G Protein-Coupled Estrogen Receptor (GPER) and ERs Are Modulated in the Testis–Epididymal Complex in the Normal and Cryptorchid Dog
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Collection
2.3. Immunohistochemistry
2.4. Western Blot
2.5. RNA Isolation, cDNA Synthesis, and Real-Time RT-PCR
2.6. Statistical Analysis
3. Results
3.1. Immunohistochemical Evaluation of GPER, ER-Alpha and ER-Beta in the Normal and Cryptorchid Testis of Dogs
3.2. Immunohistochemical Evaluation of GPER, ER-Alpha, and ER-Beta in the Normal and Cryptorchid Epididymis of Dogs
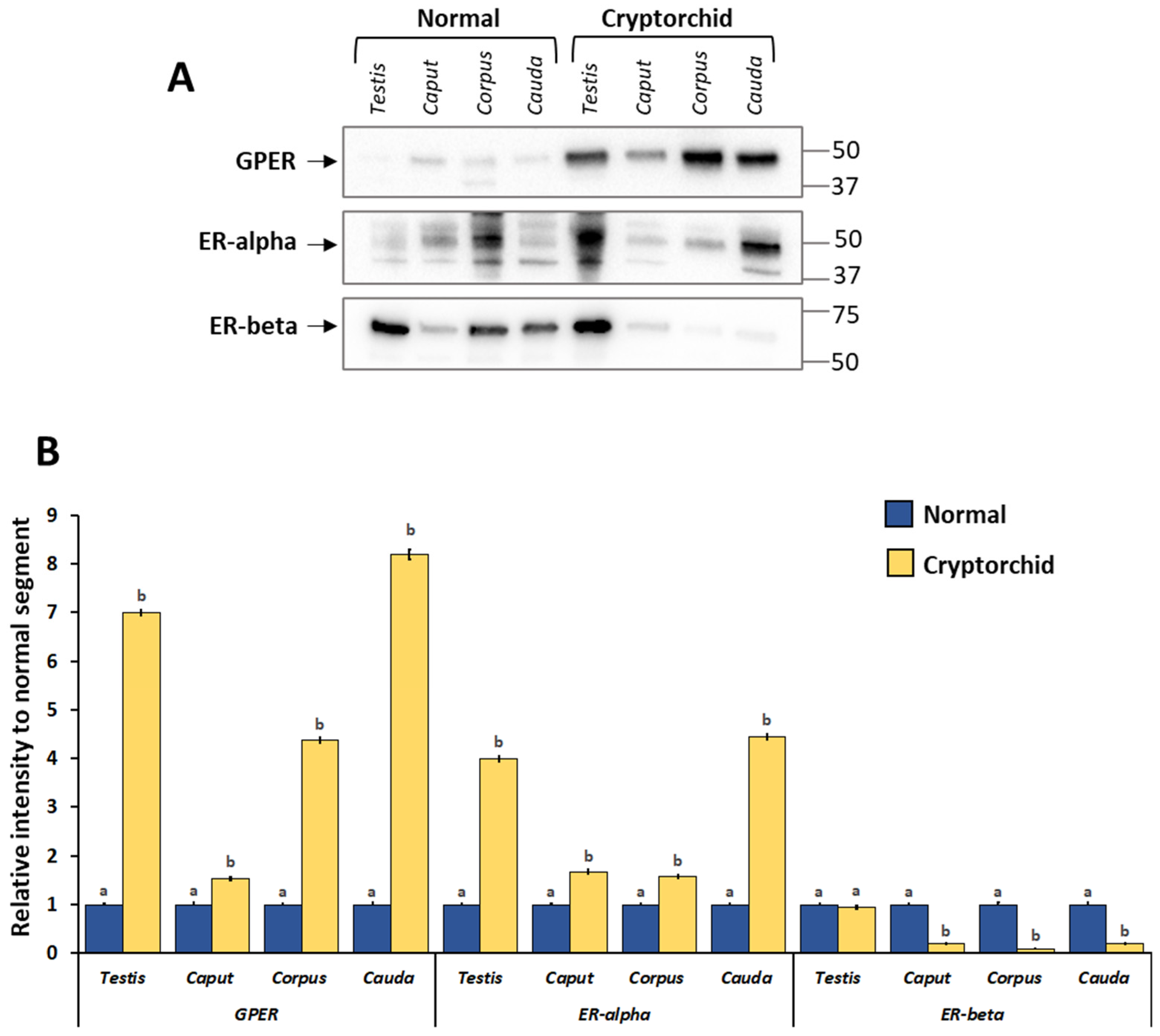
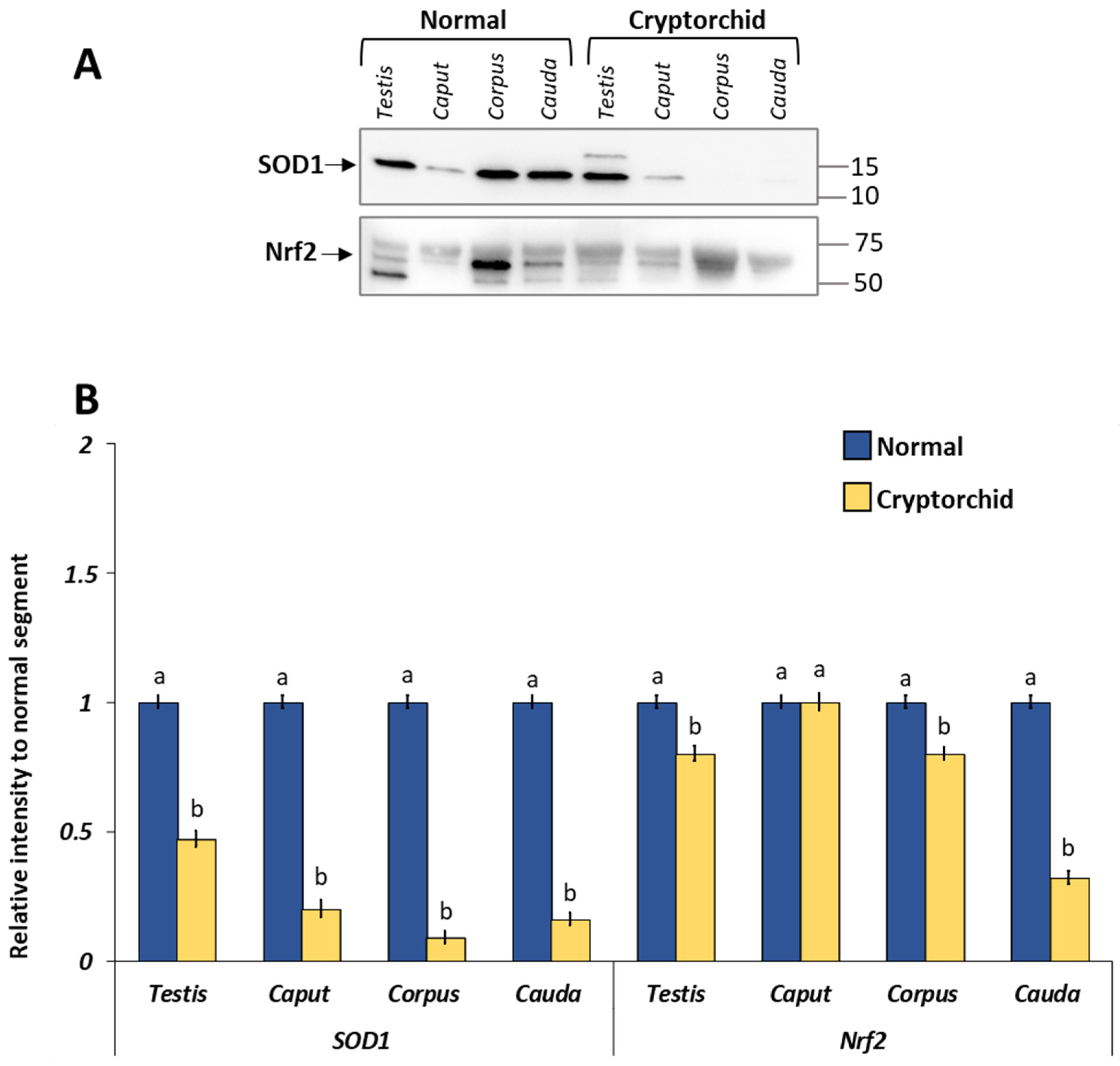
3.3. Protein Expression Levels of GPER, ER-Alpha and ER-Beta in the Normal and Cryptorchid Testis–Epididymal Complex: Western Blot and Densitometric Analysis
3.4. mRNA Expression Levels of GPER, ER-Alpha and ER-Beta in the Normal and Cryptorchid Testis–Epididymal Complex: Real-Time RT-PCR

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romagnoli, S.E. Canine cryptorchidism. Vet. Clin. N. Am. Small Anim. Pract. 1991, 21, 533–544. [Google Scholar] [CrossRef]
- Khan, F.A.; Gartley, C.J.; Khanam, A. Canine cryptorchidism: An update. Reprod. Domest. Anim. 2018, 53, 1263–1270. [Google Scholar] [CrossRef]
- Yates, D.; Hayes, G.; Heffernan, M.; Beynon, R. Incidence of cryptorchidism in dogs and cats. Vet. Rec. 2003, 152, 502–504. [Google Scholar] [CrossRef]
- Dolf, G.; Gaillard, C.; Schelling, C.; Hofer, A.; Leighton, E. Cryptorchidism and sex ratio are associated in dogs and pigs. J. Anim. Sci. 2008, 86, 2480–2485. [Google Scholar] [CrossRef]
- Sijstermans, K.; Hack, W.W.M.; Meijer, R.W.; Voort-Doedens, L.M. The frequency of undescended testis from birth to adulthood: A review. Int. J. Androl. 2008, 31, 1–11. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Y.; Yue, M.; Yue, W.; Ren, G.; Zhang, J.; Zhang, X.; He, J. Effect of cryptorchidism on the histomorphometry, proliferation, apoptosis, and autophagy in boar testes. Animals 2021, 11, 1379. [Google Scholar] [CrossRef]
- Ren, L.; Medan, M.S.; Ozu, M.; Li, C.; Watanabe, G.; Taya, K. Effects of experimental cryptorchidism on sperm motility and testicular endocrinology in adult male rats. J. Reprod. Dev. 2006, 52, 219. [Google Scholar] [CrossRef]
- Moon, J.H.; Yoo, D.Y.; Jo, Y.K.; Kim, G.A.; Jung, H.Y.; Choi, J.H.; Hwang, I.K.; Jang, G. Unilateral cryptorchidism induces morphological changes of testes and hyperplasia of Sertoli cells in a dog. Lab. Anim. Res. 2014, 30, 185–189. [Google Scholar] [CrossRef]
