Strength of Cockerels. Animals 2020, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Bahari Mo Bahari Me Khorshidi, K.J.; Abdullahpour, R.; Gharahveysi, S. Effect of using medicinal cannabis seed (Nigella sativa) in diets on performance and parameters of broiler chickens Ross 308. Adv. Environ. Biol. 2014, 8, 931–935. [Google Scholar]
- Castellini, C.; Mattioli, S.; Signorini, C.; Cotozzolo, E.; Noto, D.; Moretti, E.; Brecchia, G.; Dal Bosco, A.; Belmonte, G.; Durand, T.; et al. Effect of Dietary n—3 Source on Rabbit Male Reproduction. Oxidative Med. Cell. Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Attia, Y.A.; Hamed, R.S.; Bovera, F.; Abd El-Hamid, A.E.H.E.; Al-Harthi, M.A.; Shahba, H.A. Semen quality, antioxidant status and reproductive performance of rabbits bucks fed milk thistle seeds and rosemary leaves. Anim. Reprod. Sci. 2017, 184, 178–186. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Okehi, M.C.; Jiwuba, P.C. Effect of phytobiotic (turmeric) supplementation on semen and blood characteristics of rabbits. Comp. Clin. Pathol. 2017, 26, 817–822. [Google Scholar] [CrossRef]
- El-Ratel, I.T.; Attia, K.A.H.; El-Raghi, A.A.; Fouda, S.F. Relief of the negative effects of heat stress on semen quality, reproductive efficiency and oxidative capacity of rabbit bucks using different natural antioxidants. Anim. Biosci. 2021, 34, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L.; Johnson, L.A. Viability Assessment of Mammalian Sperm Using SYBR-14 and Propidium Iodide. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Merino, O.; Dumorné, K.; Leidy, S.V.; Figueroa, E.; Valdebenito, I.; Farías, J.G.; Risopatrón, J. Short-term storage sperm of coho salmon (Oncorhynchus kisutch) at 4 °C: Effect of sperm: Extender dilution ratios and antioxidant butyl-hydroxytoluene (BHT) on sperm function. Cryobiology 2020, 95, 44–50. [Google Scholar] [CrossRef]
- Peña, F.J.; Saravia, F.; Johannisson, A.; Walgren, M.; Rodríguez-Martínez, H. A new and simple method to evaluate early membrane changes in frozen-thawed boar spermatozoa. Int. J. Androl. 2005, 28, 107–114. [Google Scholar] [CrossRef]
- Tao, J.; Critser, E.S.; Critser, J.K. Evaluation of mouse sperm acrosomal status and viability by flow cytometry. Mol. Reprod. Dev. 1993, 36, 183–194. [Google Scholar] [CrossRef]
- Garner, D.L.; Thomas, C.A.; Joerg, H.W.; DeJarnette, J.M.; Marshall, C.E. Fluorometric Assessments of Mitochondrial Function and Viability in Cryopreserved Bovine Spermatozoa. Biol. Reprod. 1997, 57, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Chávez, J.C.; De La Vega-Beltráln, J.L.; José, O.; Torres, P.; Nishigaki, T.; Treviño, C.L.; Darszon, A. Acrosomal alkalization triggers Ca 2+ release and acrosome reaction in mammalian spermatozoa. J. Cell. Physiol. 2018, 233, 4735–4747. [Google Scholar] [CrossRef]
- Ichikawa, T.; Oeda, T.; Ohmori, H.; Schill, W.B. Reactive oxygen species influence the acrosome reaction but not acrosin activity in human spermatozoa. Int. J. Androl. 1999, 22, 37–42. [Google Scholar] [CrossRef]
