Effect of the Probiotic Bacillus subtilis DE-CA9TM on Fecal Scores, Serum Oxidative Stress Markers and Fecal and Serum Metabolome in Healthy Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Dogs
2.2. Study Design
2.3. Preparation of Samples for Microbiome Analysis
2.4. Untargeted Metabolomic Analysis of Fecal and Serum Samples
2.4.1. Sample Preparation for Metabolome Analysis
2.4.2. Metabolome Analysis by GC–MS
2.5. Analysis of Serum Antioxidant Status
2.6. Measurement of Oxidant Status
2.7. Statistical Analysis of Fecal and Serum Metabolites
2.8. Statistical Analysis of Fecal Scores
2.9. Statistical Analysis of Oxidative Stress Markers and Microbiome Markers
3. Results
3.1. Effect of DE-CA9TM vs. Placebo on Physical Examination Findings, Complete Blood Counts (CBC), Serum Biochemistry, and Canine Pancreatic Specific Lipase (cPLI) Measurements
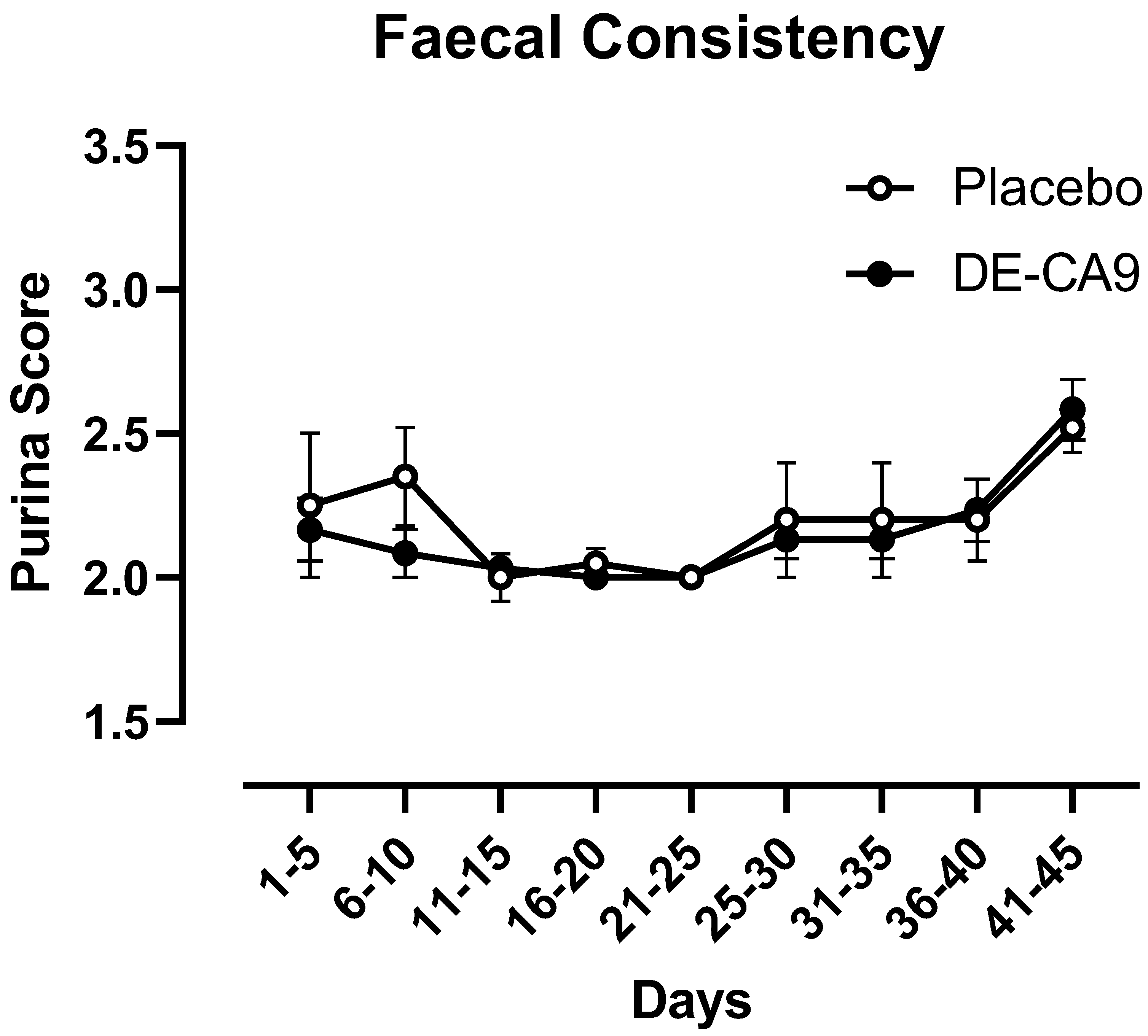
3.2. Effect of DE-CA9TM vs. Placebo on Fecal Scoring
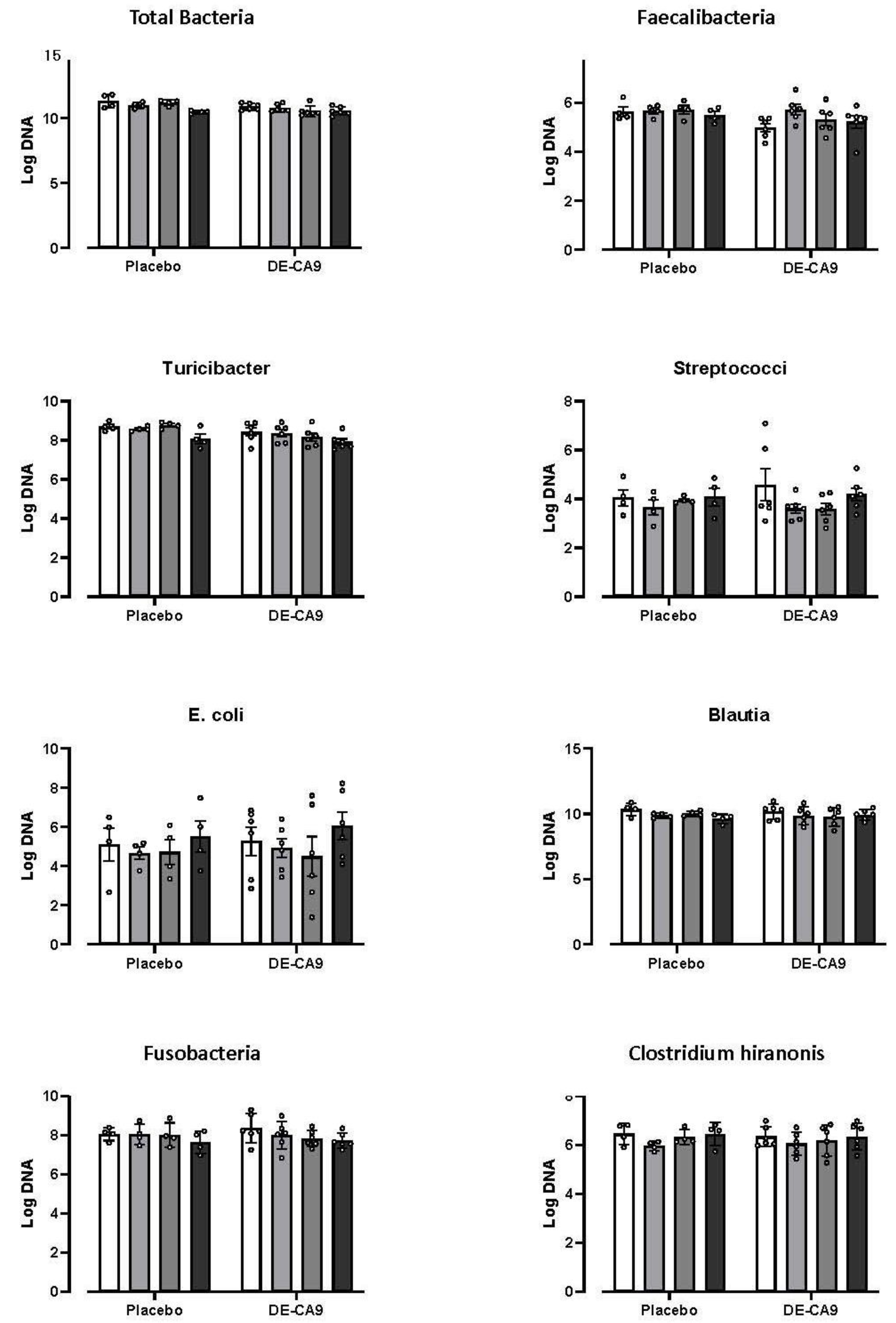
3.3. Effect of DE-CA9TM on Gut Microbiota
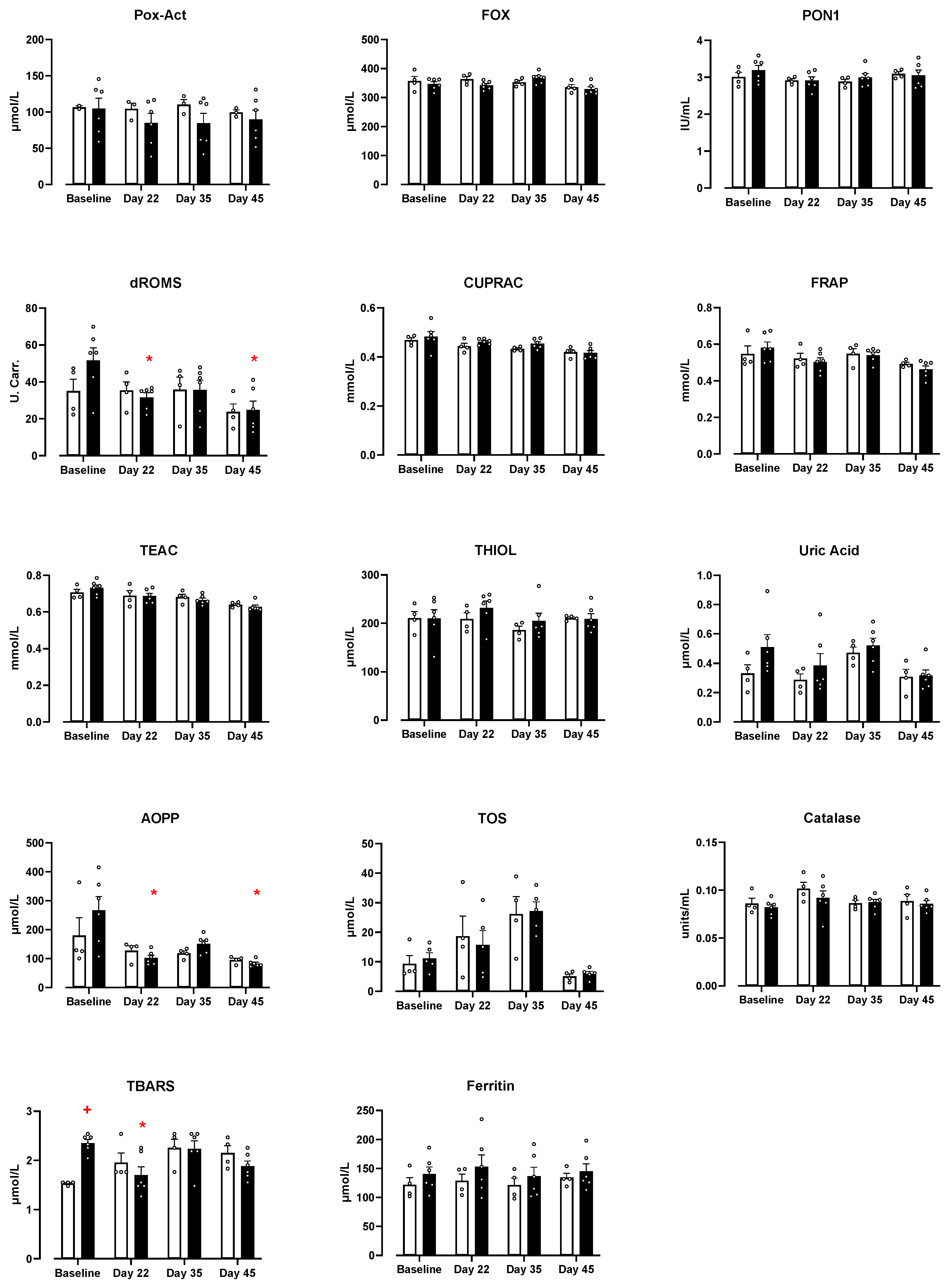
3.4. Effect of DE-CA9TM vs. Placebo on Serum Markers of Oxidative Stress
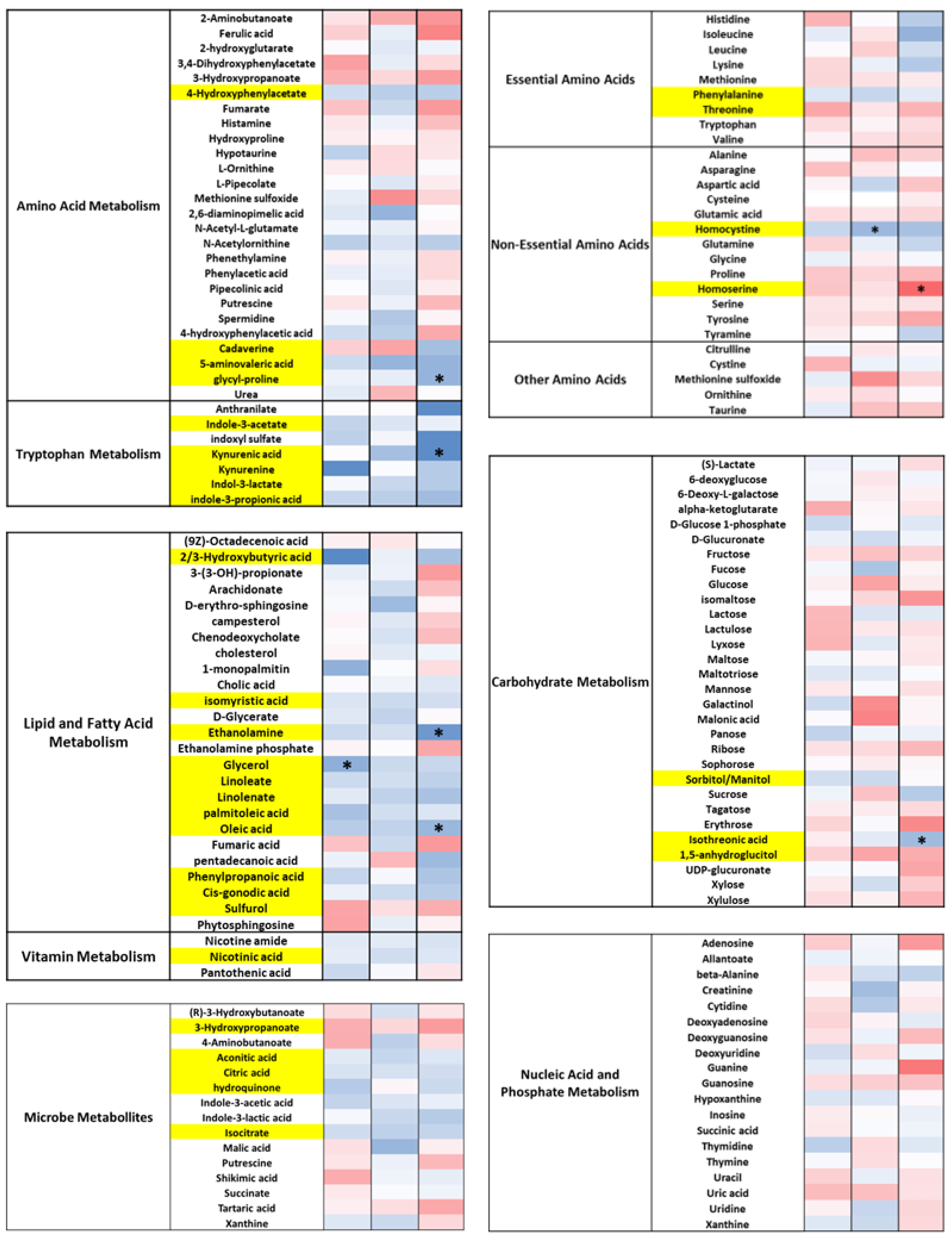
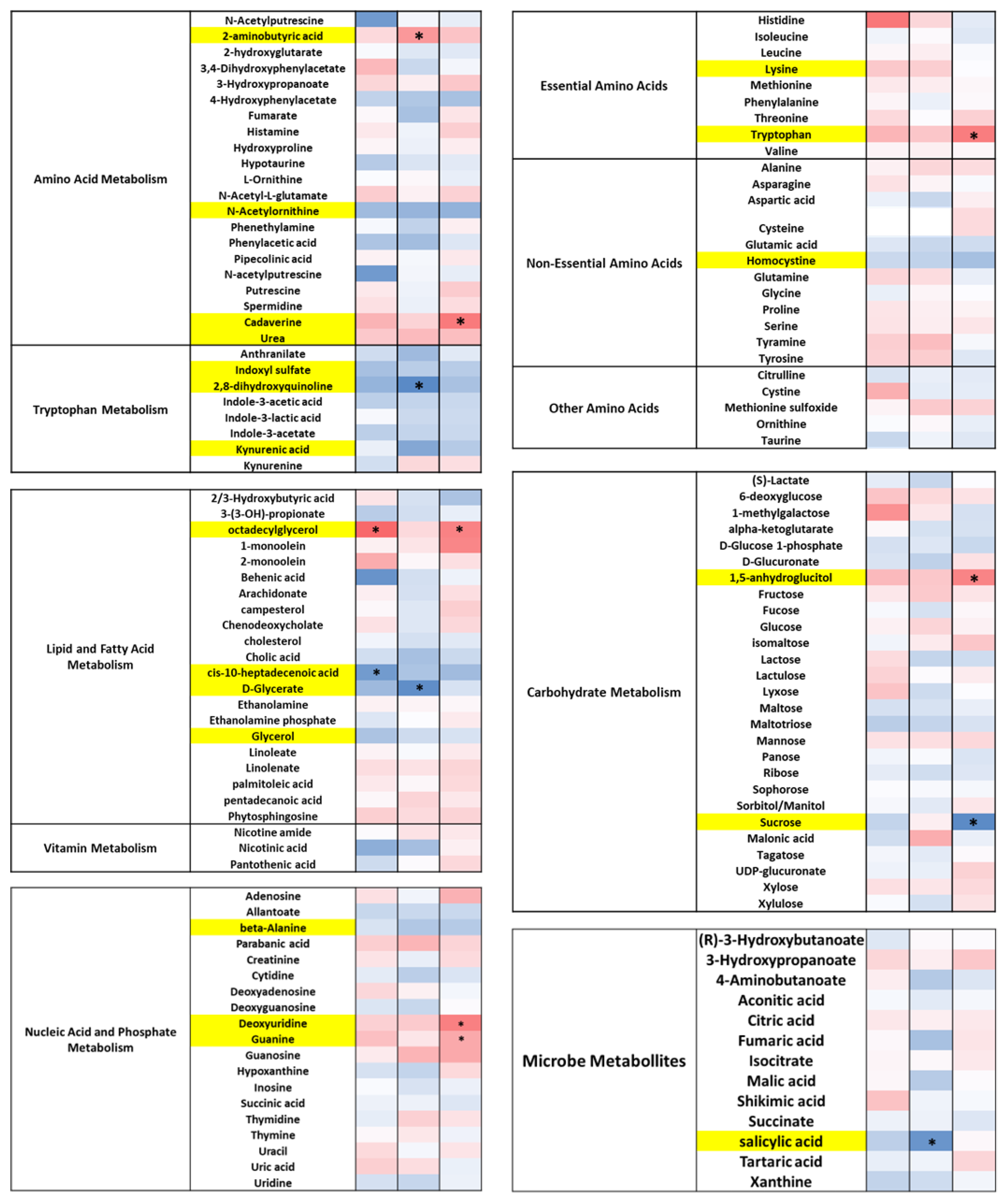
3.5. DE-CA9TM Has an Overall Effect in the Fecal Metabolites Associated with Tryptophan, Lipid, and Fatty Acid Metabolism, as Well as Amino Acids
3.6. DE-CA9TM Has an Overall Effect on Amino Acid, Fatty Acid, Vitamin, and Microbial Metabolism in Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Hu, J.; Kim, I.-H. Effect of Bacillus subtilis C-3102 Spores as a Probiotic Feed Supplement on Growth Performance, Nutrient Digestibility, Diarrhea Score, Intestinal Microbiota, and Excreta Odor Contents in Weanling Piglets. Animals 2022, 12, 316. [Google Scholar] [CrossRef] [PubMed]
- Colom, J.; Freitas, D.; Simon, A.; Brodkorb, A.; Buckley, M.; Deaton, J.; Winger, A.M. Presence and Germination of the Probiotic Bacillus subtilis DE111® in the Human Small Intestinal Tract: A Randomized, Crossover, Double-Blind, and Placebo-Controlled Study. Front. Microbiol. 2021, 12, 715863. [Google Scholar] [CrossRef]
- Lahiri, K.R.; Singh, R.; Apte, M.; Patil, M.; Taksande, A.; Varona, R.; Chatterjee, G.; Verma, M.; Brette, S.; Perez, M.I. Efficacy and Safety of Bacillus Clausii (O/C, N/R, SIN, T) Probiotic Combined with Oral Rehydration Therapy (ORT) and Zinc in Acute Diarrhea in Children: A Randomized, Double-Blind, Placebo-Controlled Study in India. Trop. Dis. Travel. Med. Vaccines 2022, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A Universal Cell Factory for Industry, Agriculture, Biomaterials and Medicine. Microb. Cell Fact. 2020, 19, 173. [Google Scholar] [CrossRef]
