Microbes on Clipper Blades after Use and Disinfection in Small Animal- and Equine Practice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection for Bacteria
2.3. Sample Collection for Fungi
2.4. Storage of Clippers and Blades
2.5. Bacterial Cultures
2.6. Fungal Cultures
2.7. Typing of Bacteria
2.8. Quantification of Bacteria
2.9. Statistical Analysis
3. Results
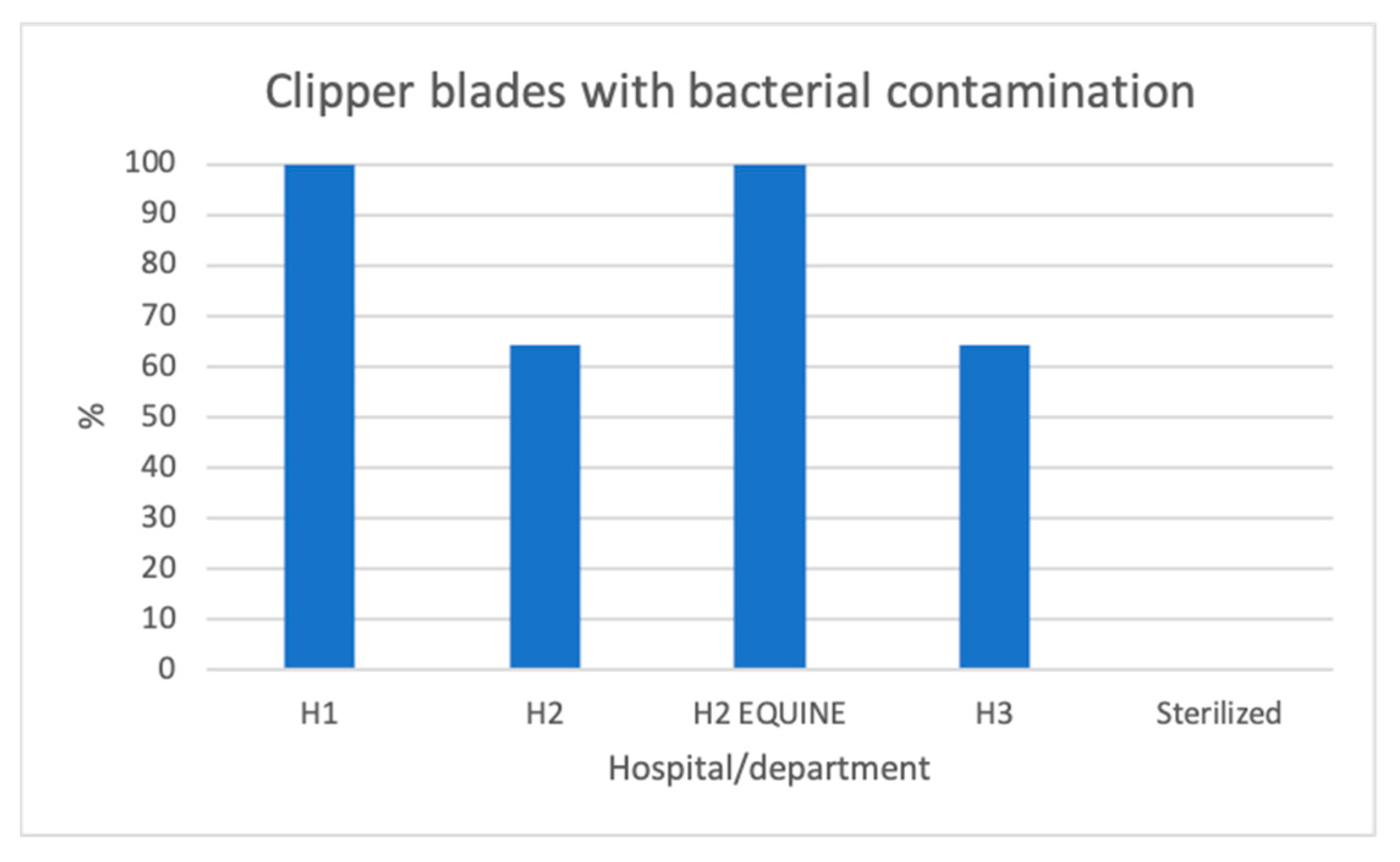
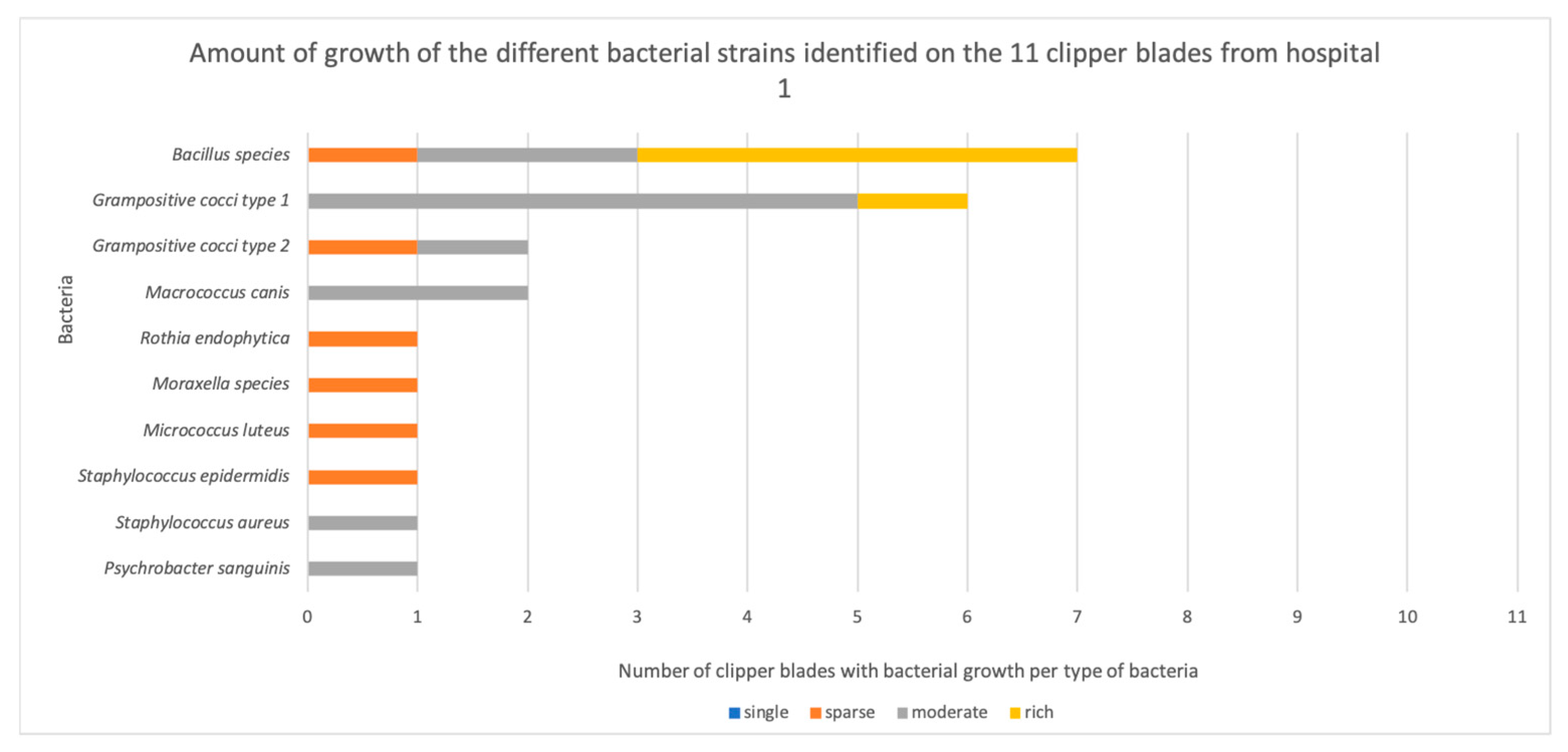
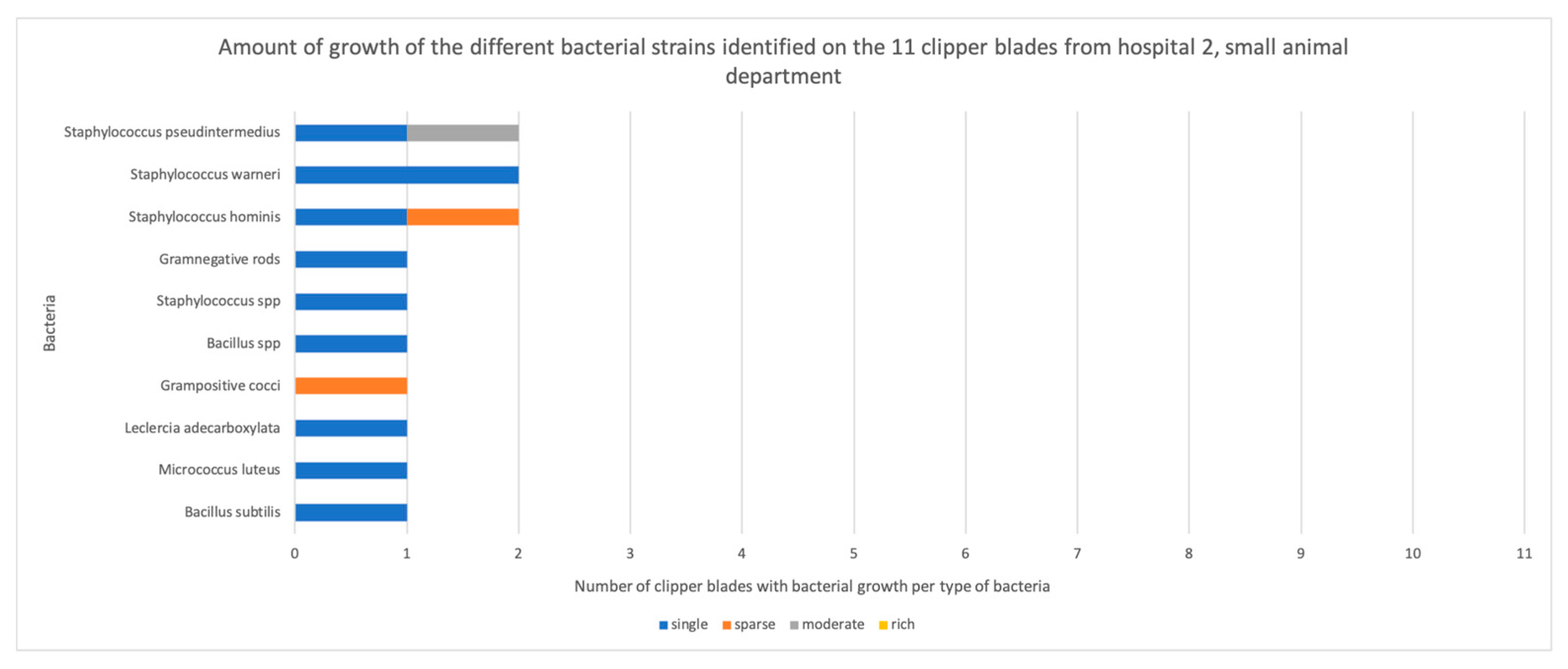
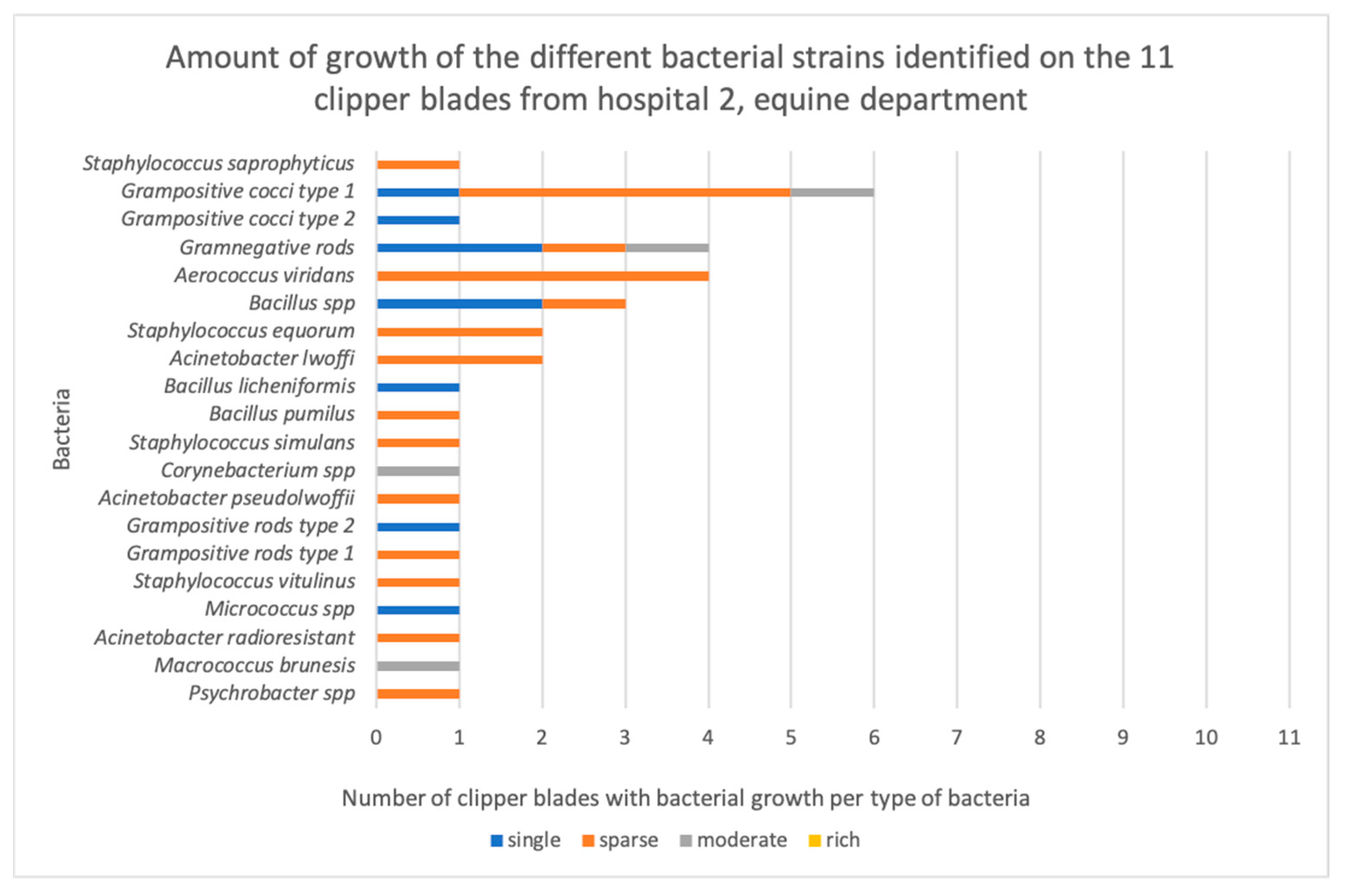
3.1. Bacterial Contamination
3.2. Fungal Contamination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Clean the machine of all hair and dirt with the help of a toothbrush.
- Use an antibacterial wipe to clean the machine.
- Carefully roll the cord around the machine.
- The small machines shall be unscrewed and cleaned between patients.
- Clean by brushing the toothbrush on both the clipper blade and clipper to access small corners.
- The blade consists of two rows of tines; push the upper one to the side and brush, then push it to the other side and brush.
- After that, use Ytdes 50 + special (NOT LIFECLEAN), and wipe the machine and clipper blade.
- Use oil for the blades.
- Let it dry on the “clean” side.Comment: Ytdes 50 + contains isopropanol (40%) and etanol (10%).Small clippers:
- Clean by brushing the toothbrush on both the clipper blade and machine to access small corners.
- Unscrew the blade from the machine, and brush inside the clipper machine.
- Put the screw in a designated container.
