Macroscopic Skin Examination Can Determine the Number of Strips Necessary to Study the stratum corneum in Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Stratum Corneum Sampling
2.3. Evaluation of the Depth of Remaining Stratum Corneum
2.4. Statistical Analysis
3. Results
3.1. An Increased Number of D-squames® Discs Is Associated with a Decreased Thickness of Remaining Stratum Corneum Layers
3.2. There Was No Significant Difference between Sites 1 and 2 or between Sites 3 and 4
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hillier, A.; Griffin, C.E. ACVD task force on canine atopic dermatitis (I): Incidence and prevalence. Vet. Immunol. Immunopathol. 2001, 81, 147–151. [Google Scholar] [CrossRef]
- Labib, A.; Yosipovitch, G.; Olivry, T. What can we learn from treating atopic itch in dogs? J. Allergy Clin. Immunol. 2022, 150, 284–286. [Google Scholar] [CrossRef]
- Asahina, R.; Maeda, S. A review of the roles of keratinocyte-derived cytokines and chemokines in the pathogenesis of atopic dermatitis in humans and dogs. Vet. Dermatol. 2017, 28, 16–25. [Google Scholar] [CrossRef]
- Freudenberg, J.M.; Olivry, T.; Mayhew, D.N.; Rubenstein, D.S.; Rajpal, D.K. The Comparison of Skin Transcriptomes Confirms Canine Atopic Dermatitis Is a Natural Homologue to the Human Disease. J. Investig. Dermatol. 2019, 139, 968–971. [Google Scholar] [CrossRef]
- Santoro, D.; Rodrigues Hoffmann, A. Canine and Human Atopic Dermatitis: Two Faces of the Same Host-Microbe Interaction. J. Investig. Dermatol. 2016, 136, 1087–1089. [Google Scholar] [CrossRef][Green Version]
- Renert-Yuval, Y.; Thyssen, J.P.; Bissonnette, R.; Bieber, T.; Kabashima, K.; Hijnen, D.; Guttman-Yassky, E. Biomarkers in atopic dermatitis—A review on behalf of the International Eczema Council. J. Allergy Clin. Immunol. 2021, 147, 1174–1190.e1. [Google Scholar] [CrossRef]
- Olivry, T.; Mayhew, D.; Paps, J.S.; Linder, K.E.; Peredo, C.; Rajpal, D.; Hofland, H.; Cote-Sierra, J. Early Activation of Th2/Th22 Inflammatory and Pruritogenic Pathways in Acute Canine Atopic Dermatitis Skin Lesions. J. Investig. Dermatol. 2016, 136, 1961–1969. [Google Scholar] [CrossRef]
- Hughes, A.J.; Tawfik, S.S.; Baruah, K.P.; O’Toole, E.A.; O’Shaughnessy, R.F.L. Tape strips in dermatology research. Br. J. Dermatol. 2021, 185, 26–35. [Google Scholar] [CrossRef]
- Hulshof, L.; Hack, D.; Hasnoe, Q.; Dontje, B.; Jakasa, I.; Riethmüller, C.; McLean, W.; Aalderen, W.; Land, B.V.; Kezic, S.; et al. A minimally invasive tool to study immune response and skin barrier in children with atopic dermatitis. Br. J. Dermatol. 2019, 180, 621–630. [Google Scholar] [CrossRef]