- Hayes, H.M., Jr.; Pendergrass, T.W. Canine testicular tumors: Epidemiologic features of 410 dogs. Int. Cancer 1976, 18, 482–487. [Google Scholar] [CrossRef]
- Liao, A.T.; Chu, P.Y.; Yeh, L.S.; Lin, C.T.; Liu, C.H. A 12-year retrospective study of canine testicular tumors. J. Vet. Med. Sci. 2009, 71, 919–923. [Google Scholar] [CrossRef]
- Hornakova, L.; Vrbovska, T.; Pavlak, M.; Valencakova-Agyagosova, A.; Halo, M.; Hajurka, J. The evaluation of blood concentrations of testosterone, 17β-oestradiol and anti-Mullerian hormone in dogs with cryptorchidism and testicular tumours. Pol. J. Vet. Sci. 2017, 20, 677–685. [Google Scholar] [CrossRef]
- Mischke, R.; Meurer, D.; Hoppen, H.O.; Ueberschär, S.; Hewicker-Trautwein, M. Blood plasma concentrations of oestradiol-17beta, testosterone and testosterone/oestradiol ratio in dogs with neoplastic and degenerative testicular diseases. Res. Vet. Sci. 2002, 73, 267–272. [Google Scholar] [CrossRef]
- Cooke, P.S.; Nanjappa, M.K.; Ko, C.; Prins, G.S.; Hess, R.A. Estrogens In Male Physiology. Physiol. Rev. 2017, 97, 995–1043. [Google Scholar] [CrossRef]
- Hess, R.A.; Cooke, P.S. Estrogen in the male: A historical perspective. Biol. Reprod. 2018, 99, 27–44. [Google Scholar] [CrossRef]
- Hejmej, A.; Bilińska, B. The effects of cryptorchidism on the regulation of steroidogenesis and gap junctional communication in equine testes. Endokrynol. Pol. 2008, 59, 112–118. [Google Scholar]
- Assisi, L.; Pelagalli, A.; Squillacioti, C.; Liguori, G.; Annunziata, C.; Mirabella, N. Orexin A-mediated modulation of reproductive activities in testis of normal and cryptorchid dogs: Possible model for studying relationships between energy metabolism and reproductive control. Front. Endocrinol. 2019, 10, 816. [Google Scholar] [CrossRef]
- Carmeci, C.; Thompson, D.A.; Ring, H.Z.; Francke, U.; Weigel, R.J. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 1997, 45, 607–617. [Google Scholar] [CrossRef]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. The G protein-coupled oestrogen receptor GPER in health and disease: An update. Nat. Rev. Endocrinol. 2023, 19, 407–424. [Google Scholar] [CrossRef]
- Luo, J.; Liu, D. Does GPER Really Function as a G Protein-Coupled Estrogen Receptor in vivo? Front. Endocrinol. 2020, 11, 148. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef]
- Chimento, A.; De Luca, A.; Nocito, M.C.; Avena, P.; La Padula, D.; Zavaglia, L.; Pezzi, V. Role of GPER-Mediated Signaling in Testicular Functions and Tumorigenesis. Cells 2020, 9, 2115. [Google Scholar] [CrossRef]