- Escada-Rebelo, S.; Mora, F.; Sousa, A.; Almeida-Santos, T.; Paiva, A.; Ramalho-Santos, J. Fluorescent probes for the detection of reactive oxygen species in human spermatozoa. Asian J. Androl. 2020, 22, 465. [Google Scholar]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Schuel, H.; Goldstein, E.; Mechoulam, R.; Zimmerman, A.M.; Zimmerman, S. Anandamide (arachidonylethanolamide), a brain cannabinoid receptor agonist, reduces sperm fertilizing capacity in sea urchins by inhibiting the acrosome reaction. Proc. Natl. Acad. Sci. USA 1994, 91, 7678–7682. [Google Scholar] [CrossRef]
- Schuel, H.; Burkman, L.J.; Lippes, J.; Crickard, K.; Forester, E.; Piomelli, D.; Giuffrida, A. N-Acylethanolamines in human reproductive fluids. Chem. Phys. Lipids 2002, 121, 211–227. [Google Scholar] [CrossRef]
- Rossato, M.; Ion Popa, F.; Ferigo, M.; Clari, G.; Foresta, C. Human Sperm Express Cannabinoid Receptor Cb 1, the Activation of Which Inhibits Motility, Acrosome Reaction, and Mitochondrial Function. J. Clin. Endocrinol. Metab. 2005, 90, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Juráček, M.; Vašeková, P.; Massányi, P.; Kováčik, A.; Bíro, D.; Šimko, M.; Gálik, B.; Rolinec, M.; Hanušovský, O.; Kolláthová, R.; et al. The Effect of Dried Grape Pomace Feeding on Nutrients Digestibility and Serum Biochemical Profile of Wethers. Agriculture 2021, 11, 1194. [Google Scholar] [CrossRef]
- Kovacik, A.; Gasparovic, M.; Tvrda, E.; Tokarova, K.; Kovacikova, E.; Rolinec, M.; Rumanova, L.; Capcarova, M.; Galik, B. Effects of humic acid diet on the serum biochemistry and oxidative status markers in pheasants. Veterinární Medicína 2020, 65, 258–268. [Google Scholar] [CrossRef]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I. A review on the use of agro-industrial CO-products in animals’ diets. Ital. J. Anim. Sci. 2022, 21, 577–594. [Google Scholar] [CrossRef]
- Winders, T.M.; Holman, D.B.; Schmidt, K.N.; Luecke, S.M.; Smith, D.J.; Neville, B.W.; Dahlen, C.R.; Swanson, K.C.; Amaz, S. Feeding hem-pseed cake alters the bovine gut, respiratory and reproductive microbiota. Sci. Rep. 2023, 13, 8121. [Google Scholar] [CrossRef] [PubMed]
- Šalavardić, Ž.K.; Novoselec, J.; Đidara, M.; Steiner, Z.; Ćavar, S.; Modić Šabić, A.; Antunović, Z. Effect of dietary hempseed cake on milk performance and haemato-chemicals in lactating Alpine dairy goats. Animal 2021, 15, 100255. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hempseed in food industry: Nutritional value, health benefits, and industrial appli-cations. Compr. Rev. Food Sci. Food Saf. 2020, 19, 282–308. [Google Scholar] [CrossRef]
- Callaway, J.C.; Pate, D.W. Hempseed Oil. In Gourmet and Health-Promoting Specialty Oils; RA, M., Kamal-Eldin, A., Eds.; Academic Press and AOCS Press: Kuopio, Finland, 2009; pp. 185–213. [Google Scholar]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enteral. Nutr. 2015, 39, 18–32. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P. Omega-3 polyunsaturated fatty acids and human health outcomes. BioFactors 2009, 35, 266–272. [Google Scholar] [CrossRef]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Ruxton, C.H.S.; Reed, S.C.; Simpson, M.J.A.; Millington, K.J. The health benefits of omega-3 polyunsaturated fatty acids: A review of the evidence. J. Hum. Nutr. Diet. 2004, 17, 449–459. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Lou, H.; Fan, P. Chemical constituents of hemp (Cannabis sativa L.) seed with potential an-ti-neuroinflammatory activity. Phytochem. Lett. 2018, 23, 57–61. [Google Scholar] [CrossRef]
