Investigation into the Effects of Allergen Exposure and Topical Vinegar and Water Spray on Skin Barrier Parameters in Atopic Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Allergen Exposure
2.3. Scoring of Dermatitis
2.4. Assessment of Skin Barrier Parameters
2.4.1. Treatments
2.4.2. Statistics
3. Results
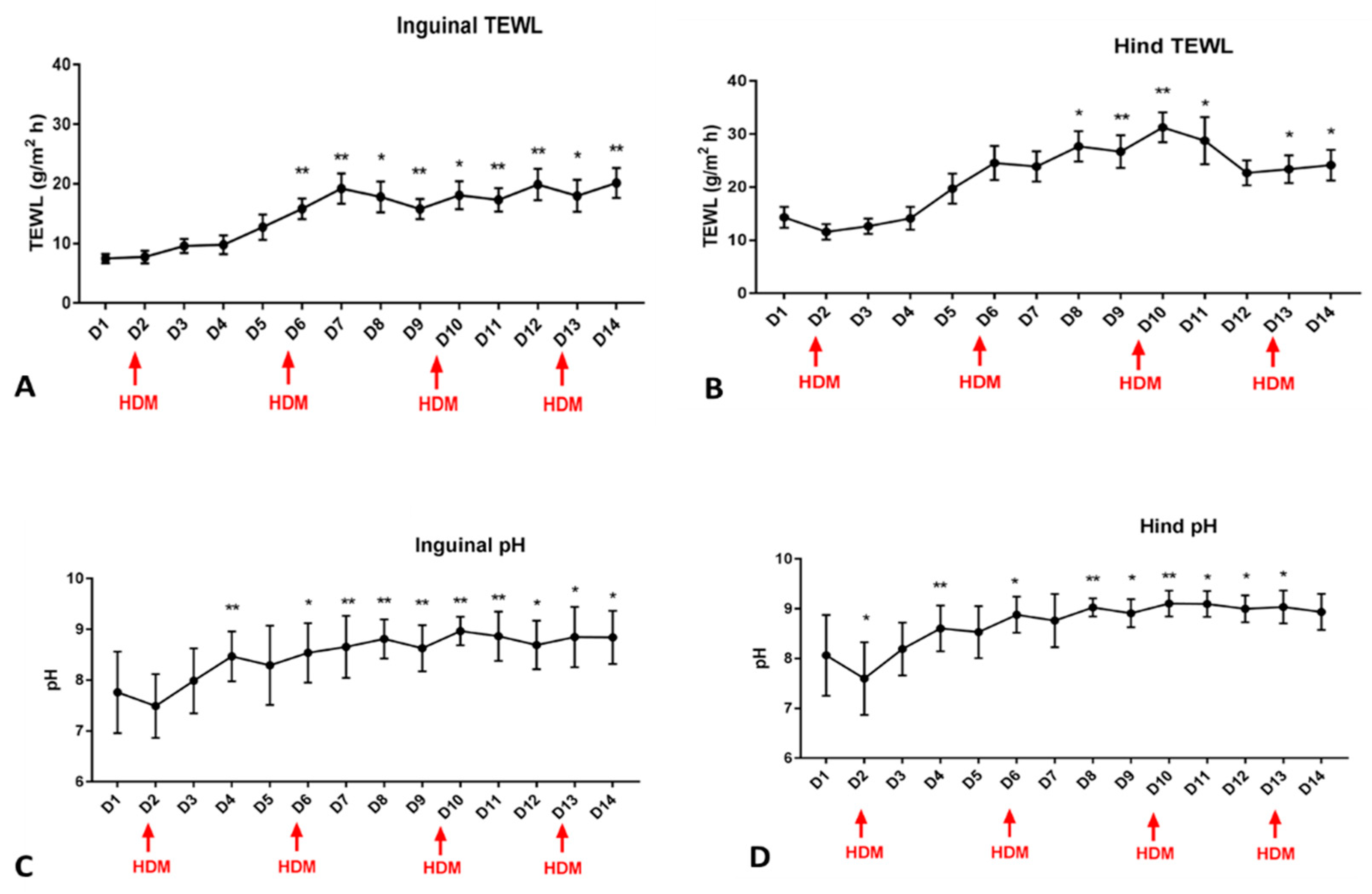
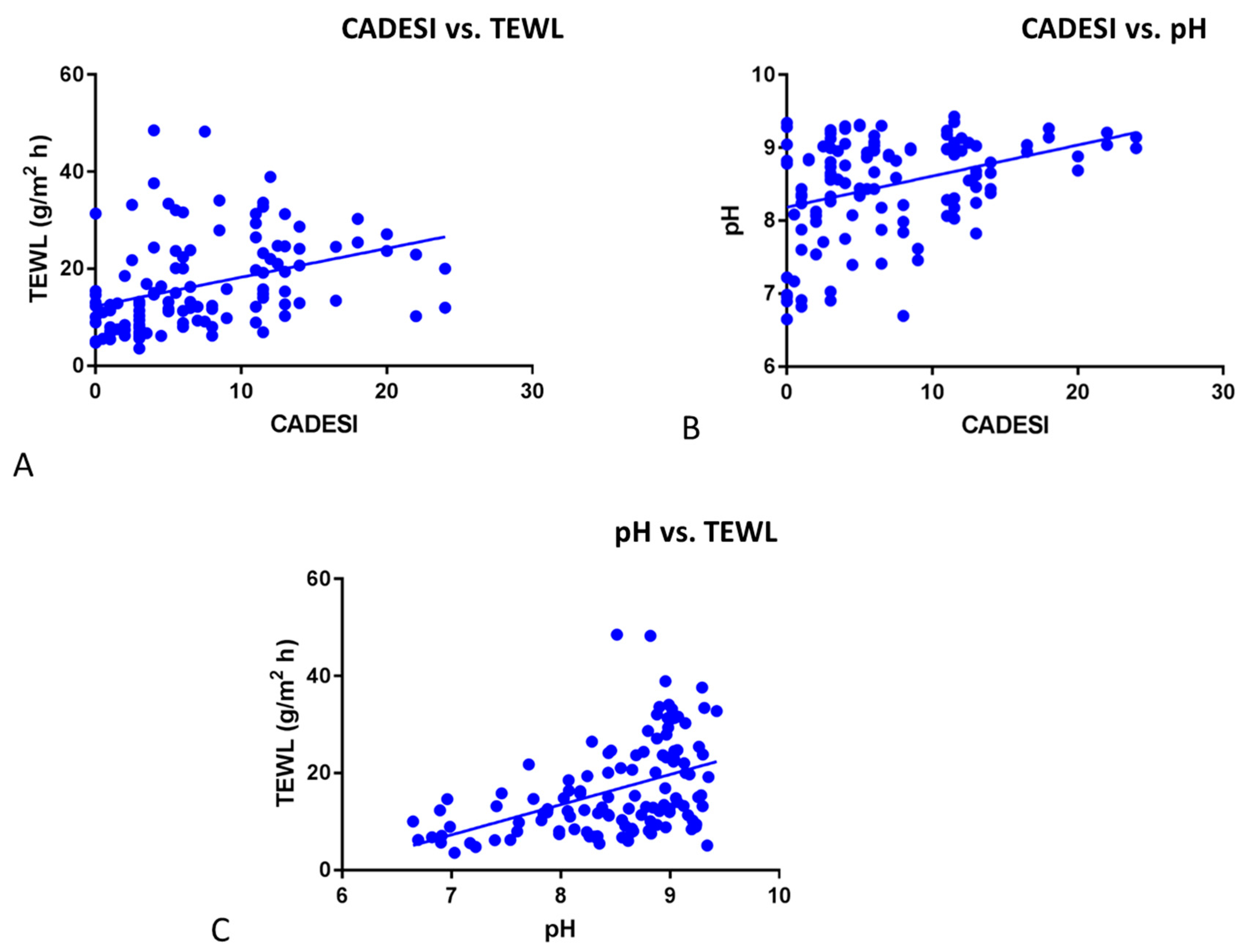
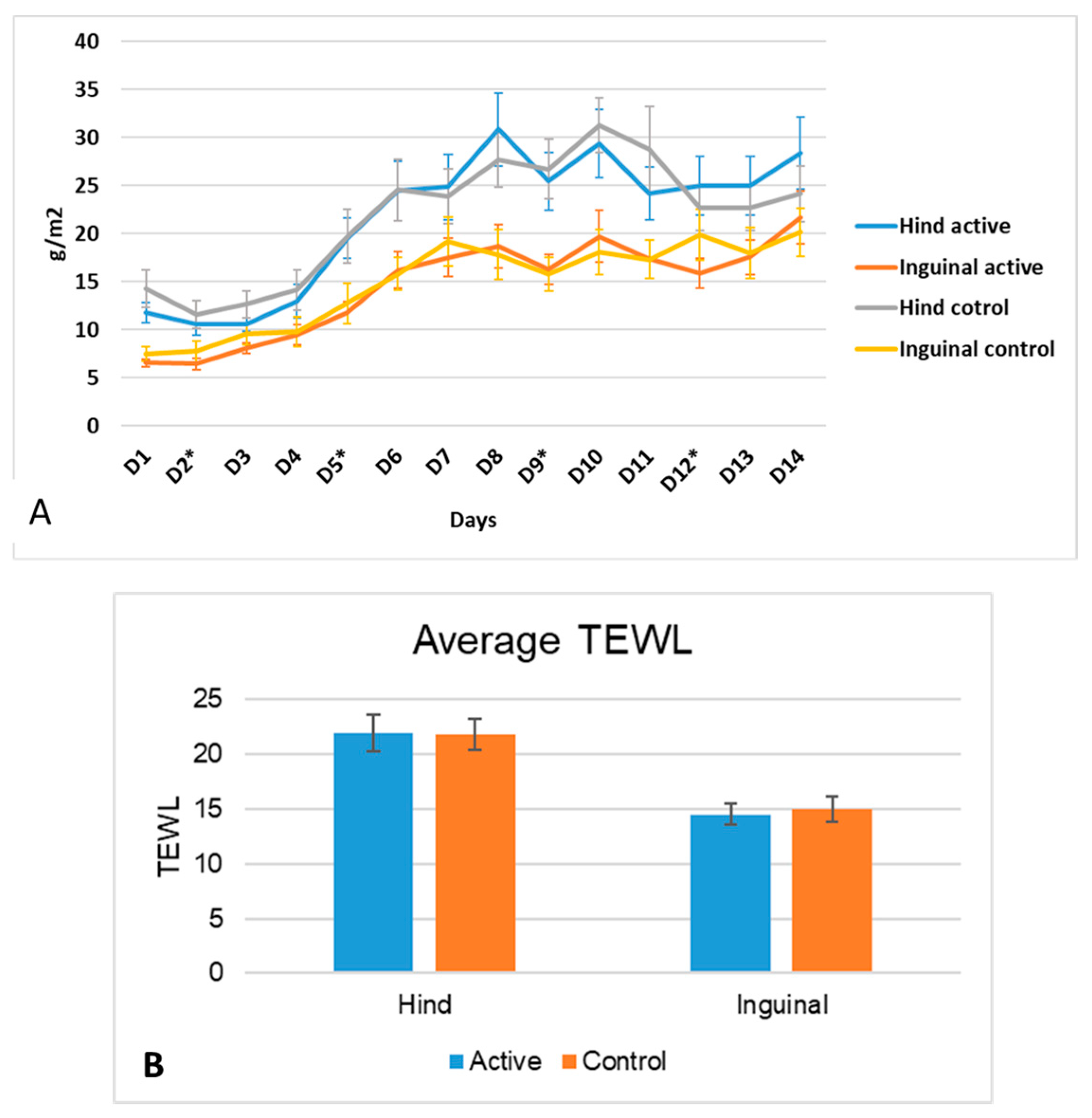
3.1. pH and TEWL
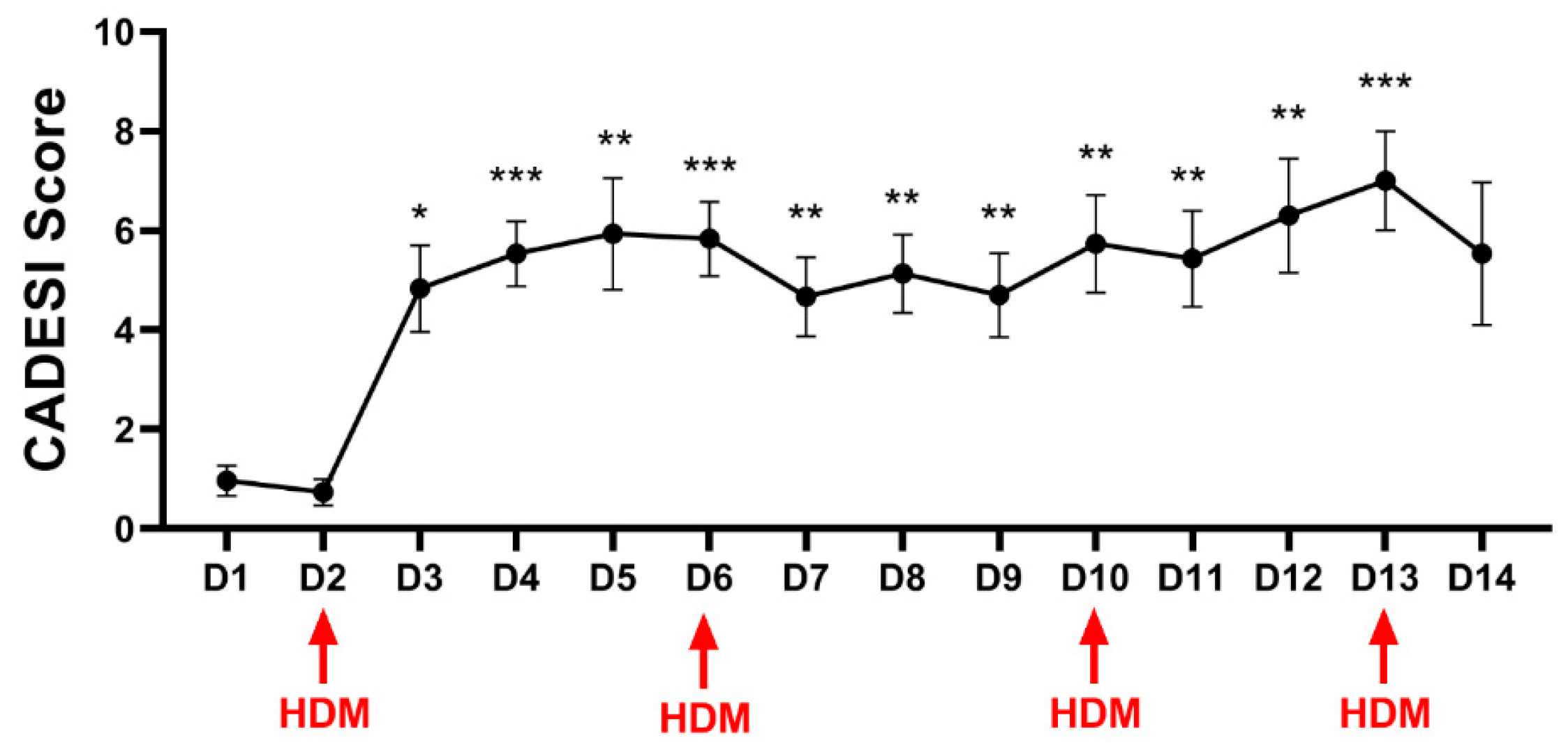
3.2. Clinical Scores
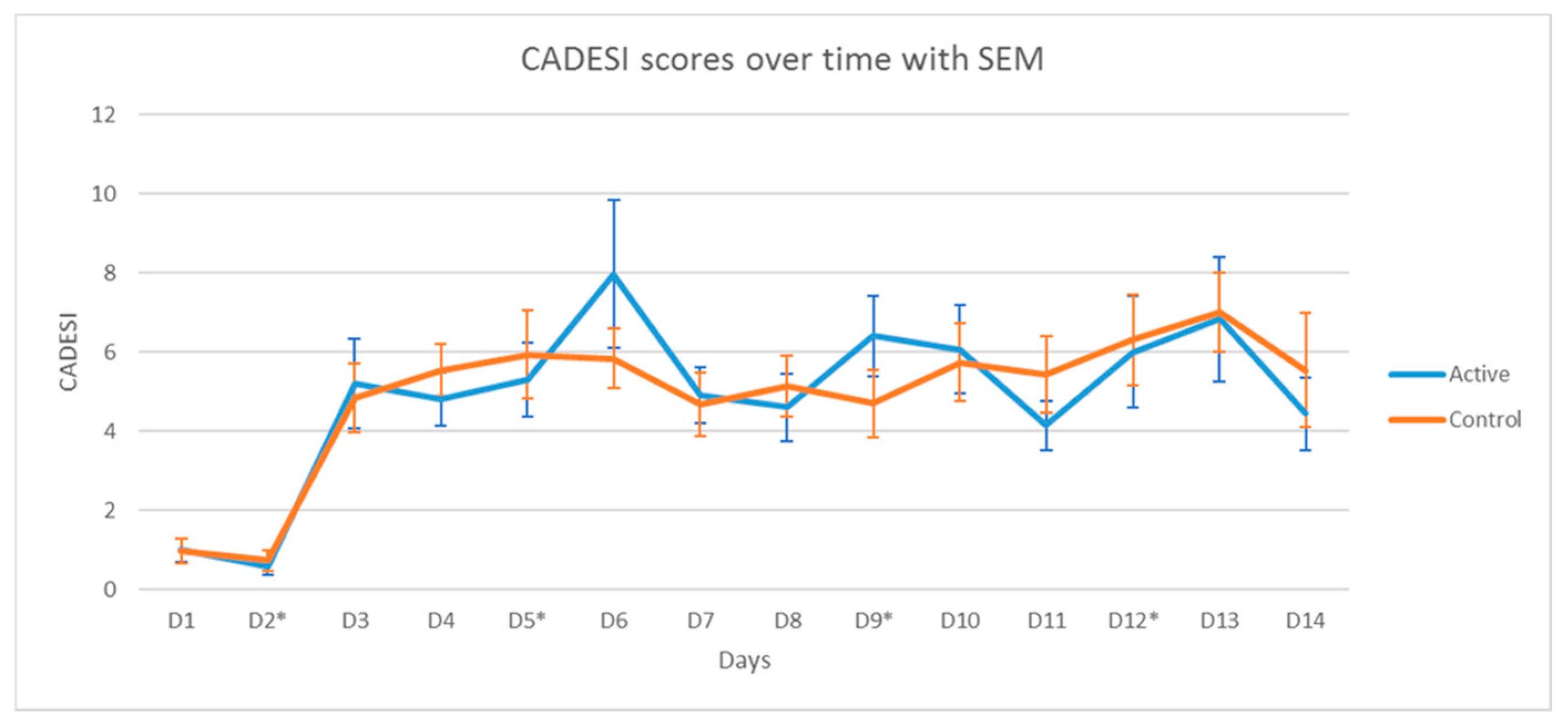
3.2.1. Clinical Scores
3.2.2. pH
3.2.3. TEWL
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef]
- Santoro, D.; Saridomichelakis, M.; Eisenschenk, M.; Tamamoto-Mochizuki, C.; Hensel, P.; Pucheu-Haston, C.; International Committee on Allergic Diseases of Animals (ICADA). Update on the skin barrier, cutaneous microbiome and host defence peptides in canine atopic dermatitis. Vet. Dermatol. 2024, 35, 5–14. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Song, Y.; Kim, T.J.; Lee, S.H.; Kim, H.J. Indoor particulate matter induces epigenetic changes in companion atopic dogs. Ecotoxicol. Environ. Saf. 2023, 266, 115544. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, B.T.; Kim, H.J. Effect of indoor air pollution on atopic dermatitis in dogs. Allergy 2023, 78, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Bize, C.; Le Gélébart, E.; Moga, A.; Payré, B.; Garcia, C. Barrier disruption, dehydration and inflammation: Investigation of the vicious circle underlying dry skin. Int. J. Cosmet. Sci. 2021, 43, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Greugny, E.T.; Bensaci, J.; Fages, F.; Stamatas, G.N. Computational modelling predicts impaired barrier function and higher sensitivity to skin inflammation following pH elevation. Exp. Dermatol. 2023, 32, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Wendtner, M.H.; Korting, H.C. The pH of the skin surface and its impact on the barrier function. Skin. Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Wohlrab, J.; Gebert, A.; Neubert, R.H.H. Lipids in the Skin and pH. Curr. Probl. Dermatol. 2018, 54, 64–70. [Google Scholar]
- Lee, H.J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef]
- Sparavigna, A.; Setaro, M.; Gualandri, V. Cutaneous pH in children affected by atopic dermatitis and in healthy children: A multicenter study. Skin Res. Technol. 1999, 5, 221–227. [Google Scholar] [CrossRef]
- Seidenari, S.; Giusti, G. Objective assessment of the skin of children affected by atopic dermatitis: A Study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm. Venereol. 1995, 75, 429–433. [Google Scholar] [CrossRef]
- Paul, L.B. Role of Skin pH in Psoriasis. Curr. Probl. Dermatol. 2018, 54, 108–114. [Google Scholar]