- Then, use Ytdes + 50 special (NOT LIFECLEAN) and wipe the machine and clipper blade.
- Then, use oil for the blades.
- Let it dry on the “clean”-side, with the small machine upside down.
- Screw together when dried.
- Pour 2-3 cm BLADE WASH in the bottle’s lid.
- Run the clipper blade (still attached to the machine) in the solution for 5–10 s, just dip the blade.
- Detach the blade and let it stay for some minute in the solution.
- Brush with the tooth brush between all the small areas that are possible. If the dirt doesn’t disappear, dip the clipper blade in BLADE WASH again and leave it for a bit.
- When has been cleaned with the tooth brush, blow it dry with the device for compressed air.
- Spray the whole clipper blade with CLIPPERCIDE SPRAY and put it in the designated parking for clipper blades.
- Brush the blade clean from fur with a toothbrush.
- While the machine is running, flush the blade with surface disinfectant (YTDES). Wipe the clipper and the clipper machine.
- When the disinfection has evaporated, start the machine and oil the blade. Do not overdose; three drops are enough!
- Put the clipper with the blade facing downwards on a tray.
- If you need to charge the machine, make sure that it is clean, and that the disinfectant has evaporated before you place it in the charger.
- The toothbrush and tray need to be changed every day. Throw the used ones away. Mark the new ones with the date.
- Clean the clipper blades daily with Bladewash as well.
Appendix B
| Clipper Blade | Bacteria | Growth |
|---|---|---|
| H1.1 | Grampositive cocci Grampositive cocci | Moderate Sparse |
| H1.2 | Bacillus species | Rich |
| H1.3 | Grampositive cocci Psychrobacter sanguinis | Moderate Moderate |
| H1.4 | Grampositive cocci Macroccus canis Bacillus species | Rich Moderate Moderate |
| H1.5 | Bacillus species (cereus group) Grampositive cocci | Rich Moderate |
| H1.6 | Bacillus species Staphylococcus aureus | Moderate Moderate |
| H1.7 | Bacillus species (cereus group) | Rich |
| H1.8 | Staphylococcus epidermidis Bacillus species (cereus group) Micrococcus luteus Moraxella species | Sparse Sparse Sparse Sparse |
| H1.9 | Grampositive cocci Grampositive cocci | Moderate Moderate |
| H1.10 | Grampositive cocci Macrococcus canis | Moderate Moderate |
| H1.11 | Bacillus species (cereus group) Rothia endophytica | Rich Sparse |
| Clipper Blade | Bacteria | Growth | After Enrichment |
|---|---|---|---|
| H2.1 | Bacillus subtilis | Single colonies | |
| H2.2 | No growth | Grampositive cocci | |
| H2.3 | Micrococcus luteus | Single colonies | Staphylococcus epidermidis |
| H2.4 | No growth | Staphylococcus saprophyticus Staphylococcus hominis | |
| H2.5 | Staphylococcus warneri Staphylococcus hominis | Single colonies Single colonies | |
| H2.6 | Staphylococcus pseudintermedius Leclercia adecarboxylata Staphylococcus hominis Grampositive cocci | Moderate Single colonies Sparse Sparse | |
| H2.7 | Bacillus species (cereus group) Staphylococcus pseudintermedius | Single colonies Single colonies | |
| H2.8 | Staphylococcus warneri Staphylococcus species | Single colonies Single colonies | |
| H2.9 | No growth | Bacillus pumilus | |
| H2.10 | No growth | Bacillus species (cereus group) | |
| H2.11 | Gramnegative rods | Single colonies |
| Clipper Blade | Bacteria | Growth |
|---|---|---|