- Prociuk, M.; Edel, A.; Gavel, N.; Deniset, J.; Ganguly, R.; Austria, J.; Ander, B.; Lukas, A.; Pierce, G. The effects of dietary hempseed on cardiac ischemia/reperfusion injury in hypercholesterolemic rabbits. Exp. Clin. Cardiol. 2006, 11, 198–205. [Google Scholar]
- Li, X.Y.; Liu, Y.H.; Wang, B.; Chen, C.Y.; Zhang, H.M.; Kang, J.X. Identification of a sustainable two-plant diet that effectively prevents age-related metabolic syndrome and extends lifespan in aged mice. J. Nutr. Biochem. 2018, 51, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Palade, L.M.; Habeanu, M.; Marin, D.E.; Chedea, V.S.; Pistol, G.C.; Grosu, I.A.; Gheorghe, A.; Ropota, M.; Taranu, I. Effect of Dietary Hemp Seed on Oxidative Status in Sows during Late Gestation and Lactation and Their Offspring. Animals 2019, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Vodolazska, D.; Lauridsen, C. Effects of dietary hemp seed oil to sows on fatty acid profiles, nutritional and immune status of piglets. J. Anim. Sci. Biotechnol. 2020, 11, 28. [Google Scholar]
- Rakha, A.; Rasheed, H.; Altemimi, A.B.; Tul-Muntaha, S.; Fatima, I.; Butt, M.S.; Aadil, R.M. Tapping the Nutraceutical Potential of Industrial Hemp against Arthritis and Diabetes-A Comprehensive Review. Food Biosci. 2024, 59, 104195. [Google Scholar] [CrossRef]
- Munteanu, C.; Mihai, M.; Dulf, F.; Ona, A.; Muntean, L.; Ranga, F.; Papuc, I. Biochemical changes induced by the administration of Cannabis sativa seeds in diabetic Wistar rats. Nutrients 2023, 15, 2944. [Google Scholar] [CrossRef]
- Cai, L.; Wu, S.; Jia, C.; Cui, C.; Sun-Waterhouse, D. Active peptides with hypoglycemic effect obtained from hemp (Cannabis sativa L.) protein through identification, molecular docking, and virtual screening. Food Chem. 2023, 429, 136912. [Google Scholar] [CrossRef]
- Altman, A.; Kent-Dennis, C.; Klotz, J.; McLeod, K.; Vanzant, E.; Harmon, D. Review: Utilizing industrial hemp (Cannabis sativa L.) by-products in livestock rations. Anim. Feed. Sci. Technol. 2024, 307, 115850. [Google Scholar] [CrossRef]

| Feed Ingredient Concentration (g/kg) | Control (Basal Diet) | E5 (with 5% Hempseed Cake) | EG10 (with 10% Hempseed Cake) |
|---|---|---|---|
| Lucerne meal | 360 | 342 | 342 |
| Extracted sunflower meal | 55 | 52 | 52 |
| Extracted rapeseed meal | 55 | 52 | 52 |
| Hempseed cake | 0 | 50 | 100 |
| Wheat bran | 90 | 85 | 81 |
| Oats | 130 | 124 | 117 |
| Malt sprouts | 150 | 143 | 135 |
| Maize | 50 | 47.5 | 45 |
| Sodium chloride | 3 | 3 | 3 |
| Mineral–vitamin mixture * | 17 | 17 | 17 |
| Barley grains | 80 | 75 | 72 |
| Limestone | 10 | 10 | 9 |