- Castañeda, C.D.; Gamble, J.N.; Wamsley, K.G.S.; McDaniel, C.D.; Kiess, A.S. In Ovo Administration of Bacillus subtilis Serotypes Effect Hatchability, 21-Day Performance, and Intestinal Microflora. Poult. Sci. 2021, 100, 101125. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus As Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef]
- Altcheh, J.; Carosella, M.V.; Ceballos, A.; D’Andrea, U.; Jofre, S.M.; Marotta, C.; Mugeri, D.; Sabbaj, L.; Soto, A.; Josse, C.; et al. Randomized, Direct Comparison Study of Saccharomyces Boulardii CNCM I-745 versus Multi-Strained Bacillus Clausii Probiotics for the Treatment of Pediatric Acute Gastroenteritis. Medicine 2022, 101, e30500. [Google Scholar] [CrossRef]
- Blumer, N.; Sel, S.; Virna, S.; Patrascan, C.C.; Zimmermann, S.; Herz, U.; Renz, H.; Garn, H. Perinatal Maternal Application of Lactobacillus rhamnosus GG Suppresses Allergic Airway Inflammation in Mouse Offspring. Clin. Exp. Allergy 2007, 37, 348–357. [Google Scholar] [CrossRef]
- De Castro, J.-A.; Kesavelu, D.; Lahiri, K.R.; Chaijitraruch, N.; Chongsrisawat, V.; Jog, P.P.; Liaw, Y.H.; Nguyen, G.K.; Nguyen, T.V.H.; Pai, U.A.; et al. Recommendations for the Adjuvant Use of the Poly-Antibiotic-Resistant Probiotic Bacillus Clausii (O/C, SIN, N/R, T) in Acute, Chronic, and Antibiotic-Associated Diarrhea in Children: Consensus from Asian Experts. Trop. Dis. Travel. Med. Vaccines 2020, 6, 21. [Google Scholar] [CrossRef]
- Freedman, K.E.; Hill, J.L.; Wei, Y.; Vazquez, A.R.; Grubb, D.S.; Trotter, R.E.; Wrigley, S.D.; Johnson, S.A.; Foster, M.T.; Weir, T.L. Examining the Gastrointestinal and Immunomodulatory Effects of the Novel Probiotic Bacillus subtilis DE111. Int. J. Mol. Sci. 2021, 22, 2453. [Google Scholar] [CrossRef]
- Gharib-Naseri, K.; Dorigam, J.C.P.; Doranalli, K.; Morgan, N.; Swick, R.A.; Choct, M.; Wu, S.-B. Bacillus Amyloliquefaciens CECT 5940 Improves Performance and Gut Function in Broilers Fed Different Levels of Protein and/or under Necrotic Enteritis Challenge. Anim. Nutr. 2021, 7, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Sadiq, F.A.; Liu, W.; Cao, L.; Shen, Z. Protective Effects of Bacillus subtilis ASAG 216 on Growth Performance, Antioxidant Capacity, Gut Microbiota and Tissues Residues of Weaned Piglets Fed Deoxynivalenol Contaminated Diets. Food Chem. Toxicol. 