- Andersson, A.M.; Sølberg, J.; Koch, A.; Skov, L.; Jakasa, I.; Kezic, S.; Thyssen, J.P. Assessment of biomarkers in pediatric atopic dermatitis by tape strips and skin biopsies. Allergy 2022, 77, 1499–1509. [Google Scholar] [CrossRef]
- Clausen, M.L.; Kezic, S.; Olesen, C.M.; Agner, T. Cytokine concentration across the stratum corneum in atopic dermatitis and healthy controls. Sci. Rep. 2020, 10, 21895. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Diaz, A.; Pavel, A.B.; Fernandes, M.; Lefferdink, R.; Erickson, T.; Canter, T.; Rangel, S.; Peng, X.; Li, R.; et al. Use of Tape Strips to Detect Immune and Barrier Abnormalities in the Skin of Children with Early-Onset Atopic Dermatitis. JAMA Dermatol. 2019, 155, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Lyubchenko, T.; Collins, H.K.; Goleva, E.; Leung, D.Y.M. Skin tape sampling technique identifies proinflammatory cytokines in atopic dermatitis skin. Ann. Allergy Asthma Immunol. 2021, 126, 46–53.e2. [Google Scholar] [CrossRef]
- Pinkus, H. Examination of the Epidermis by the Strip Method of Removing Horny Layers: I. Observations on Thickness of the Horny Layer, and on Mitotic Activity After Stripping. J. Investig. Dermatol. 1951, 16, 383–386. [Google Scholar] [CrossRef]
- Hunter, R.; Pinkus, H.; Steele, C.H. Examination of the Epidermis by the Strip Method. J. Investig. Dermatol. 1956, 27, 31–34. [Google Scholar] [CrossRef]
- McEwan, N.A.; Lu, Y.F.; Nuttall, T. A two-dimensional morphological study of corneocytes from healthy dogs and cats and from dogs with atopic dermatitis. Vet. Dermatol. 2009, 20, 360–368. [Google Scholar] [CrossRef]
- Jakasa, I.; Verberk, M.M.; Esposito, M.; Bos, J.D.; Kezic, S. Altered Penetration of Polyethylene Glycols into Uninvolved Skin of Atopic Dermatitis Patients. J. Investig. Dermatol. 2007, 127, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, U.; Meykadeh, N.; Sterry, W.; Lademann, J. Effect of the vehicle on the amount of stratum corneum removed by tape stripping. JDDG J. Dtsch. Dermatol. Ges. 2003, 1, 884–889. [Google Scholar] [CrossRef]
- Escobar-Chavez, J.J.; Merino-Sanjuán, V.; López-Cervantes, M.; Urban-Morlan, Z.; Piñón-Segundo, E.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. The Tape-Stripping Technique as a Method for Drug Quantification in Skin. J. Pharm. Pharm. Sci. 2008, 11, 104–130. [Google Scholar] [CrossRef]
- El Gammal, C.; Pagnoni, A.; Kligman, A.M.; el Gammal, S. A model to assess the efficacy of moisturizers--the quantification of soap-induced xerosis by image analysis of adhesive-coated discs (D-squames). Clin. Exp. Dermatol. 1996, 21, 338–343. [Google Scholar] [CrossRef]