- Witkowski, M.; Pardyak, L.; Pawlicki, P.; Galuszka, A.; Profaska-Szymik, M.; Plachno, B.J.; Kantor, S.; Duliban, M.; Kotula-Balak, M. The G-Protein-coupled membrane estrogen receptor is present in horse cryptorchid testes and mediates downstream pathways. Int. J. Mol. Sci. 2021, 22, 7131. [Google Scholar] [CrossRef]
- Lucas, T.F.; Royer, C.; Siu, E.R.; Lazari, M.F.; Porto, C.S. Expression and signaling of G protein-coupled estrogen receptor 1 (GPER) in rat sertoli cells. Biol. Reprod. 2010, 83, 307–317. [Google Scholar] [CrossRef]
- Chimento, A.; Sirianni, R.; Delalande, C.; Silandre, D.; Bois, C.; Andò, S.; Maggiolini, M.; Carreau, S.; Pezzi, V. 17 beta-estradiol activates rapid signaling pathways involved in rat pachytene spermatocytes apoptosis through GPR30 and ER alpha. Mol. Cell. Endocrinol. 2010, 320, 136–144. [Google Scholar] [CrossRef]
- Chimento, A.; Sirianni, R.; Zolea, F.; Bois, C.; Delalande, C.; Andò, S.; Maggiolini, M.; Aquila, S.; Carreau, S.; Pezzi, V. Gper and ESRs are expressed in rat round spermatids and mediate oestrogen-dependent rapid pathways modulating expression of cyclin B1 and Bax. Int. J. Androl. 2011, 34 Pt 1, 420–429. [Google Scholar] [CrossRef]
- Walczak-Jędrzejowska, R.; Forma, E.; Oszukowska, E.; Bryś, M.; Marchlewska, K.; Kula, K.; Słowikowska-Hilczer, J. Expression of G-Protein-coupled estrogen receptor (GPER) in whole testicular tissue and laser-capture microdissected testicular compartments of men with normal and aberrant spermatogenesis. Biology 2022, 11, 373. [Google Scholar] [CrossRef]
- Malivindi, R.; Aquila, S.; Rago, V. Immunolocalization of G Protein-Coupled Estrogen Receptor in the Pig Epididymis. Anat. Rec. 2018, 301, 1467–1473. [Google Scholar] [CrossRef]
- Rago, V.; Romeo, F.; Giordano, F.; Malivindi, R.; Pezzi, V.; Casaburi, I.; Carpino, A. Expression of oestrogen receptors (GPER, ESR1, ESR2) in human ductuli efferentes and proximal epididymis. Andrology 2018, 6, 192–198. [Google Scholar] [CrossRef]
- Martínez-Traverso, G.B.; Pearl, C.A. Immunolocalization of G protein-coupled estrogen receptor in the rat epididymis. Reprod. Biol. Endocrinol. 2015, 13, 48. [Google Scholar] [CrossRef]