| Nutrients g·kg−1; * mg·kg−1 | Hempseed Cake | Basal Diet | Basal Diet with 5% Hempseed Cake | Basal Diet with 10% Hempseed Cake |
|---|---|---|---|---|
| Crude protein | 331.12 | 152.54 | 165.39 | 162.79 |
| Crude fibre | 286.17 | 156.63 | 162.39 | 153.99 |
| Fat | 113.87 | 28.13 | 33.97 | 33.05 |
| Ash | 58.04 | 77.31 | 80.14 | 84.16 |
| Starch | 10.85 | 188.29 | 189.00 | 181.62 |
| Organic matter | 841.78 | 810.21 | 817.41 | 821.71 |
| ADF | 325.33 | 145.53 | 182.19 | 185.43 |
| NDF | 382.52 | 334.36 | 334.08 | 328.44 |
| Calcium | 1.91 | 11.74 | 9.06 | 12.06 |
| Phosphorus | 7.57 | 5.89 | 6.34 | 6.82 |
| Magnesium (Mg) | 4.95 | 2.86 | 2.18 | 2.55 |
| Sodium (Na) | 0.23 | 1.31 | 1.61 | 1.23 |
| Potassium (K) | 8.60 | 10.17 | 10.37 | 9.82 |
| Copper (Cu) * | 23.03 | 22.32 | 23.14 | 21.18 |
| Iron (Fe) * | 150.16 | 503.63 | 624.49 | 577.00 |
| ME MJ·kg−1 | 9.22 | 10.74 | 11.17 | 10.99 |
| Weight Gain per Week (g) | Total Weight Gains (g) | |
|---|---|---|
| C | 271 ± 249 | 727 ± 555 |
| E5 | 177 ± 131 | 533 ± 378 |
| E10 | 166 ± 124 | 498 ± 305 |
| Sperm Concentration (×109) | D0 | D30 | D60 | D90 |
|---|---|---|---|---|
| C (n = 20) | 1.47 ± 0.83 | 1.25 ± 0.32 | 0.82 ± 0.39 | 1.75 ± 0.69 |
| E5 (n = 20) | 1.55 ± 0.56 | 0.97 ± 0.32 | 0.89 ± 0.42 | 1.26 ± 0.70 |
| E10 (n = 20) | 1.12 ± 0.63 | 1.29 ± 0.98 | 0.97 ± 0.67 | 1.63 ± 1.28 |
| Sperm Motility (%) | D0 | D30 | D60 | D90 |
|---|---|---|---|---|
| C (n = 20) | 50.0 ± 10.0 | 62.3 ± 11.7 | 49.7 ± 24.6 | 77.6 ± 5.25 |
| E5 (n = 20) | 49.8 ± 17.7 | 52.8 ± 12.8 | 54.4 ± 16.4 | 64.5 ± 17.8 |
| E10 (n = 20) | 50.5 ± 10.6 | 69.2 ± 13.5 | 65.6 ± 21.8 | 77.2 ± 11.3 |
| Sperm Progressive Motility (%) | D0 | D30 | D60 | D90 |
|---|---|---|---|---|
| C (n = 20) | 31.1 ± 12.3 | 42.7 ± 17.4 | 33.5 ± 20.6 | 60.3 ± 9.1 |
| E5 (n = 20) | 31.2 ± 20.1 | 28.3 ± 19.5 | 37.2 ± 19.8 | 51.3 ± 16.8 |
| E10 (n = 20) | 33.2 ± 19.8 | 46.6 ± 22.4 | 44.6 ± 26.1 | 55.6 ± 24.7 |
| Viability | Apoptosis-like Changes | Acrosomal Status | MA | Capacitation | ROS | |||
|---|---|---|---|---|---|---|---|---|
| Sample | SYBR-14+ | SYTOX Green+ | YoPro-1+ | Caspase 3/7+ | PNA+ | MT Green+ | FLUO-4+ | CellROX+ |
| C | 83.8 ± 7.7 | 15.0 ± 3.4 | 17.7 ± 3.6 | 21.7 ± 2.93 | 11.8 ± 3.6 | 57.6 ± 16.5 | 16.6 ± 8.6 | 16.8 ± 13.8 |
| E5 | 79.9 ± 1.3 | 21.7 ± 11. | 26.4 ± 6.2 | 25.0 ± 10.2 | 19.5 ± 7.6 | 58.4 ± 11.7 | 15.1 ± 8.5 | 19.3 ± 13.6 |
| E10 | 83.4 ± 1.7 | 16.6 ± 0.8 | 16.5 ± 3.5 | 10.8 ± 1.65 | 10.3 ± 5.4 | 58.3 ± 17.3 | 15.9 ± 6.1 | 23.9 ± 15.3 |
| Haematological Parameter | C (10) | E5 (10) | E10 (10) | p-Value | Reference Ranges 1 |
|---|---|---|---|---|---|
| WBC (109·L−1) | 9.56 ± 1.72 | 8.37 ± 2.98 | 9.59 ± 1.23 | ns | 6–12 |
| LYM (109·L−1) | 2.38 ± 1.96 | 3.82 ± 2.37 | 4.00 ± 1.42 | ns | 1.6–10.6 |
| MON (109·L−1) | 0.48 ± 0.31 | 0.38 ± 0.38 | 0.45 ± 0.14 | ns | 0.05–0.5 |
| NEU (109·L−1) | 6.70 ± 3.08 | 4.18 ± 3.13 | 5.15 ± 1.93 | ns | 1–9.4 |
| LYM (%) | 27.6 ± 23.13 | 47.6 ± 23.7 | 42.4 ± 16.9 | ns | 30–85 |
| MON (%) | 4.85 ± 2.44 | 4.10 ± 2.40 | 4.70 ± 1.70 | ns | 1–4 |
| NEU (%) | 67.5 ± 21.8 | 48.2 ± 22.4 | 52.8 ± 16.0 | ns | 20–60 |
