A Preliminary Study on the Relationship between Gastric Lesions and Anti-Inflammatory Drug Usage in Heavy Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Pig Breeding and Feeding Information
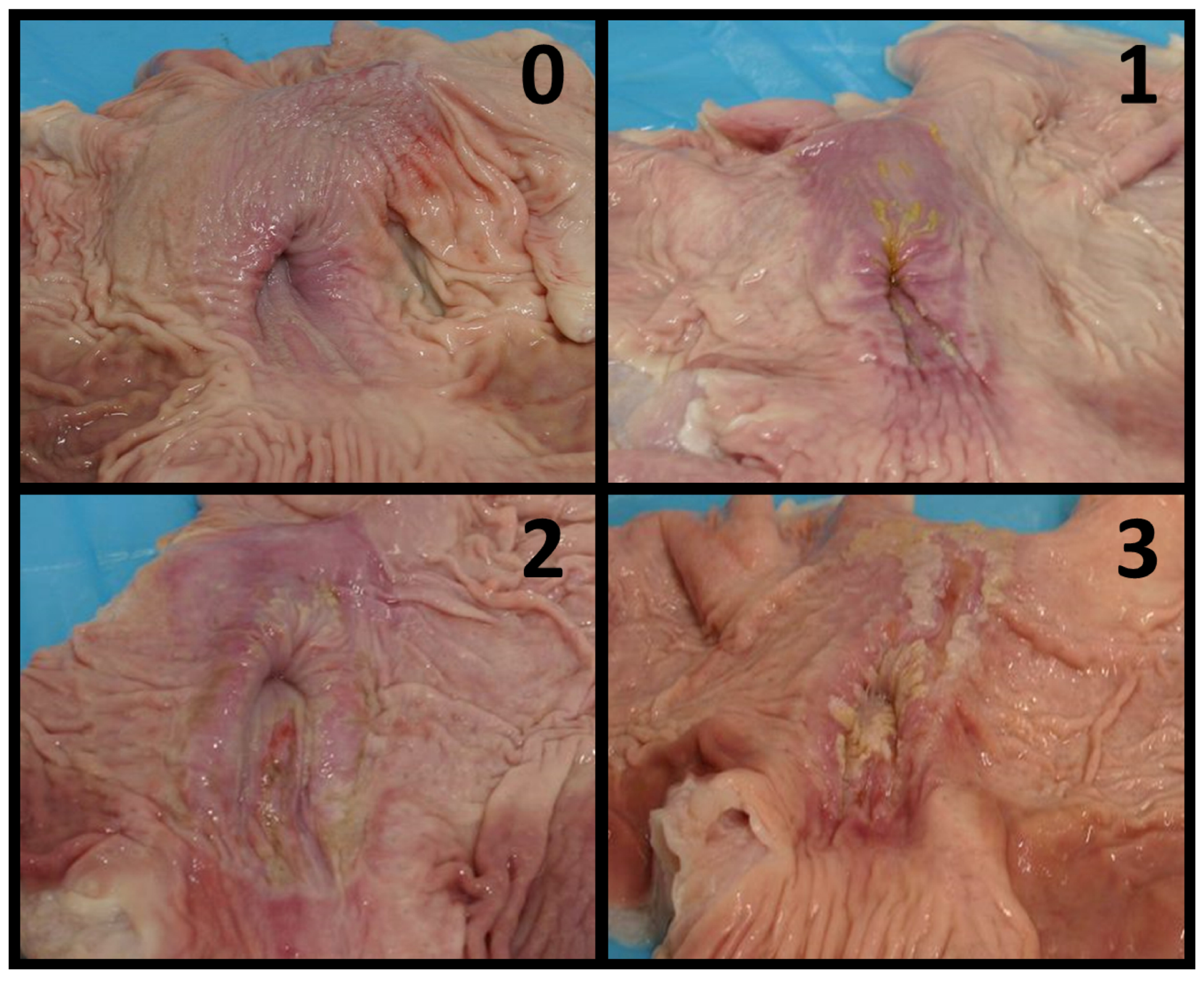
2.2. Sample Collection and Stomach Classification
2.3. Use of Anti-Inflammatory Drugs
2.4. Statistical Analysis
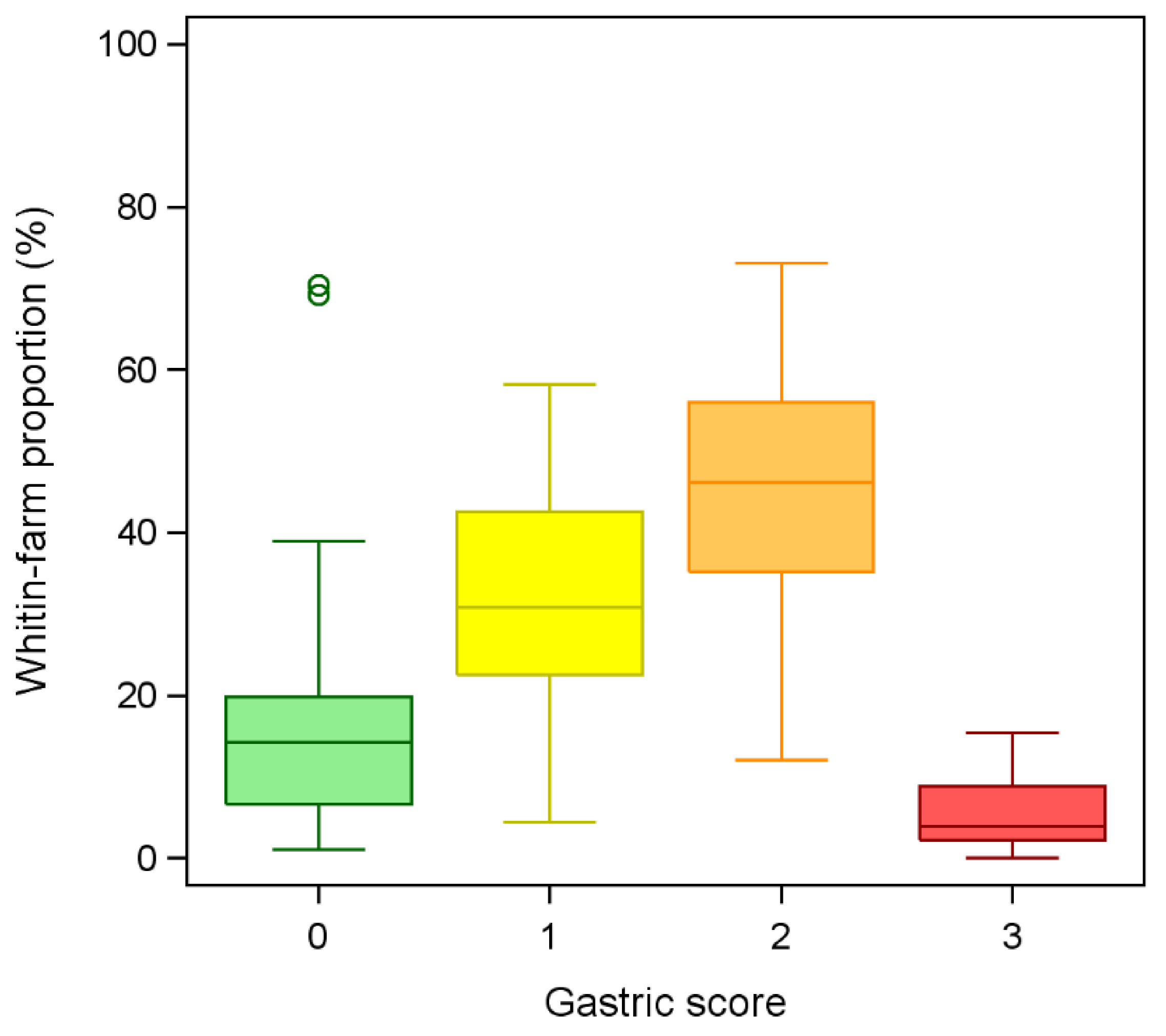
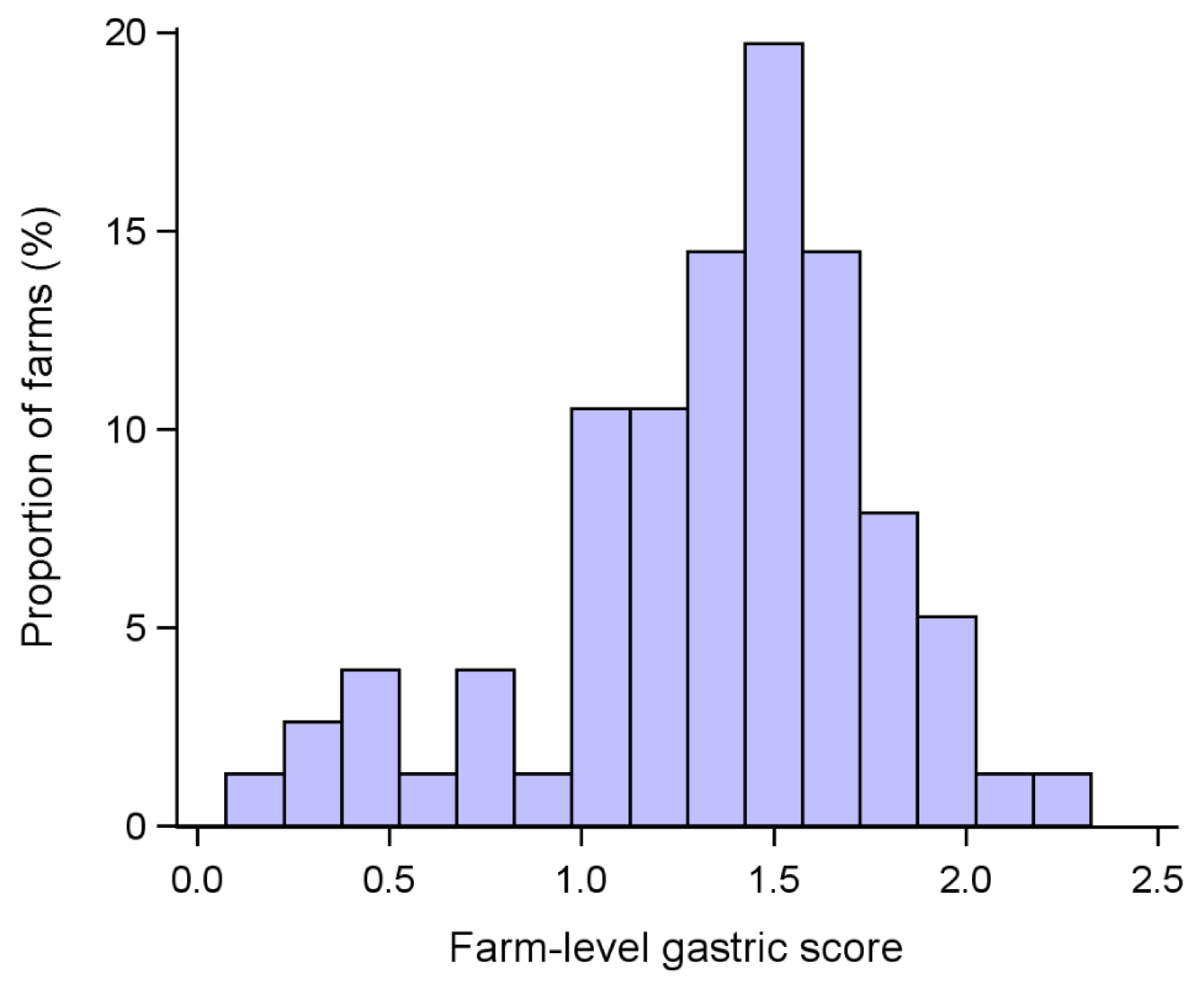
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melnichouk, S.I. Mortality associated with gastric ulceration in swine. Can. Vet. J. 2002, 43, 223–225. [Google Scholar] [PubMed]
- Rutherford, K.M.D.; Thompson, C.S.; Thomson, J.R.; Lawrence, A.B.; Nielsen, E.O.; Busch, M.E.; Haugegaard, S.; Sandoe, P. A study of associations between gastric ulcers and the behaviour of finisher pigs. Livest. Sci. 2018, 212, 45–51. [Google Scholar] [CrossRef]
- Cybulski, P.; Larska, M.; Wozniak, A.; Jablonski, A.; Stadejek, T. The Dietary Risk Factors of Gastric Ulcers in Finishing Pigs from 16 Polish Farms. Agriculture 2021, 11, 719. [Google Scholar] [CrossRef]
- Gottardo, F.; Scollo, A.; Contiero, B.; Bottacini, M.; Mazzoni, C.; Edwards, S.A. Prevalence and risk factors for gastric ulceration in pigs slaughtered at 170 kg. Animal 2017, 11, 2010–2018. [Google Scholar] [CrossRef]
- Swaby, H.; Gregory, N.G. A note on the frequency of gastric ulcers detected during post-mortem examination at a pig abattoir. Meat Sci. 2012, 90, 269–271. [Google Scholar] [CrossRef]
- Roels, S.; Ducatelle, R.; Broekaert, D. Keratin pattern in hyperkeratotic and ulcerated gastric Pars oesophagea in pigs. Res. Vet. Sci. 1997, 62, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.R.; Friendship, R.M. Digestive System. In Diseases of Swine, 10th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Stevenson, G.W., Schwartz, K.J., Eds.; Wiley-Blackwell: Chichester, UK, 2012; pp. 199–226. [Google Scholar]
- Robertson, I.D.; Accioly, J.M.; Moore, K.M.; Driesen, S.J.; Pethick, D.W.; Hampson, D.J. Risk factors for gastric ulcers in Australian pigs at slaughter. Prev. Vet. Med. 2002, 53, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Wondra, K.J.; Hancock, J.D.; Behnke, K.C.; Hines, R.H.; Stark, C.R. Effects of Particle-Size and Pelleting on Growth-Performance, Nutrient Digestibility, and Stomach Morphology in Finishing Pigs. J. Anim. Sci. 1995, 73, 757–763. [Google Scholar] [CrossRef]