- Galuszka, A.; Pawlicki, P.; Pardyak, L.; Chmurska-Gąsowska, M.; Pietsch-Fulbiszewska, A.; Duliban, M.; Turek, W.; Dubniewicz, K.; Ramisz, G.; Kotula-Balak, M. Abundance of estrogen receptors involved in non-canonical signaling in the dog testis. Anim. Reprod. Sci. 2021, 235, 106888. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.; Zhou, Q.; Jassim, E.; Saunders, P.T.; Hess, R.A. Differential expression of estrogen receptors alpha and beta in the reproductive tracts of adult male dogs and cats. Biol. Reprod. 2002, 66, 1161–1168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jung, H.Y.; Yoo, D.Y.; Jo, Y.K.; Kim, G.A.; Chung, J.Y.; Choi, J.H.; Jang, G.; Hwang, I.K. Differential expression of estrogen receptor α and progesterone receptor in the normal and cryptorchid testis of a dog. Lab. Anim. Res. 2016, 32, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Prapaiwan, N.; Manee-In, S.; Moonarmart, W.; Srisuwatanasagul, S. The expressions in oxytocin and sex steroid receptors in the reproductive tissues of normal and unilateral cryptorchid dogs. Theriogenology 2017, 100, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.T.; Sharpe, R.M.; Williams, K.; Macpherson, S.; Urquart, H.; Irvine, D.S.; Millar, M.R. Differential expression of oestrogen receptor alpha and beta proteins in the testes and male reproductive system of human and non-human primates. Mol. Hum. Reprod. 2001, 7, 227–236. [Google Scholar] [CrossRef]
- Hess, R.A.; Gist, D.H.; Bunick, D.; Lubahn, D.B.; Farrell, A.; Bahr, J.; Cooke, P.S.; Greene, G.L. Estrogen receptor (alpha and beta) expression in the excurrent ducts of the adult male rat reproductive tract. J. Androl. 1997, 18, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, M.K.; Koiri, R.K.; Srivastava, R. Expression of estrogen receptor alpha in response to stress and estrogen antagonist tamoxifen in the shell gland of Gallus gallus domesticus: Involvement of anti-oxidant system and estrogen. Stress 2021, 24, 261–272. [Google Scholar] [CrossRef]
- Aitken, R.J.; Roman, S.D. Antioxidant systems and oxidative stress in the testes. Oxid. Med. Cell. Longev. 2008, 1, 15–24. [Google Scholar] [CrossRef]
- Del Prete, C.D.; Ciani, F.; Tafuri, S.; Pasolini, M.P.; Valle, G.D.; Palumbo, V.; Abbondante, L.; Calamo, A.; Barbato, V.; Gualtieri, R. Effect of superoxide dismutase, catalase, and glutathione peroxidase supplementation in the extender on chilled semen of fertile and hypofertile dogs. J. Vet. Sci. 2018, 19, 667–675. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J. Biol. Chem. 2017, 292, 16817–16824. [Google Scholar] [CrossRef]
- Kirby, J.; Halligan, E.; Baptista, M.J.; Allen, S.; Heath, P.R.; Holden, H.; Barber, S.C.; Loynes, C.A.; Wood-Allum, C.A.; Lunec, J.; et al. Mutant SOD1 alters the motor neuronal transcriptome: Implications for familial ALS. Brain 2005, 128, 1686–1706. [Google Scholar] [CrossRef] [PubMed]