| RBC (1012·L−1) | 6.59 ± 0.53 | 7.58 ± 1.64 | 7.24 ± 0.63 | ns | 4–7 |
| HGB (g·dL−1) | 13.3 ± 0.81 | 12.9 ± 2.38 | 14.1 ± 0.67 | ns | 8–15 |
| HCT (%) | 39.64 ± 2.19 | 44.29 ± 8.79 | 41.83 ± 1.96 | ns | - |
| MCV (fL) | 60.4 ± 2.92 | 58.7 ± 2.31 | 58.0 ± 3.60 | ns | 58–67 |
| MCH (pg) | 20.2 ± 0.80 | 17.9 ± 4.56 | 19.5 ± 1.27 | ns | 17.1–23.5 |
| MCHC (g·dL−1) | 33.5 ± 0.48 | 29.6 ± 7.95 | 33.6 ± 0.18 | ns | 29–37 |
| RDWc (%) | 15.4 ± 0.49 | 16.1 ± 1.27 | 15.6 ± 0.55 | ns | - |
| RDWs (fL) | 37.8 ± 1.32 | 38.1 ± 2.10 | 36.5 ± 1.23 | ns | - |
| PLT (109·L−1) | 245 ± 96.3 | 264 ± 64.9 | 278 ± 47.6 | ns | 250–650 |
| MPV (fl) | 6.03 ± 0.40 | 5.98 ± 0.28 | 6.18 ± 0.25 | ns | - |
| PCT (%) | 0.14 ± 0.05 | 0.16 ± 0.04 | 0.17 ± 0.03 | ns | - |
| PDWc (%) | 29.1 ± 1.67 | 29.1 ± 1.60 | 29.9 ± 1.14 | ns | - |
| PDWS (fl) | 7.12 ± 0.93 | 7.14 ± 0.94 | 7.50 ± 0.63 | ns | - |
| Biochemistry Parameter | C (10) | E5 (10) | E10 (10) | p-Value | Reference Ranges 1 |
|---|---|---|---|---|---|
| K (mM·L−1) | 5.16 ± 0.68 | 5.33 ± 0.65 | 5.30 ± 0.45 | ns | 3.5–6.9 |
| Cl (mM·L−1) | 102 ± 1.45 | 102 ± 2.42 | 99.6 ± 4.36 | ns | - |
| Ca (mM·L−1) | 3.53 ± 0.18 | 3.65 ± 0.18 | 3.79 ± 0.22 | ns | 2.7–3.5 |
| P (mM·L−1) | 4.90 ± 0.91 | 4.84 ± 1.14 | 5.33 ± 0.45 | ns | 1.3–2.1 |
| Mg (mM·L−1) | 1.03 ± 0.09 | 1.10 ± 0.11 | 1.12 ± 0.13 | ns | - |
| Albumin (g·L−1) | 39.8 ± 8.26 | 45.2 ± 4.01 | 46.6 ± 4.90 | ns | 36–48 |
| ALP (U·L−1) | 114 ± 44.6 | 139 ± 20.7 | 120 ± 41.3 | ns | 41–92 |
| ALT (U·L−1) | 45.4 ± 14.2 | 60.3 ± 45.3 | 67.4 ± 22.6 | ns | 45–80 |
| AST (U·L−1) | 39.61 ± 18.1 | 37.88 ± 8.68 | 45.56 ± 6.41 | ns | 35–130 |
| Bili (µM·L−1) | 1.27 ± 0.76 | 1.58 ± 0.57 | 1.46 ± 0.69 | ns | 0–12 |
| Creat (µM·L−1) | 62.6 ± 8.32 | 71.9 ± 23.2 | 60.2 ± 12.4 | ns | 44.2–221 |
| Glu (mM·L−1) | 7.86 ± 0.48 a | 8.56 ± 0.59 b | 8.38 ± 0.50 a,b | 0.0368 | 4.1–8.6 |
| Chol. (mM·L−1) | 0.90 ± 0.40 | 1.10 ± 0.30 | 1.15 ± 0.49 | ns | 0.3–2.1 |
| TP (g·L−1) | 70.3 ± 15.2 | 74.3 ± 14.9 | 74.8 ± 12.7 | ns | 54–75 |
| TG (mM·L−1) | 0.65 ± 0.32 | 0.79 ± 0.33 | 0.82 ± 0.34 | ns | - |
| Uric acid (µM·L−1) | 3.56 ± 2.47 | 2.61 ± 2.20 | 4.19 ± 1.14 | ns | - |
| Globulin (g·L−1) | 30.5 ± 16.2 | 29.0 ± 12.4 | 28.2 ± 9.34 | ns | 16–29 |
| Alb/Glob ratio | 1.77 ± 1.15 | 1.81 ± 0.72 | 1.85 ± 0.75 | ns | - |
| Haematological Parameter | C (10) | E5 (10) | E10 (10) | p-Value | Reference Ranges 1 |
|---|---|---|---|---|---|
| WBC (109·L−1) | 7.64 ± 1.14 | 8.25 ± 2.38 | 10.374.71 | ns | 6–12 |
| LYM (109·L−1) | 2.56 ± 0.96 | 2.96 ± 2.05 | 3.11 ± 1.38 | ns | 1.6–10.6 |
| MON (109·L−1) | 0.42 ± 0.13 | 0.51 ± 0.18 | 0.29 ± 0.22 | ns | 0.05–0.5 |
| NEU (109·L−1) | 4.65 ± 1.15 | 4.78 ± 3.33 | 6.84 ± 4.64 | ns | 1–9.4 |
| LYM (%) | 33.78±13.45 | 39.2 ± 31.16 | 34.83±21.36 | ns | 30–85 |
| MON (%) | 5.48 ± 1.21 | 6.48 ± 2.60 | 4.23 ± 2.04 | ns | 1–4 |
| NEU (%) | 60.7 ± 12.9 | 54.4 ± 31.8 | 60.9 ± 20.2 | ns | 20–60 |
| RBC (1012·L−1) | 6.18 ± 0.19 | 6.29 ± 0.59 | 5.92 ± 0.81 | ns | 4–7 |
| HGB (g·dL−1) | 13.3 ± 0.37 | 12. ± 1.14 | 12.1 ± 1.18 | ns | 8–15 |