| H2.E1 | Grampositive cocci Acinetobacter lwoffii Psychrobacter species | Sparse Sparse Sparse |
| H2.E2 | Macrococcus brunesis Grampositive cocci | Moderate Moderate |
| H2.E3 | Acinetobacter radioresistant Acinetobacter lwoffi Micrococcus species | Sparse Sparse Single colonies |
| H2.E4 | Grampositive cocci Bacillus species | Single colonies Single colonies |
| H2.E5 | Staphylococcus vitulinus Aerococcus viridans Grampositive rods Grampositive rods | Sparse Sparse Sparse Single colonies |
| H2.E6 | Grampositive cocci Acinetobacter pseudolwoffii Aerococcus viridans Bacillus species | Sparse Sparse Sparse Sparse |
| H2.E7 | Gramnegative rods Aerococcus viridans Corynebacterium species | Moderate Sparse Moderate |
| H2.E8 | Staphylococcus simulans Bacillus pumilus Staphylococcus equorum Gramnegative rods | Sparse Sparse Sparse Single colonies |
| H2.E9 | Bacillus licheniformis Bacillus species (cereus group) | Single colonies Single colonies |
| H2.E10 | Staphylococcus equorum Aerococcus viridans Grampositive cocci Gramnegative rods | Sparse Sparse Sparse Single colonies |
| H2.E11 | Grampositive cocci Staphylococcus saprophyticus Gramnegative rods Grampositive cocci | Sparse Sparse Sparse Single colonies |
| Clipper Blade | Bacteria | Growth | After Enrichment |
|---|---|---|---|
| H3.1 | Grampositive diplococci Grampositive cocci | Rich Moderate | |
| H3.2 | Moraxella osloensis Grampositive cocci Staphylococcus pseudintermedius | Sparse Sparse Sparse | |
| H3.3 | Micrococcus luteus Staphylococcus epidermidis | Single colonies Single colonies | |
| H3.4 | No growth | No growth | |
| H3.5 | Grampositive cocci Gramnegative rods Grampositive cocci Staphylococcus petrasii | Sparse Sparse Sparse Sparse | |
| H3.6 | No growth | No growth | |
| H3.7 | Staphylococcus capitis | Single colonies | |
| H3.8 | No growth | No growth | |
| H3.9 | Grampositive cocci Moraxella species | Sparse Single colonies | |
| H3.10 | Enterococcus faecium | Single colonies | |
| H3.11 | No growth | Bacillus species |
| Clipper Blade | Bacteria | Growth |
|---|---|---|
| AUT1 | No growth | |
| AUT2 | No growth | |
| AUT3 | No growth | |
| AUT4 | No growth | |
| AUT5 | No growth |
References
- Messiaen, Y.; MacLellan, J.D.; Pelsue, D.H. Evaluation of the number of colony forming units on the skin of dogs after clipping the hair with two sizes of clipper blades. Am. J. Vet. Res. 2019, 80, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E.; Sargeant, J.M.; Weese, J.S. Video observation of hand hygiene practices during routine companion animal appointments and the effect of a poster intervention on hand hygiene compliance. BMC Vet. Res. 2014, 10, 106. [Google Scholar] [CrossRef]
- Boerlin, P.; Eugster, S.; Gaschen, F.; Straub, R.; Schawalder, P. Transmission of opportunistic pathogens in a veterinary teaching hospital. Vet. Microbiol. 2001, 82, 347–359. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Hamilton, E.; Kaneene, J.B.; May, K.J.; Kruger, J.M.; Schall, W.; Beal, M.W.; Hauptman, J.G.; DeCamp, C.E. Prevalence and antimicrobial resistance of Enterococcus spp. and Staphylococcus spp. isolated from surfaces in a veterinary teaching hospital. J. Am. Vet. Med. Assoc. 2012, 240, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.L.; Rosenkrantz, W.S.; Ghubash, R.M.; Neradilek, B.; Polissar, N.L. Evaluation of otoscope cone disinfection techniques and contamination level in small animal private practice. Vet. Dermatol. 2010, 21, 175–183. [Google Scholar] [CrossRef]