- Daniel Maroto-Morales, D.; Montero-Vilchez, T.; Arias-Santiago, S. Study of Skin Barrier Function in Psoriasis: The Impact of Emollients. Life 2021, 11, 651. [Google Scholar] [CrossRef]
- Panther, D.J.; Jacob, S.E. The Importance of Acidification in Atopic Eczema: An Underexplored Avenue for Treatment. J. Clin. Med. 2015, 4, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.H.; Kang, H. Importance of Stratum Corneum Acidification to Restore Skin Barrier Function in Eczematous Diseases. Ann. Dermatol. 2024, 36, 1–8. [Google Scholar] [CrossRef]
- Cobiella, D.; Archer, L.; Bohannon, M.; Santoro, D. Pilot study using five methods to evaluate skin barrier function in healthy dogs and in dogs with atopic dermatitis. Vet. Dermatol. 2019, 30, 121-e34. [Google Scholar] [CrossRef]
- Zając, M.; Szczepanik, M.P.; Wilkołek, P.M.; Adamek, L.R.; Pomorski, Z.J.H.; Sitkowski, W.; Gołyński, M. Assessment of a correlation between Canine Atopic Dermatitis Extent and Severity Index (CADESI-03) and selected biophysical skin measures (skin hydration, pH, and erythema intensity) in dogs with naturally occurring atopic dermatitis. Can. J. Vet. Res. 2015, 79, 136–140. [Google Scholar]