2021, 148, 111962. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.C.; Townsend, J.R.; Johnson, S.B.; Toy, A.M.; Vantrease, W.C.; Bender, D.; Crimi, C.C.; Stowers, K.L.; Ruiz, M.D.; VanDusseldorp, T.A.; et al. Effects of Probiotic (Bacillus subtilis) Supplementation During Offseason Resistance Training in Female Division I Athletes. J. Strength. Cond. Res. 2020, 34, 3173–3181. [Google Scholar] [CrossRef] [PubMed]
- Wauters, L.; Slaets, H.; De Paepe, K.; Ceulemans, M.; Wetzels, S.; Geboers, K.; Toth, J.; Thys, W.; Dybajlo, R.; Walgraeve, D.; et al. Efficacy and Safety of Spore-Forming Probiotics in the Treatment of Functional Dyspepsia: A Pilot Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Gastroenterol. Hepatol. 2021, 6, 784–792. [Google Scholar] [CrossRef]
- Santos, F.D.S.; Maubrigades, L.R.; Gonçalves, V.S.; Alves Ferreira, M.R.; Brasil, C.L.; Cunha, R.C.; Conceição, F.R.; Leite, F.P.L. Immunomodulatory Effect of Short-Term Supplementation with Bacillus Toyonensis BCT-7112T and Saccharomyces Boulardii CNCM I-745 in Sheep Vaccinated with Clostridium Chauvoei. Vet. Immunol. Immunopathol. 2021, 237, 110272. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Huang, K.; Zhu, M.; Liu, Q.; Liu, G.; Chen, F.; Zhang, H.; Qin, S. Selenium Enriched Bacillus subtilis Yb-1114246 Activated the TLR2-NF-ΚB1 Signaling Pathway to Regulate Chicken Intestinal β-Defensin 1 Expression. Food Funct. 2021, 12, 5913–5926. [Google Scholar] [CrossRef]
- Memon, F.U.; Yang, Y.; Leghari, I.H.; Lv, F.; Soliman, A.M.; Zhang, W.; Si, H. Transcriptome Analysis Revealed Ameliorative Effects of Bacillus Based Probiotic on Immunity, Gut Barrier System, and Metabolism of Chicken under an Experimentally Induced Eimeria Tenella Infection. Genes 2021, 12, 536. [Google Scholar] [CrossRef]
- Fritsch, D.A.; Jackson, M.I.; Wernimont, S.M.; Feld, G.K.; Badri, D.V.; Brejda, J.J.; Cochrane, C.-Y.; Gross, K.L. Adding a Polyphenol-Rich Fiber Bundle to Food Impacts the Gastrointestinal Microbiome and Metabolome in Dogs. Front. Vet. Sci. 2022, 9, 1039032. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Jha, A.R.; Oba, P.M.; Yotis, S.M.; Shmalberg, J.; Honaker, R.W.; Swanson, K.S. Longitudinal Fecal Microbiome and Metabolite Data Demonstrate Rapid Shifts and Subsequent Stabilization after an Abrupt Dietary Change in Healthy Adult Dogs. Anim. Microbiome 2022, 4, 46. [Google Scholar] [CrossRef]