- Squillacioti, C.; De Luca, A.; Liguori, G.; Alì, S.; Germano, G.; Vassalotti, G.; Navas, L.; Mirabella, N. Urocortinergic system in the testes of normal and cryptorchid dogs. Ann. Anat. Anat. Anz. 2016, 207, 91. [Google Scholar] [CrossRef] [PubMed]
- Kotula-Balak, M.; Pawlicki, P.; Milon, A.; Tworzydlo, W.; Sekula, M.; Pacwa, A.; Gorowska-Wojtowicz, E.; Bilinska, B.; Pawlicka, B.; Wiater, J. The role of G-protein-coupled membrane estrogen receptor in mouse Leydig cell function-in vivo and in vitro evaluation. Cell Tissue Res. 2018, 374, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Rago, V.; Romeo, F.; Giordano, F.; Maggiolini, M.; Carpino, A. Identification of the estrogen receptor GPER in neoplastic and non-neoplastic human testes. Reprod. Biol. Endocrinol. 2011, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Vaucher, L.; Funaro, M.G.; Mehta, A.; Mielnik, A.; Bolyakov, A.; Prossnitz, E.R.; Schlegel, P.N.; Paduch, D.A. Activation of GPER-1 estradiol receptor downregulates production of testosterone in isolated rat Leydig cells and adult human testis. PLoS ONE 2014, 9, e92425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Nie, R.; Prins, G.S.; Saunders, P.T.; Katzenellenbogen, B.S.; Hess, R.A. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J. Androl. 2002, 23, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, F.; Zhang, S.; Sheng, X.; Han, X.; Weng, Q.; Yuan, Z. Seasonal expression of androgen receptor, aromatase, and estrogen receptor alpha and beta in the testis of the wild ground squirrel (Citellus dauricus Brandt). Eur. J. Histochem. 2015, 59, 2456. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.N.; Gorelick, D.A. Crosstalk between nuclear and G protein-coupled estrogen receptors. Gen. Comp. Endocrinol. 2018, 261, 190–197. [Google Scholar] [CrossRef]
- Chevalier, N.; Hinault, C.; Clavel, S.; Paul-Bellon, R.; Fenichel, P. GPER and Testicular Germ Cell Cancer. Front. Endocrinol. 2020, 11, 600404. [Google Scholar] [CrossRef]
- Chevalier, N.; Vega, A.; Bouskine, A.; Siddeek, B.; Michiels, J.F.; Chevallier, D.; Fénichel, P. GPR30, the non-classical membrane G protein related estrogen receptor, is overexpressed in human seminoma and promotes seminoma cell proliferation. PLoS ONE 2012, 7, e34672. [Google Scholar] [CrossRef]