| HCT (%) | 37.2 ± 1.02 | 36.4 ± 3.18 | 34.8 ± 2.97 | ns | - |
| MCV (fL) | 60.2 ± 2.22 | 57.9 ± 2.35 | 59.3 ± 3.20 | ns | 58–67 |
| MCH (pg) | 21.5 ± 0.82 | 20.2 ± 0.92 | 20.5 ± 0.97 | ns | 17.1–23.5 |
| MCHC (g·dL−1) | 35.6 ± 0.49 | 34.9 ± 0.59 | 34.7 ± 1.04 | ns | 29–37 |
| RDWc (%) | 15.6 ± 0.53 | 15.4 ± 0.69 | 16.1 ± 0.97 | ns | - |
| RDWs (fL) | 38.3 ± 1.47 a | 36.1 ± 0.69 b | 38.3 ± 2.30 a,b | 0.0200 | - |
| PLT (109·L−1) | 218. ± 58.3 | 221 ± 48.5 | 218 ± 74.7 | ns | 250–650 |
| MPV (fl) | 5.65 ± 0.21 | 5.90 ± 0.36 | 5.98 ± 0.24 | ns | - |
| PCT (%) | 0.12 ± 0.03 | 0.12 ± 0.04 | 0.13 ± 0.04 | ns | - |
| PDWc (%) | 27.8 ± 1.71 | 28.8 ± 1.15 | 29.4 ± 1.52 | ns | - |
| PDWS (fl) | 6.50 ± 0.79 | 6.97 ± 0.57 | 7.28 ± 0.79 | ns | - |
| Biochemical Parameter | C (10) | E5 (10) | E10 (10) | p-Value | Reference Ranges 1 |
|---|---|---|---|---|---|
| K (mM·L−1) | 4.37 ± 0.37 | 4.54 ± 0.51 | 4.48 ± 0.36 | ns | 3.5–6.9 |
| Cl (mM·L−1) | 104. ± 1.77 | 104 ± 2.26 | 105 ± 1.02 | ns | - |
| Ca (mM·L−1) | 3.68 ± 0.11 | 3.58 ± 0.29 | 3.47 ± 0.12 | ns | 2.7–3.5 |
| P (mM·L−1) | 1.34 ± 0.14 a | 1.55 ± 0.15 b | 1.55 ± 0.13 b | 0.0072 | 1.3–2.1 |
| Mg (mM·L−1) | 1.06 ± 0.05 | 1.04 ± 0.18 | 1.06 ± 0.05 | ns | - |
| Albumin (g·L−1) | 47.7 ± 1.76 | 46.9 ± 5.48 | 46.1 ± 2.78 | ns | 36–48 |
| ALP (U·L−1) | 77.0 ± 28.5 | 73.9 ± 25.4 | 69.7 ± 34.2 | ns | 41–92 |
| ALT (U·L−1) | 51.6 ± 13.5 | 42.7 ± 12.2 | 54.2 ± 13.7 | ns | 45–80 |
| AST (U·L−1) | 28.8 ± 6.91 | 27.645.75 | 29.3 ± 7.08 | ns | 35–130 |
| Bili (µM·L−1) | 0.85 ± 0.23 | 0.73 ± 0.22 | 0.93 ± 0.25 | ns | 0–12 |
| Creat (µM·L−1) | 54.6 ± 10.5 | 57.1 ± 14.5 | 52.1 ± 9.03 | ns | 44.2–221 |
| Glu (mM·L−1) | 7.88 ± 0.32 | 8.03 ± 0.79 | 8.02 ± 0.76 | ns | 4.1–8.6 |
| Chol. (mM·L−1) | 0.68 ± 0.28 | 0.77 ± 0.76 | 0.69 ± 0.19 | ns | 0.3–2.1 |
| TP (g·L−1) | 69.0 ± 4.08 | 70.3 ± 4.26 | 66.3 ± 2.01 | ns | 54–75 |
| TG (mM·L−1) | 1.23 ± 1.09 | 1.03 ± 0.74 | 1.33 ± 0.51 | ns | - |
| Uric acid (µM·L−1) | 3.28 ± 3.03 | 2.19 ± 2.18 | 1.71 ± 1.17 | ns | - |
| Globulin (g·L−1) | 21.3 ± 4.97 | 23.5 ± 8.62 | 20.2 ± 2.57 | ns | 16–29 |
| Alb/Glob ratio | 2.34 ± 0.52 | 2.24 ± 0.79 | 2.32 ± 0.39 | ns | - |
| Haematological Parameter | C (10) | E5 (10) | E10 (10) | p-Value | Reference Ranges 1 |
|---|---|---|---|---|---|
| WBC (109·L−1) | 10.0 ± 2.83 | 7.67 ± 2.79 | 10.4 ± 2.12 | ns | 6–12 |
| LYM (109·L−1) | 4.62 ± 1.08 | 4.77 ± 2.35 | 4.15 ± 1.66 | ns | 1.6–10.6 |
| MON (109·L−1) | 0.43 ± 0.33 | 0.42 ± 0.19 | 0.51 ± 0.16 | ns | 0.05–0.5 |
| NEU (109·L−1) | 4.95 ± 3.37 | 2.48 ± 2.44 | 5.62 ± 2.87 | ns | 1–9.4 |
| LYM (%) | 49.8 ± 18.9 | 62.6 ± 19.5 | 42.7 ± 19.8 | ns | 30–85 |
| MON (%) | 4.52 ± 4.05 | 5.59 ± 1.59 | 4.90 ± 0.97 | ns | 1–4 |
| NEU (%) | 45.6 ± 40.0 | 31.8 ± 19.4 | 52.4 ± 18.9 | ns | 20–60 |
| RBC (1012·L−1) | 6.26 ± 6.21 | 6.53 ± 0.32 | 6.55 ± 0.67 | ns | 4–7 |
| HGB (g·dL−1) | 13.1 ± 13.05 | 13.2 ± 0.75 | 12.70 ± 1.51 | ns | 8–15 |
| HCT (%) | 39.9 ± 39.0 | 39.6 ± 2.47 | 37.6 ± 4.08 | ns | - |
| MCV (fL) | 63.8 ± 63.0 a | 60.8 ± 2.49 a | 57.4 ± 2.77 b | 0.0003 | 58–67 |
| MCH (pg) | 20.9 ± 20.90 a | 20.2 ± 0.76 a,b | 19.4 ± 1.34 b | 0.0300 | 17.1–23.5 |