- Galler, A.I.; Klavins, K.; Burgener, I.A. A Preliminary Metabolomic Study of Yorkshire Terrier Enteropathy. Metabolites 2022, 12, 264. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The Implications of Oxidative Stress and Antioxidant Therapies in Inflammatory Bowel Disease: Clinical Aspects and Animal Models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.M.; Rush, J.E.; Milbury, P.E.; Blumberg, J.B. Antioxidant Status and Biomarkers of Oxidative Stress in Dogs with Congestive Heart Failure. J. Vet. Intern. Med. 2005, 19, 537–541. [Google Scholar] [CrossRef]
- Almela, R.M.; Rubio, C.P.; Cerón, J.J.; Ansón, A.; Tichy, A.; Mayer, U. Selected Serum Oxidative Stress Biomarkers in Dogs with Non-Food-Induced and Food-Induced Atopic Dermatitis. Vet. Dermatol. 2018, 29, 229-e82. [Google Scholar] [CrossRef]
- Pugliese, M.; Biondi, V.; Merola, G.; Landi, A.; Passantino, A. Oxidative Stress Evaluation in Dogs Affected with Canine Monocytic Ehrlichiosis. Antioxidants 2022, 11, 328. [Google Scholar] [CrossRef]
- Rubio, C.P.; Martínez-Subiela, S.; Hernández-Ruiz, J.; Tvarijonaviciute, A.; Cerón, J.J.; Allenspach, K. Serum Biomarkers of Oxidative Stress in Dogs with Idiopathic Inflammatory Bowel Disease. Vet. J. 2017, 221, 56–61. [Google Scholar] [CrossRef]
- Segarra, S.; Martínez-Subiela, S.; Cerdà-Cuéllar, M.; Martínez-Puig, D.; Muñoz-Prieto, A.; Rodríguez-Franco, F.; Rodríguez-Bertos, A.; Allenspach, K.; Velasco, A.; Cerón, J. Oral Chondroitin Sulfate and Prebiotics for the Treatment of Canine Inflammatory Bowel Disease: A Randomized, Controlled Clinical Trial. BMC Vet. Res. 2016, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Purina 9-Point Fecal Scoring Chart. Available online: https://www.purinainstitute.com/centresquare/nutritional-and-clinical-assessment/purina-fecal-scoring-chart (accessed on 22 July 2023).
- AlShawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A Dysbiosis Index to Assess Microbial Changes in Fecal Samples of Dogs with Chronic Inflammatory Enteropathy. FEMS Microbiol. Ecol. 2017, 93, fix136. [Google Scholar] [CrossRef]