- Virtanen, H.E.; Toppari, J. Cryptorchidism and Fertility. Endocrinol. Metab. Clin. N. Am. 2015, 44, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, E.; Hirano, T.; Hori, T.; Tsutsui, T. Testicular superoxide dismutase activity, heat shock protein 70 concentration and blood plasma inhibin-alpha concentration of dogs with a Sertoli cell tumor in a unilateral cryptorchid testis. J. Vet. Med. Sci. 2007, 69, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Takihara, H.; Matsuyama, H. Elevated scrotal temperature, but not varicocele grade, reflects testicular oxidative stress-mediated apoptosis. World J. Urol. 2010, 28, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Tafuri, S.; Ciani, F.; Iorio, E.L.; Esposito, L.; Cocchia, N. Reactive Oxygen Species (ROS) and Male Fertility. In New Discoveries in Embryology; Wu, B., Ed.; IntechOpen: London, UK, 2015; pp. 19–40. [Google Scholar]
- Zhao, H.; Song, L.; Ma, N.; Liu, C.; Dun, Y.; Zhou, Z.; Yuan, D.; Zhang, C. The dynamic changes of Nrf2 mediated oxidative stress, DNA damage and base excision repair in testis of rats during aging. Exp. Gerontol. 2021, 152, 111460. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Y.; Piao, Y.; Nagaoka, K.; Watanabe, G.; Taya, K.; Li, C.M. Protective effects of nuclear factor erythroid 2-related factor 2 on whole body heat stress-induced oxidative damage in the mouse testis. Reprod. Biol. Endocrinol. 2013, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A.; Fernandes, S.A.; Gomes, G.R.; Oliveira, C.A.; Lazari, M.F.; Porto, C.S. Estrogen and its receptors in efferent ductules and epididymis. J. Androl. 2011, 32, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A. The efferent ductules: Structure and functions. In The Epididymis: From Molecules to Clinical Practice; Robaire, B., Hinton, B., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; pp. 49–80. [Google Scholar]
- Krejčířová, R.; Maňasová, M.; Sommerová, V.; Langhamerová, E.; Rajmon, R.; Maňásková-Postlerová, P. G protein-coupled estrogen receptor (GPER) in adult boar testes, epididymis and spermatozoa during epididymal maturation. Int. J. Biol. Macromol. 2018, 116, 113–119. [Google Scholar] [CrossRef]
- Lu, P.; Wang, F.; Song, X.; Liu, Y.; Zhang, K.; Cao, N. Relative abundance of G protein-coupled receptor 30 and localization in testis and epididymis of sheep at different developmental stages. Anim. Reprod. Sci. 2016, 175, 10–17. [Google Scholar] [CrossRef]
- Filippi, S.; Luconi, M.; Granchi, S.; Vignozzi, L.; Bettuzzi, S.; Tozzi, P.; Ledda, F.; Forti, G.; Maggi, M. Estrogens, but not androgens, regulate expression and functional activity of oxytocin receptor inrabbit epididymis. Endocrinology 2002, 143, 4271. [Google Scholar] [CrossRef][Green Version]