- Shimada, K.; Yoon, J.S.; Yoshihara, T.; Iwasaki, T.; Nishifuji, K. Increased transepidermal water loss and decreased ceramide content in lesional and non-lesional skin of dogs with atopic dermatitis. Vet. Dermatol. 2009, 20, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L.H. Transepidermal water loss (TEWL) and corneometry with hydrogel vehicle in the treatment of atopic dermatitis: A randomized, investigator-blind pilot study. J. Drugs Dermatol. 2012, 11, 180–184. [Google Scholar]
- Lau-Gillard, P.J.; Hill, P.B.; Chesney, C.J.; Budleigh, C.; Immonen, A. Evaluation of a hand-held evaporimeter (VapoMeter) for the measurement of transepidermal water loss in healthy dogs. Vet. Dermatol. 2010, 21, 136–145. [Google Scholar] [CrossRef]
- Marsella, R. Are transepidermal water loss and clinical signs correlated in canine atopic dermatitis? A compilation of studies Vet. Dermatol. 2012, 23, 238-e49. [Google Scholar] [CrossRef] [PubMed]
- Zając, M.; Szczepanik, M.P.; Wilkołek, P.M.; Adamek, L.R.; Pomorski, Z.J.; Sitkowski, W.; Gołyński, M.G. Assessment of the relationship between transepidermal water loss (TEWL) and severity of clinical signs (CADESI-03) in atopic dogs. Vet. Dermatol. 2014, 25, 503–506.e83. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Feschuk, A.M.; Kashetsky, N.; Maibach, H.I. “Normal” TEWL-how can it be defined? A systematic review. Exp. Dermatol. 2022, 31, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–2300.e1. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, J.L.; Stefaniak, A.B.; Wilhelm, K.P. Measurement of Skin Surface pH. Curr. Probl. Dermatol. 2018, 54, 19–25. [Google Scholar]