- Cole, J.T.; Gookin, J.L.; Gayle, J.M.; Eisemann, J.H.; Argenzio, R.A.; Blikslager, A.T. Endoscopy via a gastric cannula to monitor the development of ulcers in the pars esophagea in pigs after consumption of a finely ground feed combined with a period of withholding of feed. Am. J. Vet. Res. 2002, 63, 1076–1082. [Google Scholar] [CrossRef]
- Mosseler, A.K.; Wintermann, M.F.; Beyerbach, M.; Kamphues, J. Effects of grinding intensity and pelleting of the diet—Fed either dry or liquid—on intragastric milieu, gastric lesions and performance of swine. Anim. Feed Sci. Technol. 2014, 194, 113–120. [Google Scholar] [CrossRef]
- Overholt, M.F.; Lowell, J.E.; Wilson, K.B.; Matulis, R.J.; Stein, H.H.; Dilger, A.C.; Boler, D.D. Effects of feeding pelleted diets without or with distillers dried grains with solubles on fresh belly characteristics, fat quality, and commercial bacon slicing yields of finishing pigs. J. Anim. Sci. 2016, 94, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Amory, J.R.; Mackenzie, A.M.; Pearce, G.P. Factors in the housing environment of finisher pigs associated with the development of gastric ulcers. Vet. Rec. 2006, 158, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Eisemann, J.H.; Morrow, W.E.M.; See, M.T.; Davies, P.R.; Zering, K. Effect of feed withdrawal prior to slaughter on prevalence of gastric ulcers in pigs. J. Am. Vet. Med. Assoc. 2002, 220, 503–506. [Google Scholar] [CrossRef]
- Peralvo-Vidal, J.M.; Weber, N.R.; Nielsen, J.P.; Bache, J.K.; Haugegaard, S.; Pedersen, A.O. Risk factors for gastric ulceration in nursery pigs. Prev. Vet. Med. 2021, 189, 105298. [Google Scholar] [CrossRef]
- Takeuchi, K. Pathogenesis of NSAID-induced gastric damage: Importance of cyclooxygenase inhibition and gastric hypermotility. World J. Gastroenterol. 2012, 18, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Radi, Z.A.; Khan, N.K. Effects of cyclooxygenase inhibition on the gastrointestinal tract. Exp. Toxicol. Pathol. 2006, 58, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Schoos, A.; Devreese, M.; Maes, D.G.D. Use of non-steroidal anti-inflammatory drugs in porcine health management. Vet. Rec. 2019, 185, 172. [Google Scholar] [CrossRef]