- White, R.; Atherly, T.; Guard, B.; Rossi, G.; Wang, C.; Mosher, C.; Webb, C.; Hill, S.; Ackermann, M.; Sciabarra, P.; et al. Randomized, Controlled Trial Evaluating the Effect of Multi-Strain Probiotic on the Mucosal Microbiota in Canine Idiopathic Inflammatory Bowel Disease. Gut Microbes 2017, 8, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Garvey, W.T.; Newman, J.W.; Lok, K.H.; Hoppel, C.L.; Adams, S.H. Plasma Metabolomic Profiles Reflective of Glucose Homeostasis in Non-Diabetic and Type 2 Diabetic Obese African-American Women. PLoS ONE 2010, 5, e15234. [Google Scholar] [CrossRef]
- Campos, C.; Guzmán, R.; López-Fernández, E.; Casado, Á. Evaluation of the Copper(II) Reduction Assay Using Bathocuproinedisulfonic Acid Disodium Salt for the Total Antioxidant Capacity Assessment: The CUPRAC–BCS Assay. Anal. Biochem. 2009, 392, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.P.; Tvarijonaviciute, A.; Martinez-Subiela, S.; Hernández-Ruiz, J.; Cerón, J.J. Validation of an Automated Assay for the Measurement of Cupric Reducing Antioxidant Capacity in Serum of Dogs. BMC Vet. Res. 2016, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.P.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Arnao, M.B.; Ceron, J.J. Validation of Three Automated Assays for Total Antioxidant Capacity Determination in Canine Serum Samples. J. Vet. Diagn. Investig. 2016, 28, 693–698. [Google Scholar] [CrossRef] [PubMed]
- González-Arostegui, L.G.; Muñoz-Prieto, A.; Tvarijonaviciute, A.; Cerón, J.J.; Rubio, C.P. Measurement of Redox Biomarkers in the Whole Blood and Red Blood Cell Lysates of Dogs. Antioxidants 2022, 11, 424. [Google Scholar] [CrossRef]
- Johansson, L.H.; Håkan Borg, L.A. A Spectrophotometric Method for Determination of Catalase Activity in Small Tissue Samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced Oxidation Protein Products as a Novel Marker of Oxidative Stress in Uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Arostegui, L.G.G.; Prieto, A.M.; Marín, L.P.; López, G.G.; Tvarijonaviciute, A.; Madrigal, J.J.C.; Rubio, C.P. Changes in Biomarkers of Redox Status in Serum and Saliva of Dogs with Hypothyroidism. BMC Vet. Res. 2023, 19, 33. [Google Scholar] [CrossRef]
- Nybroe, S.; Horsman, P.B.; Krag, K.; Hosbjerg, T.G.; Stenberg, K.; Khakimov, B.; Baymler, J.; Bjørnvad, C.R.; Kieler, I.N. Alterations in Healthy Adult Canine Faecal Microbiome and Selected Metabolites as a Result of Feeding a Commercial Complete Synbiotic Diet with Enterococcus faecium NCIMB 10415. Animals 2022, 13, 144. [Google Scholar] [CrossRef]