- Perkins, A.V.; Sellon, D.C.; Gay, J.M.; Lofgren, E.T.; Moore, D.A.; Jones, L.P.; Davis, M.A. Prevalence of methicillin-resistant Staphylococcus pseudintermedius on hand-contact and animal-contact surfaces in companion animal community hospitals. Can. Vet. J. 2020, 61, 613–620. [Google Scholar]
- Moriello, K.A.; Coyner, K.; Paterson, S.; Mignon, B. Diagnosis and treatment of dermatophytosis in dogs and cats.: Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2017, 28, 266-e68. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.W.; Miller, W.H., Jr. Equine Dermatology, 2nd ed.; Elsevier: Maryland Heights, MO, USA, 2011; pp. 172–183. [Google Scholar]
- Sparkes, A.H.; Werrett, G.; Stokes, C.R.; Gruffydd-Jones, T.J. Microsporum canis: Inapparent carriage by cats and the viability of arthrospores. J. Small Anim. Pract. 1994, 35, 397–401. [Google Scholar] [CrossRef]
- Moriello, K. Feline dermatophytosis: Aspects pertinent to disease management in single and multiple cat situations. J. Feline Med. Surg. 2014, 16, 419–431. [Google Scholar] [CrossRef]
- Mancianti, F.; Papini, R. Isolation of keratinophilic fungi from the floors of private veterinary clinics in Italy. Vet. Res. Commun. 1996, 20, 161–166. [Google Scholar] [CrossRef]
- Bağcigil, A.F.; Ikiz, S.; Ozgür, N.Y.; Ilgaz, A. Recovery of dermatophytes in pet grooming tools from veterinary clinics and pet grooming salons. J. Small Anim. Pract. 2010, 51, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Mount, R.; Schick, A.E.; Lewis, T.P., 2nd; Newton, H.M. Evaluation of Bacterial Contamination of Clipper Blades in Small Animal Private Practice. J. Am. Anim. Hosp. Assoc. 2016, 52, 95–101. [Google Scholar] [CrossRef]
- Ley, B.; Silverman, E.; Peery, K.; Dominguez, D. Evaluation of Commonly Used Products for Disinfecting Clipper Blades in Veterinary Practices: A Pilot Study. J. Am. Anim. Hosp. Assoc. 2016, 52, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Gould, A.P.; Coyner, K.S.; Trimmer, A.M.; Weese, J.S.; Budke, C.M. Recovery of meticillin-resistant Staphylococcus species from pet-grooming salons. Vet. Dermatol. 2020, 31, 262-e60. [Google Scholar] [CrossRef]
- Loeffler, A.; Lloyd, D.H. Companion animals: A reservoir for methicillin-resistant Staphylococcus aureus in the community? Epidemiol. Infect. 2010, 138, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Baptiste, K.E.; Williams, K.; Willams, N.J.; Wattret, A.; Clegg, P.D.; Dawson, S.; Corkill, J.E.; O’Neill, T.; Hart, C.A. Methicillin-resistant staphylococci in companion animals. Emerg. Infect. Dis. 2005, 11, 1942–1944. [Google Scholar] [CrossRef] [PubMed]
- Sveriges Veterinärförbund. Sveriges Veterinärförbunds Riktlinjer för Infektionskontroll Inom Smådjurssjukvården. Available online: https://www.svf.se/media/oq4oq5yw/svfs-riktlinje-g%C3%A4llande-infektionskontroll-inom-sm%C3%A5djurssjukv%C3%A5rden-2012.pdf (accessed on 20 March 2023).
- Sveriges Veterinärförbund och Hästsektionen inom Sveriges Veterinärmedicinska Sällskap SVS:s Riktlinjer för Infektionskontroll Inom Hästsjukvård. Available online: https://www.svf.se/media/10mbpucp/infektionskontroll_inom_hastsjukvard.pdf/ (accessed on 20 March 2023).