- Nixon, E.; Carlson, A.R.; Routh, P.A.; Hernandez, L.; Almond, G.W.; Baynes, R.E.; Messenger, K.M. Comparative effects of nonsteroidal anti-inflammatory drugs at castration and tail-docking in neonatal piglets. PLoS ONE 2021, 16, e0254409. [Google Scholar] [CrossRef]
- Nixon, E.; Chittenden, J.T.; Baynes, R.E.; Messenger, K.M. Pharmacokinetic/pharmacodynamic modeling of ketoprofen and flunixin at piglet castration and tail-docking. J. Vet. Pharmacol. Ther. 2019, 45, 450–466. [Google Scholar] [CrossRef]
- Holstege, M.M.C.; de Bont-Smolenaars, A.J.G.; Santman-Berends, I.M.G.A.; van der Linde-Witteveen, G.M.; van Schaik, G.; Velthuis, A.G.J.; Lam, T.J.G.M. Factors associated with high antimicrobial use in young calves on Dutch dairy farms: A case-control study. J. Dairy Sci. 2018, 101, 9259–9265. [Google Scholar] [CrossRef]
- Postma, M.; Stark, K.D.C.; Sjolund, M.; Backhans, A.; Beilage, E.G.; Losken, S.; Belloc, C.; Collineau, L.; Iten, D.; Visschers, V.; et al. Alternatives to the use of antimicrobial agents in pig production: A multi-country expert-ranking of perceived effectiveness, feasibility and return on investment. Prev. Vet. Med. 2015, 118, 457–466. [Google Scholar] [CrossRef]
- Ison, S.H.; Rutherford, K.M.D. Attitudes of farmers and veterinarians towards pain and the use of pain relief in pigs. Vet. J. 2014, 202, 622–627. [Google Scholar] [CrossRef]
- Consorzio del Prosciutto di Parma. Prosciutto di Parma (Parma Ham) Protected Designation of Origin Specifications. Specifications and Dossier Pursuant to Article 4 of Council Regulation EEC No. 2081/92 Dated 14 July 1992. Available online: https://www.prosciuttodiparma.com/wp-content/uploads/2019/07/Parma_Ham_Specifications_Disciplinare_Consolidato_Nov_13.pdf (accessed on 23 August 2023).
- Ministero della Salute—Direzione Generale Della Sanità Animale e dei Farmaci Veterinari. Sistema Informativo Nazionale Della Farmacosorveglianza—Ricetta Veterinaria Elettronica. Available online: https://www.ricettaveterinariaelettronica.it/ (accessed on 23 August 2023).
- Ministero della Salute—Portale dei Sistemi Informativi Veterinari. Available online: https://www.vetinfo.it/ (accessed on 23 August 2023).
- Timmerman, T.; Dewulf, J.; Catry, B.; Feyen, B.; Opsomer, G.; de Kruif, A.; Maes, D. Quantification and evaluation of antimicrobial drug use in group treatments for fattening pigs in Belgium. Prev. Vet. Med. 2006, 74, 251–263. [Google Scholar] [CrossRef] [PubMed]
