Cutaneous Canine Mast Cell Tumor: The Use of Proliferative Markers (Ki-67 and Ki-67 × AgNOR) in Cytological Samples for Diagnosis and Prognosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sampling
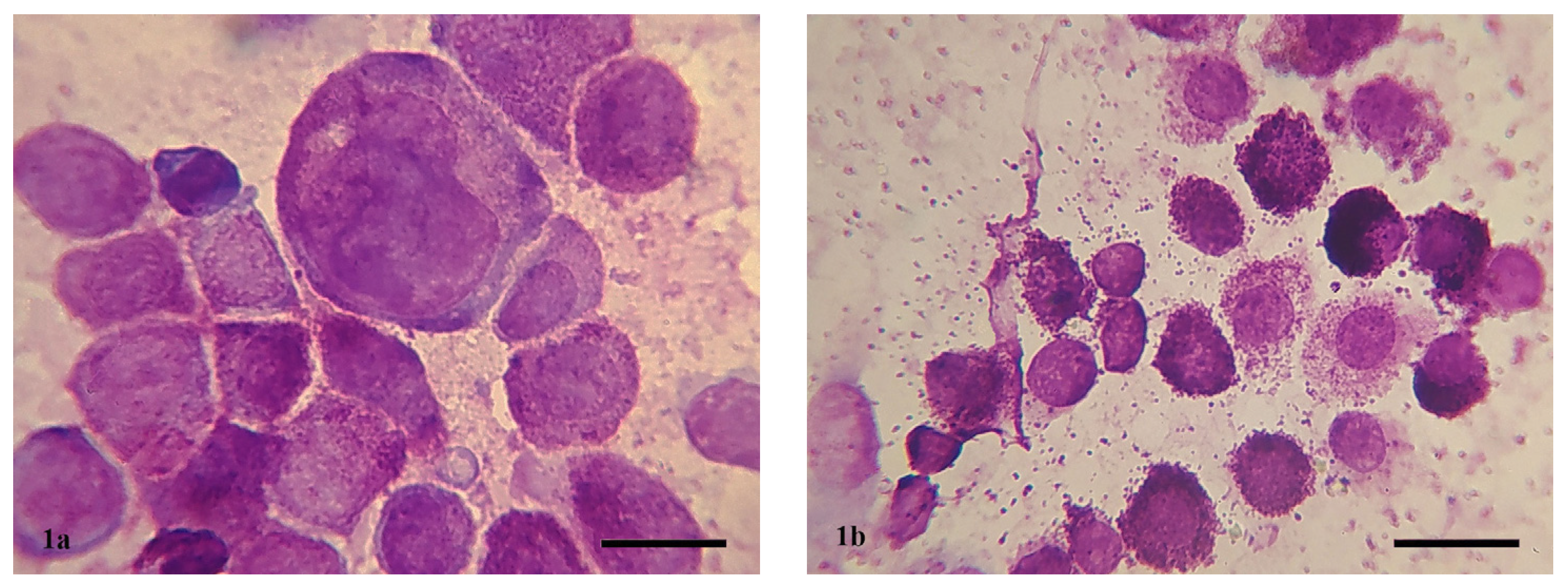
2.3. Cytologic Examination and Grading
2.4. Histopathological Examination and Grading
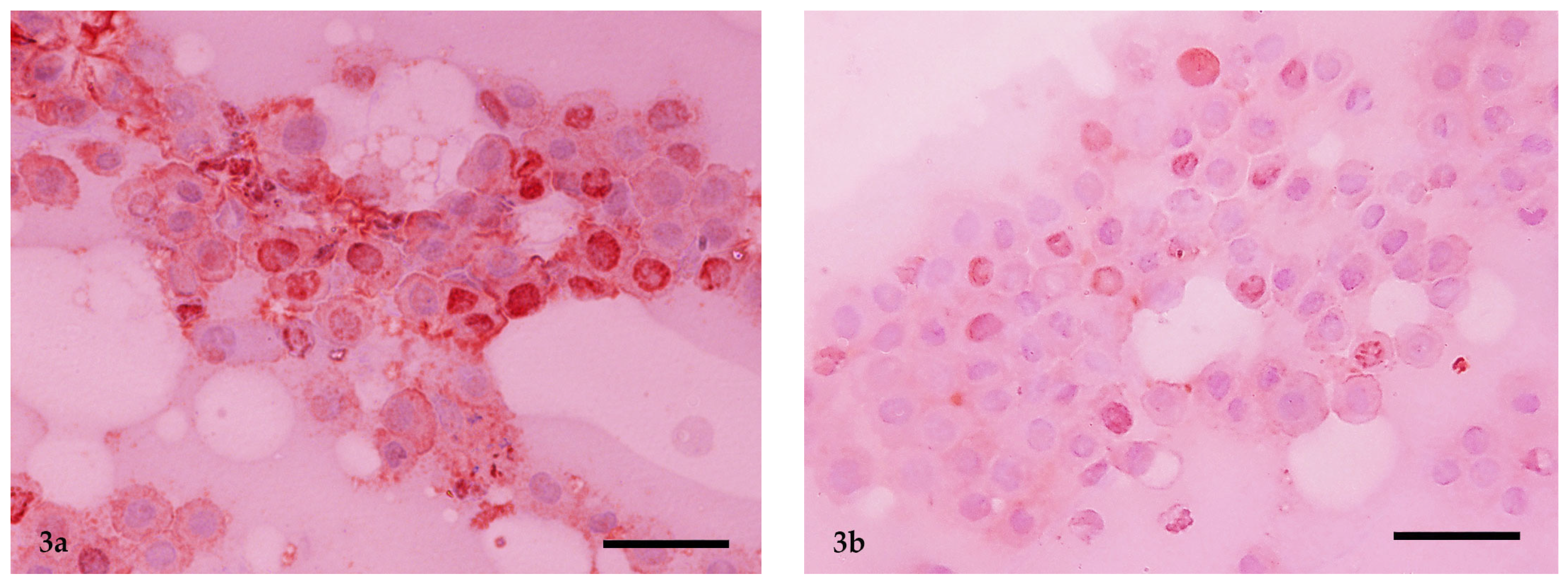
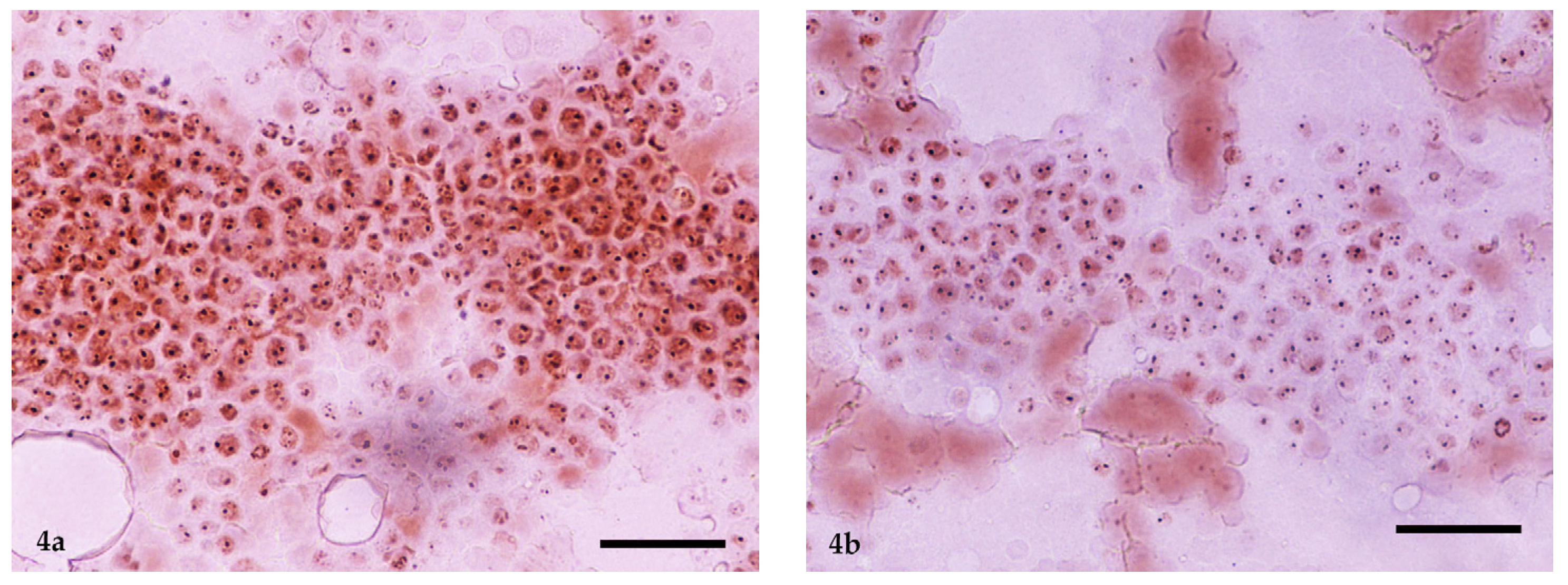
2.5. Immunocytochemistry and Ki-67 Scoring
2.6. AgNOR Cytochemical Staining and Ki-67 × AgNOR Scoring
2.7. Grading Comparisons and Statistical Analysis
3. Results
3.1. Animals
3.2. Comparison of Cytological and Histopathological Grading
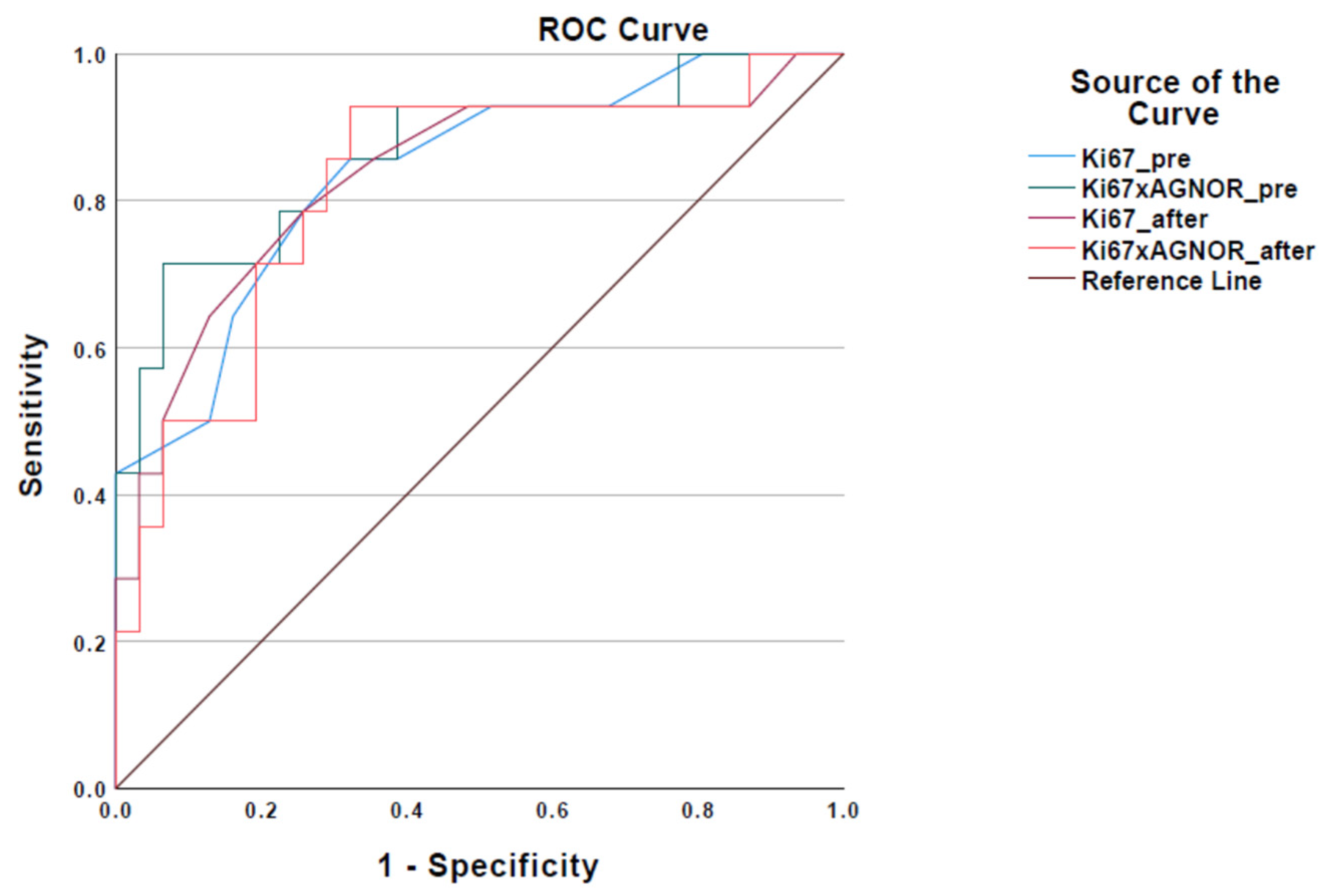
3.3. Cut-Off Values for Ki-67 and Ki-67 × AgNOR
3.4. Comparison of Immunocytochemical (Ki-67 and Ki-67 × AgNOR) and Histopathological Grading
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berlato, D.; Bulman-Fleming, J.; Clifford, C.A.; Garrett, L.; Intile, J.; Jones, P.; Kamstock, D.A.; Liptak, J.M.; Pavuk, A.; Powell, R.; et al. Value, Limitations, and Recommendations for Grading of Canine Cutaneous Mast Cell Tumors: A Consensus of the Oncology-Pathology Working Group. Vet. Pathol. 2021, 58, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Kiupel, M.; Webster, J.D.; Bailey, K.L.; Best, S.; DeLay, J.; Detrisac, C.J.; Fitzgerald, S.D.; Gamble, D.; Ginn, P.E.; Goldschmidt, M.H.; et al. Proposal of a 2-Tier Histologic Grading System for Canine Cutaneous Mast Cell Tumors to More Accurately Predict Biological Behavior. Vet. Pathol. 2011, 48, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.K.; Ehler, W.J.; Mac Ewen, E.G. Canine Cutaneous Mast Cell Tumor: Morphologic Grading and Survival Time in 83 Dogs. Vet. Pathol. 