- Asl, H.F.; Mashayekhi, F.J.; Bayat, M.; Habibi, D.; Zendedel, A.; Baazm, M. Role of epididymis and testis in nuclear factor erythroid 2-related factor 2 signaling in mouse experimental cryptorchidism. Iran. Red. Crescent. Med. J. 2019, 21, 1–7. [Google Scholar] [CrossRef]
- Chen, J.Q.; Cammarata, P.R.; Baines, C.P.; Yager, J.D. Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim. Biophys. Acta 2009, 1793, 1540–1570. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Mauvais-Jarvis, F. Rapid, nongenomic estrogen actions protect pancreatic islet survival. Islets 2009, 1, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.H.; Bozkus, F.; Uremis, N.; Uremis, M.M. The protective role of G protein-coupled estrogen receptor 1 (GPER-1) on methotrexate-induced nephrotoxicity in human renal epithelium cells. Ren Fail 2016, 38, 686–692. [Google Scholar] [CrossRef]
- Imam Aliagan, A.; Madungwe, N.B.; Tombo, N.; Feng, Y.; Bopassa, J.C. Chronic GPER1 activation protects against oxidative stress-induced cardiomyoblast death via preservation of mitochondrial integrity and deactivation of mammalian sterile-20-like kinase/yes-associated protein pathway. Front. Endocrinol. 2020, 11, 579161. [Google Scholar] [CrossRef]
- Chen, G.; Zeng, H.; Li, X.; Liu, J.; Li, Z.; Xu, R.; Ma, Y.; Liu, C.; Xue, B. Activation of G protein coupled estrogen receptor prevents chemotherapy-induced intestinal mucositis by inhibiting the DNA damage in crypt cell in an extracellular signal-regulated kinase 1- and 2- dependent manner. Cell Death Dis. 2021, 12, 1034. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Liu, K.; Liu, J.; Chai, S.; Chen, G.; Wen, S.; Ming, T.; Wang, J.; Ma, Y.; et al. Activation of the G Protein-coupled estrogen receptor prevented the development of acute colitis by protecting the crypt cell. J. Pharmacol. Exp. Ther. 2021, 376, 281–293. [Google Scholar] [CrossRef]



| Primer Name | Gene Name | Primer Sequence 5′-3′ | Genbank Accession Number | Product Size |
|---|---|---|---|---|
| dGPERfor dGPERrev | Canis lupus familiaris GPER1 | AAAGCCTGCAGTGTCTTGGTATC TGGGTACTGGTGATTCTGGACTT | XM_005621204.2 | 150 bp 1 |
| dESR1for dESR1rev | Canis lupus familiaris ESRA | TCGGAAAACTGCTCCTGTAAATG ACCACAATCTCTCGGTCAAAGAG | NM_001002936.1 | 150 bp |
| dESR2for dESR2rev | Canis lupus familiaris ESRB | CGTGCTAGAGATGAAATCGTTAATG CCCCTGTTTCCTGAGCAGTCTAT | XM_038591983.1 | 152 bp |