- AACTING. Guidelines for Collection, Analysis and Reporting of Farm-Level Antimicrobial Use, in the Scope of Antimicrobial Stewardship (Version 1.2). Available online: https://aacting.org/swfiles/files/AACTING_Guidelines_V1.2_2019.07.02_54.pdf (accessed on 20 December 2022).
- Scali, F.; Santucci, G.; Maisano, A.M.; Giudici, F.; Guadagno, F.; Tonni, M.; Amicabile, A.; Formenti, N.; Giacomini, E.; Lazzaro, M.; et al. The Use of Antimicrobials in Italian Heavy Pig Fattening Farms. Antibiotics 2020, 9, 892. [Google Scholar] [CrossRef]
- Di Martino, G.; Capello, K.; Scollo, A.; Gottardo, F.; Stefani, A.L.; Rampin, F.; Schiavon, E.; Marangon, S.; Bonfanti, L. Continuous straw provision reduces prevalence of oesophago-gastric ulcer in pigs slaughtered at 170 kg (heavy pigs). Res. Vet. Sci. 2013, 95, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Scollo, A.; Abbas, M.; Contiero, B.; Gottardo, F. Undocked Tails, Mycoplasma-like Lesions and Gastric Ulcers in Slaughtering Pigs: What Connection? Animals 2023, 13, 305. [Google Scholar] [CrossRef]
- Nieuwoudt, W.; Lawrence, A.; Tarr, M. The Economic Selling weight of pigs. Agrekon 1976, 15, 8–10. [Google Scholar] [CrossRef]
- Guise, H.J.; Carlyle, W.W.H.; Penny, R.H.C.; Abbott, T.A.; Riches, H.L.; Hunter, E.J. Gastric ulcers in finishing pigs: Their prevalence and failure to influence growth rate. Vet. Rec. 1997, 141, 563–566. [Google Scholar] [CrossRef]
- van de Weerd, H.; Ison, S. Providing effective environmental enrichment to pigs: How far have we come? Animals 2019, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Scollo, A.; Di Martino, G.; Bonfanti, L.; Stefani, A.L.; Schiavon, E.; Marangon, S.; Gottardo, F. Tail docking and the rearing of heavy pigs: The role played by gender and the presence of straw in the control of tail biting. Blood parameters, behaviour and skin lesions. Res. Vet. Sci. 2013, 95, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Gastaldo, A.; Tremolada, C.; Borciani, M.; Iotti, G.; Barbieri, S.; Canali, E. Survey on the use of manipulable material as environmental enrichment in the pig farms in Italy. Large Anim. Rev. 2014, 2, 165–168. [Google Scholar]