- Marsella, R.; Ahrens, K.; Wilkes, R.; Trujillo, A.; Dorr, M. Comparison of various treatment options for canine atopic dermatitis: A blinded, randomized, controlled study in a colony of research atopic beagle dogs. Vet. Dermatol. 2020, 31, 284-e69. [Google Scholar] [CrossRef]
- Marsella, R.; Segarra, S.; Ahrens, K.; Alonso, C.; Ferrer, L. Topical treatment with SPHINGOLIPIDS and GLYCOSAMINOGLYCANS for canine atopic dermatitis. BMC Vet. Res. 2020, 16, 92. [Google Scholar] [CrossRef]
- Olivry, T.; Marsella, R.; Iwasaki, T.; Mueller, R. International Task Force on Canine Atopic Dermatitis. Validation of CADESI-03, a severity scale for clinical trials enrolling dogs with atopic dermatitis. Vet. Dermatol. 2007, 18, 78–86. [Google Scholar] [CrossRef]
- Li, R.; Rodrigues, M.; Li, L.; Winget, J.; Wang, Y.; Wang, C.; Smith, E.; Wei, K. Association Between Skin Acid Mantle, Natural Moisturizing Factors, and Antibacterial Activity against S. aureus in the Stratum Corneum. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1595–1606. [Google Scholar] [CrossRef]
- Olivry, T.; Paps, J.S.; Amalric, N. Transient and reversible reduction of stratum corneum filaggrin degradation products after allergen challenge in experimentally mite-sensitised atopic dogs. Vet. Dermatol. 2022, 33, 62-e20. [Google Scholar] [CrossRef]