1984, 21, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Garrett, L.D. Canine mast cell tumors: Diagnosis, treatment, and prognosis. Vet. Med. 2014, 5, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Willmann, M.; Yuzbasiyan-Gurkan, V.; Marconato, L.; Dacasto, M.; Hadzijusufovic, E.; Hermine, O.; Sadovnik, I.; Gamperl, S.; Schneeweiss-Gleixner, M.; Gleixner, K.V.; et al. Proposed Diagnostic Criteria and Classification of Canine Mast Cell Neoplasms: A Consensus Proposal. Front. Vet. Sci. 2021, 8, 755258. [Google Scholar] [CrossRef] [PubMed]
- Sledge, D.G.; Webster, J.; Kiupel, M. Canine cutaneous mast cell tumors: A combined clinical and pathologic approach to diagnosis, prognosis, and treatment selection. Vet. J. 2016, 215, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Camus, M.S.; Priest, H.L.; Koehler, J.W.; Driskell, E.A.; Rakich, P.M.; Ilha, M.R.; Krimer, P.M. Cytologic Criteria for Mast Cell Tumor Grading in Dogs With Evaluation of Clinical Outcome. Vet. Pathol. 2016, 53, 1117–1123. [Google Scholar] [CrossRef]
- Hergt, F.; von Bomhard, W.; Kent, M.S.; Hirschberger, J. Use of a 2-tier histologic grading system for canine cutaneous mast cell tumors on cytology specimens. Vet. Clin. Path. 2016, 45, 477–483. [Google Scholar] [CrossRef]
- Scase, T.J.; Edwards, D.; Miller, J.; Henley, W.; Smith, K.; Blunden, A.; Murphy, S. Canine mast cell tumors: Correlation of apoptosis and proliferation markers with prognosis. J. Vet. Intern. Med. 2006, 20, 151–158. [Google Scholar] [CrossRef]
- Webster, J.D.; Yuzbasiyan-Gurkan, V.; Miller, R.A.; Kaneene, J.B.; Kiupel, M. Cellular proliferation in canine cutaneous mast cell tumors: Associations with c-KIT and its role in prognostication. Vet. Pathol. 2007, 44, 298–308. [Google Scholar] [CrossRef]