- European Commission. Commission Recommendation (EU) 2016/336 of 8 March 2016 on the application of Council Directive 2008/120/EC laying down minimum standards for the protection of pigs as regards measures to reduce the need for tail-docking. OJEU 2016, 2016, 62. [Google Scholar]
- Herskin, M.S.; Jensen, H.E.; Jespersen, A.; Forkman, B.; Jensen, M.B.; Canibe, N.; Pedersen, L.J. Impact of the amount of straw provided to pigs kept in intensive production conditions on the occurrence and severity of gastric ulceration at slaughter. Res. Vet. Sci. 2016, 104, 200–206. [Google Scholar] [CrossRef]
- Ramis, G.; Gomez, S.; Pallares, F.J.; Munoz, A. Influence of farm size on the prevalence of oesophagogastric lesions in pigs at slaughter in south-east Spain. Vet. Rec. 2004, 155, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.K.; Ingvartsen, K.L. Effects of cereal disintegration method, feeding method and straw as bedding on stomach characteristics including ulcers and performance in growing pigs. Acta Agric. Scand. Sect. A-Anim. Sci. 2000, 50, 30–38. [Google Scholar] [CrossRef]
- Helbing, M.; Terranova, M.; Kreuzer, M.; Clauss, M. Evaluation of the prevalence of stomach ulcers in slaughtered pigs in a Swiss -abattoir. Schweiz. Arch. Tierheilkd. 2022, 164, 329–338. [Google Scholar] [CrossRef]
- Krauss, I.; Schwarz, L.; Schodl, K.; Knecht, C.; Brunthaler, R.; Metzler-Zebeli, B.; Leeb, C.; Hennig-Pauka, I. Assessing Gastric Ulceration in Fattening Pigs Housed without or with Straw and Additional Space—A Macroscopic and Microscopic Study on a Conventional Austrian Farm. Slov. Vet. Res. 2018, 55, 91–100. [Google Scholar] [CrossRef]
- Jensen, K.H.; Jorgensen, L.; Haugegaard, S.; Herskin, M.S.; Jensen, M.B.; Pedersen, L.J.; Canibe, N. The dose-response relationship between the amount of straw provided on the floor and gastric ulceration of pars oesophagea in growing pigs. Res. Vet. Sci. 2017, 112, 66–74. [Google Scholar] [CrossRef]
- Laryea, M.; Emikpe, B.; Attoh-Kotoku, V.; Omotosho, O.; Asare, D.; Asenso, N.; Yeboah, R.; Adejumobi, O.; Mensah, M.; Okai, D. The occurrence of gastric lesions in slaughtered pigs at the Kumasi abattoir, Ghana. Bangladesh J. Vet. Med. 2016, 14, 85–91. [Google Scholar] [CrossRef][Green Version]
- Omotosho, O.O.; Ayoade, G.O.; Emikpe, B.O.; Adediran, O.A.; Uwalaka, E.C. Identification of predisposing and risk factors associated with gastric lesions in pigs. Asian Pac. J. Trop. Dis. 2015, 5, 825–829. [Google Scholar] [CrossRef]