- Peer, R.P.; Burli, A.; Maibach, H.I. Unbearable transepidermal water loss (TEWL) experimental variability: Why? Arch. Dermatol. Res. 2022, 314, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Danby, S.G.; Cork, M.J. pH in atopic dermatitis. Curr. Probl. Dermatol. 2018, 54, 95–107. [Google Scholar] [PubMed]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Matousek, J.L.; Campbell, K.L. A comparative review of cutaneous pH. Vet. Dermatol. 2002, 13, 293–300. [Google Scholar] [CrossRef]
- Meyer, W.; Neurand, K. Comparison of skin pH in domesticated and laboratory mammals. Arch. Dermatol. Res. 1991, 283, 16–18. [Google Scholar] [CrossRef]
- Oh, W.S.; Oh, T.H. Mapping of the dog skin based on biophysical measurements. Vet. Dermatol. 2010, 21, 367–372. [Google Scholar] [CrossRef]
- Schlake, A.; Devriendt, N.; Talloen, L.; Dadi, T.B.; de Rooster, H. Influence of age, sex, body condition score, rectal temperature, anatomical location and hair on skin pH in dogs. Vet. Dermatol. 2022, 33, 3-e2. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, N.R.; Kim, B.K.; Jung, M.; Kim, D.H.; Moniaga, C.S.; Kabashima, K.; Choi, E.H. Acidification of stratum corneum prevents the progression from atopic dermatitis to respiratory allergy. Exp. Dermatol. 2017, 26, 66–72. [Google Scholar] [CrossRef]