| MCHC (g·dL−1) | 32.82 ± 32.65 | 33.23 ± 0.87 | 33.78 ± 1.08 | ns | 29–37 |
| RDWc (%) | 15.4 ± 15.4 | 15.4 ± 1.24 | 15.5 ± 0.94 | ns | - |
| RDWs (fL) | 40.3 ± 3.94 a | 36.8 ± 1.56 a,b | 36.0 ± 2.03 b | 0.0175 | - |
| PLT (109·L−1) | 301 ± 58.3 | 232 ± 67.6 | 323 ± 101 | ns | 250–650 |
| MPV (fl) | 5.82 ± 0.25 | 5.78 ± 0.25 | 5.97 ± 0.46 | ns | - |
| PCT (%) | 0.18 ± 0.04 a,b | 0.14 ± 0.04 a | 0.19 ± 0.05 b | 0.0495 | - |
| PDWc (%) | 28.4 ± 1.54 | 28.6 ± 1.39 | 29.4 ± 1.33 | ns | - |
| PDWS (fl) | 6.75 ± 0.72 | 6.86 ± 0.67 | 7.23 ± 0.69 | ns | - |
| Biochemical Parameter | C (10) | E5 (10) | E10 (10) | p-Value | Reference Ranges 1 |
|---|---|---|---|---|---|
| K (mM·L−1) | 5.00 ± 0.65 | 4.45 ± 0.44 | 4.63 ± 0.36 | ns | 3.5–6.9 |
| Cl (mM·L−1) | 104 ± 1.29 a,b | 107 ± 2.07 a | 102 ± 3.6 b | 0.0030 | - |
| Ca (mM·L−1) | 4.57 ± 1.98 | 3.67 ± 0.16 | 3.60 ± 0.13 | ns | 2.7–3.5 |
| P (mM·L−1) | 1.29 ± 0.11 | 1.46 ± 0.33 | 1.29 ± 0.22 | ns | 1.3–2.1 |
| Mg (mM·L−1) | 1.00 ± 0.16 | 0.95 ± 0.04 | 1.02 ± 0.08 | ns | - |
| Albumin (g·L−1) | 36.2 ± 2.79 | 37.6 ± 1.51 | 34.9 ± 3.02 | ns | 36–48 |
| ALP (U·L−1) | 53.6 ± 11.9 | 65.8 ± 12.0 | 59.9 ± 25.0 | ns | 41–92 |
| ALT (U·L−1) | 29.9 ± 7.01 | 23.9 ± 5.63 | 30.4 ± 8.07 | ns | 45–80 |
| AST (U·L−1) | 30.0 ± 6.43 | 26.1 ± 3.57 | 33.9 ± 10.5 | ns | 35–130 |
| Bili (µM·L−1) | 1.24 ± 0.43 | 0.90 ± 0.21 | 1.10 ± 0.21 | ns | 0–12 |
| Creat (µM·L−1) | 62.6 ± 13.9 | 62.9 ± 9.09 | 61.7 ± 10.9 | ns | 44.2–221 |
| Glu (mM·L−1) | 7.72 ± 0.47 a | 6.73 ± 0.57 b | 5.78 ± 0.59 c | 0.0000 | 4.1–8.6 |
| Chol. (mM·L−1) | 0.93 ± 0.36 a | 0.53 ± 0.17 b | 0.79 ± 0.34 | 0.0379 | 0.3–2.1 |
| TP (g·L−1) | 69.2 ± 2.76 | 69.5 ± 6.94 | 64.9 ± 5.27 | ns | 54–75 |
| TG (mM·L−1) | 1.72 ± 1.24 a | 0.72 ± 0.35 b | 1.03 ± 0.26 a,b | 0.0311 | - |
| Uric acid (µM·L−1) | 7.84 ± 2.92 | 7.66 ± 2.52 | 7.92 ± 2.92 | ns | - |
| Globulin (g·L−1) | 32.9 ± 4.08 | 31.8 ± 7.98 | 30.0 ± 4.65 | ns | 16–29 |
| A/G ratio | 1.12 ± 0.18 | 1.23 ± 0.23 | 1.18 ± 0.20 | ns | - |
| Haematological Parameter | C (10) | E5 (10) | E10 (10) | p-Value | Reference Ranges 1 |
|---|---|---|---|---|---|
| WBC (109·L−1) | 10.5 ± 3.48 | 6.84 ± 0.91 | 7.32 ± 3.53 | ns | 6–12 |
| LYM (109·L−1) | 4.97 ± 1.46 | 4.84 ± 1.20 | 3.35 ± 1.78 | ns | 1.6–10.6 |
| MON (109·L−1) | 0.54 ± 0.15 | 0.37 ± 0.17 | 0.47 ± 0.30 | ns | 0.05–0.5 |
| NEU (109·L−1) | 4.91 ± 2.53 | 1.62 ± 1.61 | 3.51 ± 4.05 | ns | 1–9.4 |
| LYM (%) | 48.4 ± 10.21 | 72.6 ± 22.2 | 52.7 ± 22.4 | ns | 30–85 |
| MON (%) | 5.57 ± 1.75 | 5.30 ± 1.90 | 6.10 ± 1.74 | ns | 1–4 |
| NEU (%) | 46.1 ± 10.75 | 22.1 ± 21.1 | 41.2 ± 21.8 | ns | 20–60 |
| RBC (1012·L−1) | 7.16 ± 1.07 | 6.59 ± 0.65 | 5.76 ± 1.32 | ns | 4–7 |
| HGB (g·dL−1) | 12.2 ± 1.81 | 12.7 ± 1.16 | 10.5 ± 2.40 | ns | 8–15 |
| HCT (%) | 43.2 ± 6.56 a | 39.9 ± 3.21 a,b | 33.5 ± 7.21 b | 0.0175 | - |
| MCV (fL) | 60.2 ± 4.12 | 60.7 ± 1.38 | 58.7 ± 3.71 | ns | 58–67 |
| MCH (pg) | 17.5 ± 3.80 | 19.3 ± 0.52 | 18.3 ± 0.96 | ns | 17.1–23.5 |
| MCHC (g·dL−1) | 30.6 ± 1.82 | 31.8 ± 0.71 | 31.3 ± 0.95 | ns | 29–37 |