- Kopinski, J.S.; McKenzie, R.A. Oesophagogastric ulceration in pigs: A visual morphological scoring guide. Aust. Vet. J. 2007, 85, 356–361. [Google Scholar] [CrossRef]
- van den Berg, A.; Brülisauer, F.; Regula, G. Prävalenz von Veränderungen der kutanen Magenschleimhaut bei Schlachtschweinen in der Schweiz. Schweiz. Arch. Tierheilkd. 2005, 147, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Ayles, H.L.; Friendship, R.M.; Ball, R.O. Effect of dietary particle size on gastric ulcers, assessed by endoscopic examination, and relationship between ulcer severity and growth performance of individually fed pigs. Swine Health Prod 1996, 4, 211–216. [Google Scholar]
- Ramis, G.; Gomez, S.; Ballesta, M.; Munoz, A. Esophagogastric ulcer in finishing pigs from twelve large multi-site herds in southeastern Spain, 1995-2000: Descriptive epidemiology. J. Swine Health Prod. 2006, 14, 18–24. [Google Scholar] [CrossRef]
- Pardon, B.; Catry, B.; Dewulf, J.; Persoons, D.; Hostens, M.; De Bleecker, K.; Deprez, P. Prospective study on quantitative and qualitative antimicrobial and anti-inflammatory drug use in white veal calves. J. Antimicrob. Chemother. 2012, 67, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D.; Perkins, W.E.; Stetsko, P.I. Chronic Effects of Misoprostol in Combination with the Nsaid, Diclofenac, on Gastrointestinal-Tract of Pigs—Relation to Diarrheagenic Activity, Leukocyte Infiltration, and Mucosal Leukotrienes. Dig. Dis. Sci. 1995, 40, 1435–1444. [Google Scholar] [CrossRef]
- Rainsford, K.D.; Stetsko, P.I.; Sirko, S.P.; Debski, S. Gastrointestinal mucosal injury following repeated daily oral administration of conventional formulations of indometacin and other non-steroidal anti-inflammatory drugs to pigs: A model for human gastrointestinal disease. J. Pharm. Pharmacol. 2003, 55, 661–668. [Google Scholar] [CrossRef]
- Whittle, B.J.R.; Hansen, D.; Salmon, J.A. Gastric ulcer formation and cyclo-oxygenase inhibition in cat antrum follows parenteral administration of aspirin but not salicylate. Eur. J. Pharmacol. 1985, 116, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Konturek, S.J.; Brzozowski, T.; Piastucki, I.; Radecki, T. Prevention of ethanol and aspirin-induced gastric mucosal lesions by paracetamol and salicylate in rats: Role of endogenous prostaglandins. Gut 1983, 23, 536–540. [Google Scholar] [CrossRef]