- Puurunen, J.; Ottka, C.; Salonen, M.; Niskanen, J.E.; Lohi, H. Age, Breed, Sex and Diet Influence Serum Metabolite Profiles of 2000 Pet Dogs. R. Soc. Open Sci. 2022, 9, 211642. [Google Scholar] [CrossRef]
- Forster, G.M.; Stockman, J.; Noyes, N.; Heuberger, A.L.; Broeckling, C.D.; Bantle, C.M.; Ryan, E.P. A Comparative Study of Serum Biochemistry, Metabolome and Microbiome Parameters of Clinically Healthy, Normal Weight, Overweight, and Obese Companion Dogs. Top. Companion Anim. Med. 2018, 33, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.E.; Wang, Y.; Claus, S.P.; Lawler, D.; Kochhar, S.; Holmes, E.; Nicholson, J.K. Metabolic Phenotype Modulation by Caloric Restriction in a Lifelong Dog Study. J. Proteome Res. 2013, 12, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Jewell, D.E. Feeding Healthy Beagles Medium-Chain Triglycerides, Fish Oil, and Carnitine Offsets Age-Related Changes in Serum Fatty Acids and Carnitine Metabolites. PLoS ONE 2012, 7, e49510. [Google Scholar] [CrossRef] [PubMed]
- Tvarijonaviciute, A.; Ceron, J.J.; Holden, S.L.; Cuthbertson, D.J.; Biourge, V.; Morris, P.J.; German, A.J. Obesity-Related Metabolic Dysfunction in Dogs: A Comparison with Human Metabolic Syndrome. BMC Vet. Res. 2012, 8, 147. [Google Scholar] [CrossRef]
- Gheorghe, C.E.; Martin, J.A.; Manriquez, F.V.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Focus on the Essentials: Tryptophan Metabolism and the Microbiome-Gut-Brain Axis. Curr. Opin. Pharmacol. 2019, 48, 137–145. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Beumer, J.; Clevers, H. How the Gut Feels, Smells, and Talks. Cell 2017, 170, 10–11. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef]
- Chen, L.; Yang, P.; Hu, L.; Yang, L.; Chu, H.; Hou, X. Modulating Phenylalanine Metabolism by L. Acidophilus Alleviates Alcohol-Related Liver Disease through Enhancing Intestinal Barrier Function. Cell Biosci. 2023, 13, 24. [Google Scholar] [CrossRef]
- Franco, R.; Reyes-Resina, I.; Navarro, G. Dopamine in Health and Disease: Much More Than a Neurotransmitter. Biomedicines 2021, 9, 109. [Google Scholar] [CrossRef]
- Braekke, K.; Ueland, P.M.; Harsem, N.K.; Karlsen, A.; Blomhoff, R.; Staff, A.C. Homocysteine, Cysteine, and Related Metabolites in Maternal and Fetal Plasma in Preeclampsia. Pediatr. Res. 2007, 62, 319–324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roth, W.; Mohamadzadeh, M. Vitamin B12 and Gut-Brain Homeostasis in the Pathophysiology of Ischemic Stroke. EBioMedicine 2021, 73, 103676. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.B.; Baipadithaya, G.; Balakrishnan, A.; Hegde, M.; Vohra, M.; Ahamed, R.; Nagri, S.K.; Ramachandra, L.; Satyamoorthy, K. Elevated Homocysteine Levels in Type 2 Diabetes Induce Constitutive Neutrophil Extracellular Traps. Sci. Rep. 2016, 6, 36362. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.H.; Bestard-Escalas, J.; Garate, J.; Maimó-Barceló, A.; Fernández, R.; Reigada, R.; Khorrami, S.; Ginard, D.; Okazaki, T.; Fernández, J.A.; et al. Tissue-Selective Alteration of Ethanolamine Plasmalogen Metabolism in Dedifferentiated Colon Mucosa. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiong, X.; Wang, K.-X.; Zou, L.-J.; Ji, P.; Yin, Y.-L. Ethanolamine Enhances Intestinal Functions by Altering Gut Microbiome and Mucosal Anti-Stress Capacity in Weaned Rats. Br. J. Nutr. 2018, 120, 241–249. [Google Scholar] [CrossRef]
- Foerster, E.G.; Mukherjee, T.; Cabral-Fernandes, L.; Rocha, J.D.B.; Girardin, S.E.; Philpott, D.J. How Autophagy Controls the Intestinal Epithelial Barrier. Autophagy 2022, 18, 86–103. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, X.; Wang, K.; Zou, L.; Lv, D.; Yin, Y. Ethanolamine Metabolism in the Mammalian Gastrointestinal Tract: Mechanisms, Patterns, and Importance. CMM 2017, 17, 92–99. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of Glycerol (E 422) as a Food Additive. EFSA J. 2017, 15, e04720. [Google Scholar] [CrossRef]
- Zelante, T.; Puccetti, M.; Giovagnoli, S.; Romani, L. Regulation of Host Physiology and Immunity by Microbial Indole-3-Aldehyde. Curr. Opin. Immunol. 2021, 70, 27–32. [Google Scholar] [CrossRef]
- Dagenais-Lussier, X.; Loucif, H.; Beji, C.; Telittchenko, R.; Routy, J.-P.; van Grevenynghe, J. Latest Developments in Tryptophan Metabolism: Understanding Its Role in B Cell Immunity. Cytokine Growth Factor. Rev. 2021, 59, 111–117. [Google Scholar] [CrossRef]
- Jamshed, L.; Debnath, A.; Jamshed, S.; Wish, J.V.; Raine, J.C.; Tomy, G.T.; Thomas, P.J.; Holloway, A.C. An Emerging Cross-Species Marker for Organismal Health: Tryptophan-Kynurenine Pathway. Int. J. Mol. Sci. 2022, 23, 6300. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int. J. Tryptophan Res. 2018, 11, 1178646918802282. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K. Organ Co-Relationship in Tryptophan Metabolism and Factors That Govern the Biosynthesis of Nicotinamide from Tryptophan. J. Nutr. Sci. Vitaminol. 2018, 64, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Candellone, A.; Girolami, F.; Badino, P.; Jarriyawattanachaikul, W.; Odore, R. Changes in the Oxidative Stress Status of Dogs Affected by Acute Enteropathies. Vet. Sci. 2022, 9, 276. [Google Scholar] [CrossRef]