- Weese, J.S.; van Duijkeren, E. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Vet. Microbiol. 2010, 140, 418–429. [Google Scholar] [CrossRef]
- Abraham, J.L.; Morris, D.O.; Griffeth, G.C.; Shofer, F.S.; Rankin, S.C. Surveillance of healthy cats and cats with inflammatory skin disease for colonization of the skin by methicillin-resistant coagulase-positive staphylococci and Staphylococcus schleiferi ssp. schleiferi. Vet. Dermatol. 2007, 18, 252–259. [Google Scholar] [CrossRef]
- Bannoehr, J.; Ben Zakour, N.L.; Waller, A.S.; Guardabassi, L.; Thoday, K.L.; van den Broek, A.H.; Fitzgerald, J.R. Population genetic structure of the Staphylococcus intermedius group: Insights into agr diversification and the emergence of methicillin-resistant strains. J. Bacteriol. 2007, 189, 8685–8692. [Google Scholar] [CrossRef]
- Shumaker, A.K.; Angus, J.C.; Coyner, K.S.; Loeffler, D.G.; Rankin, S.C.; Lewis, T.P. Microbiological and histopathological features of canine acral lick dermatitis. Vet. Dermatol. 2008, 19, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Medleau, L.; Blue, J.L. Frequency and antimicrobial susceptibility of Staphylococcus spp. isolated from feline skin lesions. J. Am. Vet. Med. Assoc. 1988, 193, 1080–1081. [Google Scholar]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [PubMed]
- Fey, P.D.; Olson, M.E. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol. 2010, 5, 917–933. [Google Scholar] [CrossRef]
- Loeffler, A.; Lloyd, D.H. What has changed in canine pyoderma? A narrative review. Vet. J. 2018, 235, 73–82. [Google Scholar] [CrossRef]
- Teixeira, I.M.; de Oliveira Ferreira, E.; de Araújo Penna, B. Dogs as reservoir of methicillin resistant coagulase negative staphylococci strains—A possible neglected risk. Microb. Pathog. 2019, 135, 103616. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Outbreak of cutaneous Bacillus cereus infections among cadets in a university military program—Georgia, August 2004. Morb. Mortal. Wkly. Rep. 2005, 54, 1233–1235. [Google Scholar]
- Deal, A.; Klein, D.; Lopolito, P.; Schwarz, J.S. Cleaning and Disinfection of Bacillus cereus Biofilm. PDA J. Pharm. Sci. Technol. 2016, 70, 208–217. [Google Scholar] [CrossRef]
- Miller, W.H.; Griffin, C.E.; Campbell, K.L.; Muller, G.H. Chapter 5. In Muller and Kirk’s Small Animal Dermatology, 7th ed.; Elsevier: Maryland Heights, MO, USA, 2013; pp. 231–243. [Google Scholar]
- Chupia, V.; Ninsuwon, J.; Piyarungsri, K.; Sodarat, C.; Prachasilchai, W.; Suriyasathaporn, W.; Pikulkaew, S. Prevalence of Microsporum canis from Pet Cats in Small Animal Hospitals, Chiang Mai, Thailand. Vet. Sci. 2022, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Lloyd, D.H.; Lamport, A.I. Survey of dermatophytes on clinically normal cats in the southeast of England. J. Small Anim. Pract. 2005, 46, 436–439. [Google Scholar] [CrossRef]
- Kieri, S. Är Rengjorda Klippskär för Rakapparater Kontaminerade? SLU, Institutionen för Kliniska Vetenskaper: Uppsala, Sweeden, 2022. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gustafsson, L.; Wikström, C.; Mueller, R.S.; Bergvall, K. Microbes on Clipper Blades after Use and Disinfection in Small Animal- and Equine Practice. Vet. Sci. 2024, 11, 38. https://doi.org/10.3390/vetsci11010038
Gustafsson L, Wikström C, Mueller RS, Bergvall K. Microbes on Clipper Blades after Use and Disinfection in Small Animal- and Equine Practice. Veterinary Sciences. 2024; 11(1):38. https://doi.org/10.3390/vetsci11010038
Chicago/Turabian StyleGustafsson, Lina, Camilla Wikström, Ralf S. Mueller, and Kerstin Bergvall. 2024. "Microbes on Clipper Blades after Use and Disinfection in Small Animal- and Equine Practice" Veterinary Sciences 11, no. 1: 38. https://doi.org/10.3390/vetsci11010038
APA StyleGustafsson, L., Wikström, C., Mueller, R. S., & Bergvall, K. (2024). Microbes on Clipper Blades after Use and Disinfection in Small Animal- and Equine Practice. Veterinary Sciences, 11(1), 38. https://doi.org/10.3390/vetsci11010038