- Ziegler, A.; Fogle, C.; Blikslager, A. Update on the use of cyclooxygenase-2-selective nonsteroidal anti-inflammatory drugs in horses. JAVMA J. Am. Vet. Med. Assoc. 2017, 250, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Nuwer, A.; Perry, T.; Pickett, R.A.; Curtin, T. Expanded or heat-processed fractions of corn and their relative ability to elicit esophagogastric ulcers in swine. J. Anim. Sci. 1967, 26, 518–525. [Google Scholar] [CrossRef] [PubMed]
- De Jong, J.A.; DeRouchey, J.M.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; Woodworth, J.C.; Allerson, M.W. Evaluating pellet and meal feeding regimens on finishing pig performance, stomach morphology, and carcass characteristics. J. Anim. Sci. 2016, 94, 4781–4788. [Google Scholar] [CrossRef]
- Liesner, V.; Taube, V.; Leonhard-Marek, S.; Beineke, A.; Kamphues, J. Integrity of gastric mucosa in reared piglets—Effects of physical form of diets (meal/pellets), pre-processing grinding (coarse/fine) and addition of lignocellulose (0/2.5%). J. Anim. Physiol. Anim. Nutr. 2009, 93, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Holinger, M.; Fruh, B.; Stoll, P.; Kreuzer, M.; Hillmann, E. Grass silage for growing-finishing pigs in addition to straw bedding: Effects on behaviour and gastric health. Livest. Sci. 2018, 218, 50–57. [Google Scholar] [CrossRef]
| Overall Use (%) | Farms with Use > 0 | Median (Range) TI1000 † | Mean ± SD TI1000 † | |
|---|---|---|---|---|
| Dexamethasone | 11.20 | 23 | 0.18 (0–6.17) | 0.70 ± 1.31 |
| Nonsteroidal anti-inflammatory drugs | 88.80 | 20 | 0.07 (0–30.10) | 5.22 ± 8.53 |
| Acetylsalicylic acid | 50.62 | 10 | 0 (0–22.07) | 2.99 ± 6.50 |
| Ketoprofen | 0.25 | 5 | 0 (0–0.20) | 0.02 ± 0.04 |
| Meloxicam | 0.04 | 2 | 0 (0–0.07) | 0.003 ± 0.01 |
| Metamizole | 0.11 | 3 | 0 (0–0.13) | 0.01 ± 0.03 |
| Sodium Salicylate | 37.76 | 5 | 0 (0–30.01) | 2.21 ± 6.62 |
| Tolfenamic Acid | 0.01 | 1 | 0 (0–0.01) | <0.001 ± 0.002 |
| All anti-inflammatory drugs | 100 | 29 | 0.45 (0–31.6) | 5.92 ± 9.04 |
| Country | Year | No. of Stomachs | Gastric Lesions Score (Prevalence %) | Ref. | |||
|---|---|---|---|---|---|---|---|
| No Lesions | Hyperkeratosis | Erosion/Mild Ulcers | Severe Ulcers | ||||
| Italy | 2023 | 9371 | 20.3 | 30.7 | 42.1 | 6.8 | This study |