- Horta, R.S.; Lavalle, G.E.; Monteiro, L.N.; Souza, M.C.C.; Cassali, G.D.; Araújo, R.B. Assessment of Canine Mast Cell Tumor Mortality Risk Based on Clinical, Histologic, Immunohistochemical, and Molecular Features. Vet. Pathol. 2018, 55, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Sabattini, S.; Renzi, A.; Marconato, L.; Militerno, G.; Agnoli, C.; Barbiero, L.; Rigillo, A.; Capitani, O.; Tinto, D.; Bettini, G. Comparison between May-Grünwald-Giemsa and rapid cytological stains in fine-needle aspirates of canine mast cell tumour: Diagnostic and prognostic implications. Vet. Comp. Oncol. 2018, 16, 511–551. [Google Scholar] [CrossRef] [PubMed]
- Kiupel, M.; Camus, M. Diagnosis and Prognosis of Canine Cutaneous Mast Cell Tumors. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, F.; Grandi, F.; Rocha, N.S. The need for cytologic/histologic correlation studies to establish a cytologic grading system for canine mast cell tumors in veterinary medicine. Vet. Clin. Path. 2011, 40, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.; Kudnig, S.T.; Firestone, S.M. Diagnostic accuracy of pre-treatment biopsy for grading cutaneous mast cell tumours in dogs. Vet. Comp. Oncol. 2018, 16, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, F.; Sabattini, S.; Bettini, G. Cytological grading of canine cutaneous mast cell tumours: Cytological grading of canine MCTs. Vet. Comp. Oncol. 2016, 14, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Ploton, D.; Menager, M.; Jeannesson, P.; Himber, G.; Pigeon, F.; Adnet, J.J. Improvement in the staining and in the visualization of the argyrophilic proteins of the nucleolar organizer region at the optical level. Histochem. J. 1986, 18, 5–14. [Google Scholar] [CrossRef]
- Avallone, G.; Rasotto, R.; Chambers, J.K.; Miller, A.D.; Behling-Kelly, E.; Monti, P.; Berlato, D.; Valenti, P.; Roccabianca, P. Review of Histological Grading Systems in Veterinary Medicine. Vet. Pathol. 2021, 58, 809–828. [Google Scholar] [CrossRef]