| RDWc (%) | 15.3 ± 1.07 | 15.3 ± 0.53 | 15.9 ± 0.79 | ns | - |
| RDWs (fL) | 38.2 ± 2.97 | 37.9 ± 1.57 | 37.9 ± 2.07 | ns | - |
| PLT (109·L−1) | 336 ± 112 a | 185 ± 72.9 b | 193 ± 41.9 b | 0.0063 | 250–650 |
| MPV (fl) | 5.82 ± 0.28 | 5.92 ± 0.15 | 6.13 ± 0.40 | ns | - |
| PCT (%) | 0.22 ± 0.12 a | 0.11 ± 0.04 b | 0.10 ± 0.05 b | 0.0141 | - |
| PDWc (%) | 28.3 ± 0.96 | 28.9 ± 1.58 | 30.1 ± 1.76 | ns | - |
| PDWS (fl) | 6.68 ± 0.47 | 7.02 ± 0.84 | 7.64 ± 0.96 | ns | - |
| Biochemical Parameter | C (10) | E5 (10) | E10 (10) | p-Value | Reference Ranges 1 |
|---|---|---|---|---|---|
| K (mM·L−1) | 5.02 ± 0.43 | 4.75 ± 0.48 | 4.65 ± 0.22 | ns | 3.5–6.9 |
| Cl (mM·L−1) | 102 ± 3.81 | 103 ± 4.63 | 105 ± 1.58 | ns | - |
| Ca (mM·L−1) | 3.56 ± 0.19 | 3.46 ± 0.17 | 3.49 ± 0.13 | ns | 2.7–3.5 |
| P (mM·L−1) | 1.08 ± 0.16 a | 1.21 ± 0.12 a,b | 1.27 ± 0.15 b | 0.0431 | 1.3–2.1 |
| Mg (mM·L−1) | 0.98 ± 0.13 | 0.92 ± 0.07 | 0.98 ± 0.07 | ns | - |
| Albumin (g·L−1) | 33.8 ± 2.77 | 35.3 ± 3.08 | 35.5 ± 2.80 | ns | 36–48 |
| ALP (U·L−1) | 41.9 ± 12.6 | 49.1 ± 19.4 | 55.3 ± 18.3 | ns | 41–92 |
| ALT (U·L−1) | 28.7 ± 7.58 a,b | 23.2 ± 5.35 a | 35.3 ± 13.7 b | 0.0460 | 45–80 |
| AST (U·L−1) | 30.4 ± 7.01 | 30.2 ± 9.46 | 32.5 ± 13.2 | ns | 35–130 |
| Bili (µM·L−1) | 1.13 ± 0.14 a | 0.87 ± 0.18 b | 0.97 ± 0.20 a,b | 0.0210 | 0–12 |
| Creat (µM·L−1) | 60.1 ± 9.93 | 59.0 ± 9.31 | 59.5 ± 14.0 | ns | 44.2–221 |
| Glu (mM·L−1) | 7.32 ± 0.36 | 7.31 ± 0.52 | 7.07 ± 0.35 | ns | 4.1–8.6 |
| Chol. (mM·L−1) | 0.81 ± 0.57 | 0.51 ± 0.16 | 0.75 ± 0.43 | ns | 0.3–2.1 |
| TP (g·L−1) | 66.2 ± 5.26 | 66.0 ± 7.48 | 65.2 ± 2.60 | ns | 54–75 |
| TG (mM·L−1) | 1.61 ± 0.86 a | 0.85 ± 0.34 b | 0.91 ± 0.28 b | 0.0160 | - |
| Uric acid (µM·L−1) | 6.83 ± 2.88 | 7.32 ± 1.62 | 7.18 ± 1.78 | ns | - |
| Globulin (g·L−1) | 32.4 ± 6.15 | 30.8 ± 7.04 | 29.7 ± 4.25 | ns | 16–29 |
| Alb/Glob ratio | 1.08 ± 0.21 | 1.19 ± 0.24 | 1.22 ± 0.23 | ns | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baláži, A.; Svoradová, A.; Kováčik, A.; Vašíček, J.; Chrenek, P. The Effects of Adding Hempseed Cake on Sperm Traits, Body Weight, Haematological and Biochemical Parameters in Rabbit Males. Vet. Sci. 2024, 11, 509. https://doi.org/10.3390/vetsci11100509
Baláži A, Svoradová A, Kováčik A, Vašíček J, Chrenek P. The Effects of Adding Hempseed Cake on Sperm Traits, Body Weight, Haematological and Biochemical Parameters in Rabbit Males. Veterinary Sciences. 2024; 11(10):509. https://doi.org/10.3390/vetsci11100509
Chicago/Turabian StyleBaláži, Andrej, Andrea Svoradová, Anton Kováčik, Jaromír Vašíček, and Peter Chrenek. 2024. "The Effects of Adding Hempseed Cake on Sperm Traits, Body Weight, Haematological and Biochemical Parameters in Rabbit Males" Veterinary Sciences 11, no. 10: 509. https://doi.org/10.3390/vetsci11100509
APA StyleBaláži, A., Svoradová, A., Kováčik, A., Vašíček, J., & Chrenek, P. (2024). The Effects of Adding Hempseed Cake on Sperm Traits, Body Weight, Haematological and Biochemical Parameters in Rabbit Males. Veterinary Sciences, 11(10), 509. https://doi.org/10.3390/vetsci11100509