| Switzerland | 2022 | 1005 | 38.8 | 27.2 | 14.9 | 19.1 | [41] |
| Poland | 2021 | 32,264 | 28.1 | 9.2 | 7.8 | 54.9 | [3] |
| Denmark | 2018 | 447 | 5.1 | 24.7 | 25.1 | 45.2 | [2] |
| Scotland | 2018 | 78 | 20.5 | 46.1 | 24.4 | 9.0 | [2] |
| Austria | 2018 | 233 | 2.6 | 58.8 | 10.3 | 28.3 | [42] |
| Italy | 2017 | 22,551 | 16.8 | 62.5 | 16.6 | 4.1 | [4] |
| Denmark | 2017 | 712 | 27.9 | 33.2 | 27.3 | 11.6 | [43] |
| Ghana | 2016 | 75 | 74.7 | 18.7 | 6.76 | 0.0 | [44] |
| Nigeria | 2015 | 480 | 67.1 | 22.5 | 7.9 | 2.5 | [45] |
| Italy | 2013 | 635 | 24.6 | 22.7 | 36.3 | 16.4 | [30] |
| UK | 2012 | 9827 | 20.4 | 49.2 | 23.9 | 6.4 | [5] |
| Australia | 2007 | 280 | 3.9 | 35.0 | 30.0 | 31.1 | [46] |
| Switzerland | 2005 | 1897 | 40.5 | 44.4 | 5.0 | 10.1 | [47] |
| Canada | 2002 | 1021 | 6.2 | 42.5 | 35.8 | 15.5 | [1] |
| UK | 1997 | 1242 | 57.0 | 20.1 | 9.5 | 13.4 | [33] |
| USA | 1996 | 80 | 2.7 | 39.7 | 39.7 | 17.8 | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghidini, S.; Scali, F.; Romeo, C.; Guadagno, F.; Maisano, A.M.; De Luca, S.; Varrà, M.O.; Conter, M.; Ianieri, A.; Zanardi, E.; et al. A Preliminary Study on the Relationship between Gastric Lesions and Anti-Inflammatory Drug Usage in Heavy Pigs. Vet. Sci. 2023, 10, 551. https://doi.org/10.3390/vetsci10090551
Ghidini S, Scali F, Romeo C, Guadagno F, Maisano AM, De Luca S, Varrà MO, Conter M, Ianieri A, Zanardi E, et al. A Preliminary Study on the Relationship between Gastric Lesions and Anti-Inflammatory Drug Usage in Heavy Pigs. Veterinary Sciences. 2023; 10(9):551. https://doi.org/10.3390/vetsci10090551
Chicago/Turabian StyleGhidini, Sergio, Federico Scali, Claudia Romeo, Federica Guadagno, Antonio Marco Maisano, Silvio De Luca, Maria Olga Varrà, Mauro Conter, Adriana Ianieri, Emanuela Zanardi, and et al. 2023. "A Preliminary Study on the Relationship between Gastric Lesions and Anti-Inflammatory Drug Usage in Heavy Pigs" Veterinary Sciences 10, no. 9: 551. https://doi.org/10.3390/vetsci10090551
APA StyleGhidini, S., Scali, F., Romeo, C., Guadagno, F., Maisano, A. M., De Luca, S., Varrà, M. O., Conter, M., Ianieri, A., Zanardi, E., & Alborali, G. L. (2023). A Preliminary Study on the Relationship between Gastric Lesions and Anti-Inflammatory Drug Usage in Heavy Pigs. Veterinary Sciences, 10(9), 551. https://doi.org/10.3390/vetsci10090551