- Paes, P.R.O.; Horta, R.S.; Luza, L.C.; Pierezan, F.; Costa, M.P.; Lavalle, G.E. Inclusion of fibroblasts and collagen fibrils in the cytologic grading of canine cutaneous mast cell tumors. Vet. Clin. Path. 2022, 51, 339–348. [Google Scholar] [CrossRef]
- Lee, C.E.; Lindley, S.S.; Smith, A.N.; Gaillard, P.; Henderson, R.A.; Matz, B.M. Predictive ability of fine-needle aspirate cytology for incompletely resected mast cell tumor surgical sites. Can. Vet. J. 2021, 62, 141–144. [Google Scholar]
- Khan, M.Z.; Haleem, A.; Al Hassani, H.; Kfoury, H. Cytopathological grading, as a predictor of histopathological grade, in ductal carcinoma (NOS) of breast, on air-dried Diff-Quik smears. Diagn. Cytopathol. 2003, 29, 185–193. [Google Scholar] [CrossRef] [PubMed]
- de Nardi, A.B.; dos Santos Horta, R.; Fonseca-Alves, C.E.; de Paiva, F.N.; Linhares, L.C.M.; Firmo, B.F.; Ruiz Sueiro, F.A.; de Oliveira, K.D.; Lourenço, S.V.; De Francisco Strefezzi, R.; et al. Diagnosis, Prognosis and Treatment of Canine Cutaneous and Subcutaneous Mast Cell Tumors. Cells 2022, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Preziosi, R.; Morini, M.; Sarli, G. Expression of the KIT Protein (CD117) in Primary Cutaneous Mast Cell Tumors of the Dog. J. Vet. Diagn. Investig. 2004, 16, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Freytag, J.O.; Queiroz, M.R.; Govoni, V.M.; Pereira, I.V.A.; Pulz, L.H.; de Francisco Strefezzi, R.; Queiroga, F.L.; Cogliati, B. Prognostic value of immunohistochemical markers in canine cutaneous mast cell tumours: A systematic review and meta-analysis. Vet. Comp. Oncol. 2021, 19, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.D.; Bagci, P.; Ohike, N.; Saka, B.; Erbarut Seven, I.; Dursun, N.; Balci, S.; Gucer, H.; Jang, K.