- Matousek, J.L.; Campbell, K.L.; Kakoma, I.; Schaeffer, D.J. The effects of four acidifying sprays, vinegar, and water on canine cutaneous pH levels. J. Am. Anim. Hosp. Assoc. 2003, 39, 29–33. [Google Scholar] [CrossRef]
- Lee, N.R.; Lee, H.J.; Yoon, N.Y.; Kim, D.; Jung, M.; Ho, E. Acidic Water Bathing Could Be a Safe and Effective Therapeutic Modality for Severe and Refractory Atopic Dermatitis. Ann. Dermatol. 2016, 28, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Luu, L.A.; Flowers, R.H.; Kellams, A.L.; Zeichner, S.; Preston, D.C.; Zlotoff, B.J.; Wisniewski, J.A. Apple cider vinegar soaks [0.5%] as a treatment for atopic dermatitis do not improve skin barrier integrity. Pediatr. Dermatol. 2019, 36, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Hachem, J.-P.; Roelandt, T.; Schürer, N.; Pu, X.; Fluhr, J.; Giddelo, C.; Man, M.-Q.; Crumrine, D.; Roseeuw, D.; Feingold, K.R.; et al. Acute acidification of stratum corneum membrane domains using polyhydroxyl acids improves lipid processing and inhibits degradation of corneodesmosomes. J. Investig. Dermatol. 2010, 130, 500–510. [Google Scholar] [CrossRef]
- Phophi, L.; Abouelkhair, M.A.; Jones, R.; Zehr, J.; Kania, S.A. Temporal changes in antibiotic resistance and population structure of methicillin-resistant Staphylococcus pseudintermedius between 2010 and 2021 in the United States. Comp. Immunol. Microbiol. Infect. Dis. 2023, 100, 102028. [Google Scholar] [CrossRef]