| dGAPDHfor dGAPDHfor | Canis lupus familiaris GAPDH | TGTCCCCACCCCCAATG TCGTCATATTTGGCAGCTTTCTC | XM_003434387 | 69 bp |
| Cytotypes | |||||||
|---|---|---|---|---|---|---|---|
| Leydig Cells | Sertoli Cells | Pre-Meiotic Cells | Pachytene Spermatocytes | Round Spermatids | Oval Spermatids | Elongated Spermatids | |
| GPER | |||||||
| Normal | +++ | - | - | +++ | + | ++ | - |
| Cryptorchid | ++ | ++ | - | - | - | - | - |
| ER-alpha | |||||||
| Normal | ++ | - | - | - | +++ | - | - |
| Cryptorchid | +++ | ++ | - | - | - | - | - |
| ER-beta | |||||||
| Normal | ++ | +++ | - | - | - | - | +++ |
| Cryptorchid | +++ | +++ | - | - | - | - | - |
| GPER | ER-Alpha | ER-Beta | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Epididymal Segments | Immunostaining Pattern | |||||||||
| P | B | PM | P | B | PM | P | B | PM | ||
| NORMAL | Caput | + | - | - | ++ | +++ | - | ++ | +++ | - |
| Corpus | ++ | - | + | ++ | - | - | ++ | +++ | - | |
| Cauda | ++ | - | + | ++ | - | - | +++ | +++ | - | |
| Caput | ++ | - | - | ++ | - | - | ++ | +++ | - | |
| CRYPTORCHID | Corpus | + | - | ++ | ++ | - | - | + | ++ | - |
| Cauda | ++ | - | +++ | + * | - | - | +++ | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liguori, G.; Tafuri, S.; Pelagalli, A.; Ali’, S.; Russo, M.; Mirabella, N.; Squillacioti, C. G Protein-Coupled Estrogen Receptor (GPER) and ERs Are Modulated in the Testis–Epididymal Complex in the Normal and Cryptorchid Dog. Vet. Sci. 2024, 11, 21. https://doi.org/10.3390/vetsci11010021
Liguori G, Tafuri S, Pelagalli A, Ali’ S, Russo M, Mirabella N, Squillacioti C. G Protein-Coupled Estrogen Receptor (GPER) and ERs Are Modulated in the Testis–Epididymal Complex in the Normal and Cryptorchid Dog. Veterinary Sciences. 2024; 11(1):21. https://doi.org/10.3390/vetsci11010021
Chicago/Turabian StyleLiguori, Giovanna, Simona Tafuri, Alessandra Pelagalli, Sabrina Ali’, Marco Russo, Nicola Mirabella, and Caterina Squillacioti. 2024. "G Protein-Coupled Estrogen Receptor (GPER) and ERs Are Modulated in the Testis–Epididymal Complex in the Normal and Cryptorchid Dog" Veterinary Sciences 11, no. 1: 21. https://doi.org/10.3390/vetsci11010021
APA StyleLiguori, G., Tafuri, S., Pelagalli, A., Ali’, S., Russo, M., Mirabella, N., & Squillacioti, C. (2024). G Protein-Coupled Estrogen Receptor (GPER) and ERs Are Modulated in the Testis–Epididymal Complex in the Normal and Cryptorchid Dog. Veterinary Sciences, 11(1), 21. https://doi.org/10.3390/vetsci11010021