-T.; Tajiri, T.; et al. Calculation of the Ki67 index in pancreatic neuroendocrine tumors: A comparative analysis of four counting methodologies. Mod. Pathol. 2015, 28, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.R.; Engelmann, A.M.; Mello, C.B.E.; de Andrade, C.M. Adapted technique for demonstrating argyrophilic nucleolar organizer regions in the cytologic samples of canine mast cell tumors. Vet. Clin. Path. 2022, 51, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Vajdovich, P.; Psáder, R.; Tóth, Z.A.; Perge, E. Use of the Argyrophilic Nucleolar Region Method for Cytologic and Histologic Examination of the Lymph Nodes in Dogs. Vet. Pathol. 2004, 41, 338–345. [Google Scholar] [CrossRef]
- Kravis, L.D.; Vail, D.M.; Kisseberth, W.C.; Ogilvie, G.K.; Volk, L.M. Frequency of argyrophilic nucleolar organizer regions in fine-needle aspirates and biopsy specimens from mast cell tumors in dogs. J. Am. Vet. Med. Assoc. 1996, 209, 1418–1420. [Google Scholar] [CrossRef]
- Priest, H.L.; Hume, K.R.; Killick, D.; Kozicki, A.; Rizzo, V.L.; Seelig, D.; Snyder, L.A.; Springer, N.L.; Wright, Z.M.; Robat, C. The use, publication and future directions of immunocytochemistry in veterinary medicine: A consensus of the Oncology-Pathology Working Group. Vet. Comp. Oncol. 2017, 15, 868–880. [Google Scholar] [CrossRef]
- Sailasuta, A.; Ketpun, D.; Piyaviriyakul, P.; Theerawatanasirikul, S.; Theewasutrakul, P.; Rungsipipat, A. The Relevance of CD117-Immunocytochemistry Staining Patterns to Mutational Exon-11 in c-kit Detected by PCR from Fine-Needle Aspirated Canine Mast Cell Tumor Cells. Vet. Med. Int. 2014, 2014, 787498. [Google Scholar] [CrossRef]
- Marouda, C.; Anagnostou, T.; Savvas, I.; Papazoglou, L.G.; Psalla, D. Τhe Effect of Opioid Administration on Cytologic and Histopathologic Diagnosis of Canine Cutaneous Mast Cell Tumors Treated by Surgical Excision. Vet. Sci. 2022, 9, 202. [Google Scholar] [CrossRef] [PubMed]