- Hülpüsch, C.; Tremmel, K.; Hammel, G.; Bhattacharyya, M.; de Tomassi, A.; Nussbaumer, T.; Neumann, A.U.; Reiger, M.; Traidl-Hoffmann, C. Skin pH-dependent Staphylococcus aureus abundance as predictor for increasing atopic dermatitis severity. Allergy 2020, 75, 2888–2898. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsella, R. Investigation into the Effects of Allergen Exposure and Topical Vinegar and Water Spray on Skin Barrier Parameters in Atopic Dogs. Vet. Sci. 2024, 11, 459. https://doi.org/10.3390/vetsci11100459
Marsella R. Investigation into the Effects of Allergen Exposure and Topical Vinegar and Water Spray on Skin Barrier Parameters in Atopic Dogs. Veterinary Sciences. 2024; 11(10):459. https://doi.org/10.3390/vetsci11100459
Chicago/Turabian StyleMarsella, Rosanna. 2024. "Investigation into the Effects of Allergen Exposure and Topical Vinegar and Water Spray on Skin Barrier Parameters in Atopic Dogs" Veterinary Sciences 11, no. 10: 459. https://doi.org/10.3390/vetsci11100459
APA StyleMarsella, R. (2024). Investigation into the Effects of Allergen Exposure and Topical Vinegar and Water Spray on Skin Barrier Parameters in Atopic Dogs. Veterinary Sciences, 11(10), 459. https://doi.org/10.3390/vetsci11100459