- Chethan, G.E.; De, U.K.; Singh, M.K.; Chander, V.; Raja, R.; Paul, B.R.; Choudhary, O.P.; Thakur, N.; Sarma, K.; Prasad, H. Antioxidant Supplementation during Treatment of Outpatient Dogs with Parvovirus Enteritis Ameliorates Oxidative Stress and Attenuates Intestinal Injury: A Randomized Controlled Trial. Vet. Anim. Sci. 2023, 21, 100300. [Google Scholar] [CrossRef]
- Salminen, L.E.; Paul, R.H. Oxidative Stress and Genetic Markers of Suboptimal Antioxidant Defense in the Aging Brain: A Theoretical Review. Rev. Neurosci. 2014, 25, 805–819. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allenspach, K.; Sung, C.-H.; Ceron, J.J.; Peres Rubio, C.; Bourgois-Mochel, A.; Suchodolski, J.S.; Yuan, L.; Kundu, D.; Colom Comas, J.; Rea, K.; et al. Effect of the Probiotic Bacillus subtilis DE-CA9TM on Fecal Scores, Serum Oxidative Stress Markers and Fecal and Serum Metabolome in Healthy Dogs. Vet. Sci. 2023, 10, 566. https://doi.org/10.3390/vetsci10090566
Allenspach K, Sung C-H, Ceron JJ, Peres Rubio C, Bourgois-Mochel A, Suchodolski JS, Yuan L, Kundu D, Colom Comas J, Rea K, et al. Effect of the Probiotic Bacillus subtilis DE-CA9TM on Fecal Scores, Serum Oxidative Stress Markers and Fecal and Serum Metabolome in Healthy Dogs. Veterinary Sciences. 2023; 10(9):566. https://doi.org/10.3390/vetsci10090566
Chicago/Turabian StyleAllenspach, Karin, Chi-Hsuan Sung, Jose Joaquin Ceron, Camila Peres Rubio, Agnes Bourgois-Mochel, Jan S. Suchodolski, Lingnan Yuan, Debosmita Kundu, Joan Colom Comas, Kieran Rea, and et al. 2023. "Effect of the Probiotic Bacillus subtilis DE-CA9TM on Fecal Scores, Serum Oxidative Stress Markers and Fecal and Serum Metabolome in Healthy Dogs" Veterinary Sciences 10, no. 9: 566. https://doi.org/10.3390/vetsci10090566
APA StyleAllenspach, K., Sung, C.-H., Ceron, J. J., Peres Rubio, C., Bourgois-Mochel, A., Suchodolski, J. S., Yuan, L., Kundu, D., Colom Comas, J., Rea, K., & Mochel, J. P. (2023). Effect of the Probiotic Bacillus subtilis DE-CA9TM on Fecal Scores, Serum Oxidative Stress Markers and Fecal and Serum Metabolome in Healthy Dogs. Veterinary Sciences, 10(9), 566. https://doi.org/10.3390/vetsci10090566