| Grading Systems Markers | Grade | Cases | Misclassified Cases |
|---|---|---|---|
| Kiupel’s histopathological system | Low | 31/45 (68.9%) | - |
| High | 14/45 (31.1%) | - | |
| Camus’s cytological system | Low | 25/45 (55.6%) | 3/25 |
| High | 20/45 (44.4%) | 9/20 | |
| Ki-67 grading in the present study | Low | 26/45 (57.8%) | 3/26 |
| High | 19/45 (42.2%) | 8/19 | |
| Ki-67 × AgNOR grading in the present study | Low | 27/45 (60%) | 3/27 |
| High | 18/45 (40%) | 7/18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marouda, C.; Anagnostou, T.; Brunetti, B.; Savvas, I.; Papazoglou, L.G.; Psalla, D. Cutaneous Canine Mast Cell Tumor: The Use of Proliferative Markers (Ki-67 and Ki-67 × AgNOR) in Cytological Samples for Diagnosis and Prognosis. Vet. Sci. 2024, 11, 23. https://doi.org/10.3390/vetsci11010023
Marouda C, Anagnostou T, Brunetti B, Savvas I, Papazoglou LG, Psalla D. Cutaneous Canine Mast Cell Tumor: The Use of Proliferative Markers (Ki-67 and Ki-67 × AgNOR) in Cytological Samples for Diagnosis and Prognosis. Veterinary Sciences. 2024; 11(1):23. https://doi.org/10.3390/vetsci11010023
Chicago/Turabian StyleMarouda, Christina, Tilemahos Anagnostou, Barbara Brunetti, Ioannis Savvas, Lysimachos G. Papazoglou, and Dimitra Psalla. 2024. "Cutaneous Canine Mast Cell Tumor: The Use of Proliferative Markers (Ki-67 and Ki-67 × AgNOR) in Cytological Samples for Diagnosis and Prognosis" Veterinary Sciences 11, no. 1: 23. https://doi.org/10.3390/vetsci11010023
APA StyleMarouda, C., Anagnostou, T., Brunetti, B., Savvas, I., Papazoglou, L. G., & Psalla, D. (2024). Cutaneous Canine Mast Cell Tumor: The Use of Proliferative Markers (Ki-67 and Ki-67 × AgNOR) in Cytological Samples for Diagnosis and Prognosis. Veterinary Sciences, 11(1), 23. https://doi.org/10.3390/vetsci11010023