- Morhenn, V.B.; Chang, E.Y.; Rheins, L.A. A noninvasive method for quantifying and distinguishing inflammatory skin reactions. J. Am. Acad. Dermatol. 1999, 41, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Ogai, K.; Nagase, S.; Mukai, K.; Iuchi, T.; Mori, Y.; Matsue, M.; Sugitan, K.; Sugama, J.; Okamoto, S. A Comparison of Techniques for Collecting Skin Microbiome Samples: Swabbing Versus Tape-Stripping. Front. Microbiol. 2018, 9, 2362. [Google Scholar] [CrossRef] [PubMed]
- Filaggrin Breakdown Products Determine Corneocyte Conformation in Patients with Atopic Dermatitis|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S0091674915006491?token=9265275E70F59AAE2AF569DAD55A8BB7EE22E90B13B5C06CC81B901584984A9D894E5B9507D8B3BD1BF54F4C07441A49&originRegion=eu-west-1&originCreation=20230119173541 (accessed on 19 January 2023).
- Breternitz, M.; Flach, M.; Präßler, J.; Elsner, P.; Fluhr, J.W. Acute barrier disruption by adhesive tapes is influenced by pressure, time and anatomical location: Integrity and cohesion assessed by sequential tape stripping; a randomized, controlled study. Br. J. Dermatol. 2007, 156, 231–240. [Google Scholar] [CrossRef]
- Marttin, E.; Neelissen-Subnel, M.T.; De Haan, F.H.; Boddé, H.E. A critical comparison of methods to quantify stratum corneum removed by tape stripping. Skin Pharmacol. Physiol. 1996, 9, 69–77. [Google Scholar] [CrossRef]
- Bashir, S.J.; Chew, A.L.; Anigbogu, A.; Dreher, F.; Maibach, H.I. Physical and physiological effects of stratum corneum tape stripping. Skin Res. Technol. 2001, 7, 40–48. [Google Scholar] [CrossRef]
- Sølberg, J.; Ulrich, N.H.; Krustrup, D.; Ahlström, M.G.; Thyssen, J.P.; Menné, T.; Bonefeld, C.M.; Gadsbøll, A.-S.Ø.; Balslev, E.; Johansen, J.D. Skin tape stripping: Which layers of the epidermis are removed? Contact Dermat. 2019, 80, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Simon, K.; Oberender, G.; Roloff, A. Continuous removal of single cell layers by tape stripping the stratum corneum—A histological study. Eur. J. Pharm. Biopharm. 2023, 188, 48–53. [Google Scholar] [CrossRef]
- Olesen, C.M.; Fuchs, C.S.K.; Philipsen, P.A.; Hædersdal, M.; Agner, T.; Clausen, M.L. Advancement through epidermis using tape stripping technique and Reflectance Confocal Microscopy. Sci. Rep. 2019, 9, 12217. [Google Scholar] [CrossRef]
- Vidémont, E.; Mariani, C.; Vidal, S.; Pin, D. Characterization of the canine skin barrier restoration following acute disruption by tape stripping. Vet. Dermatol. 2012, 23, 103-e23. [Google Scholar] [CrossRef]
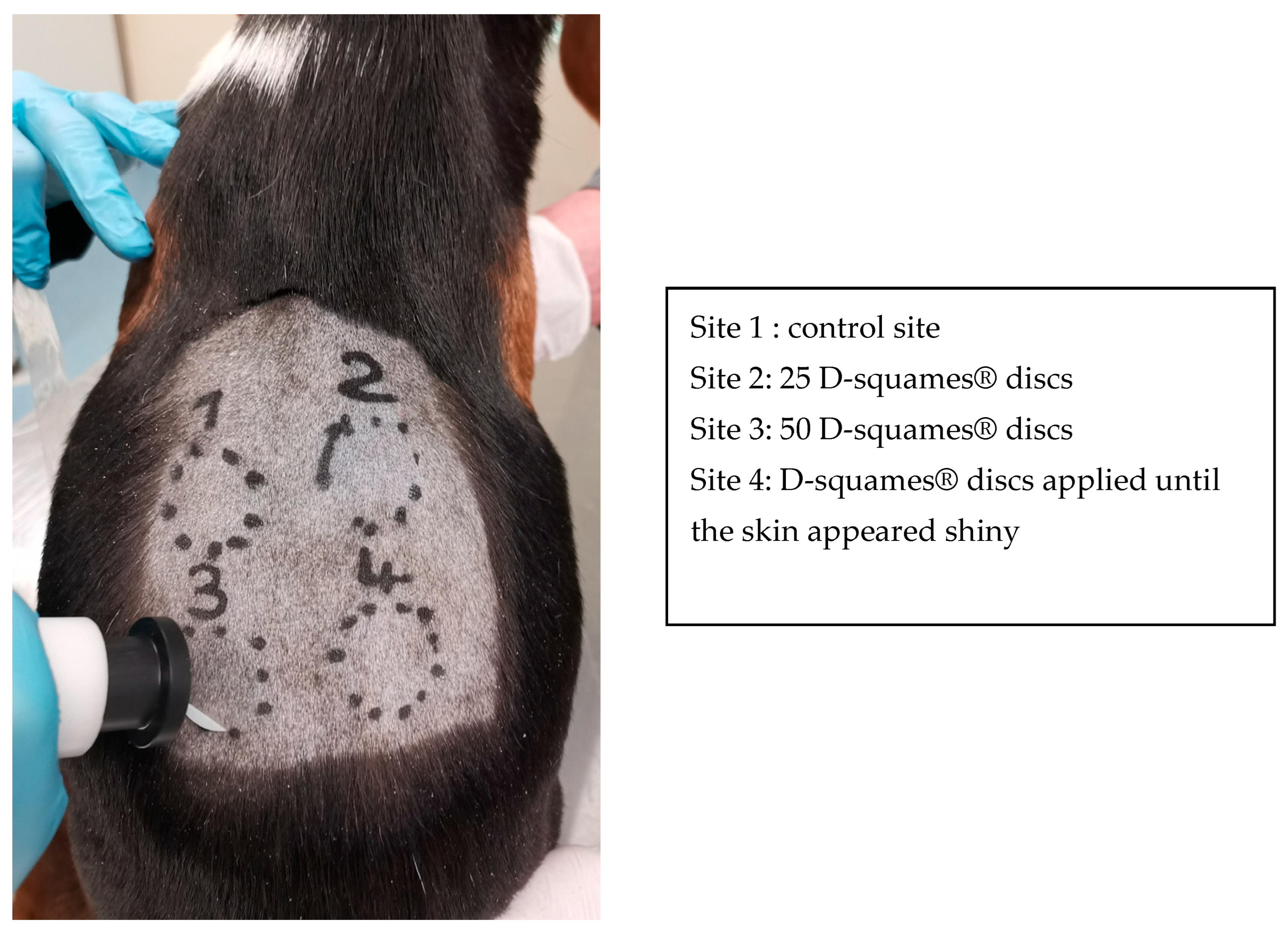
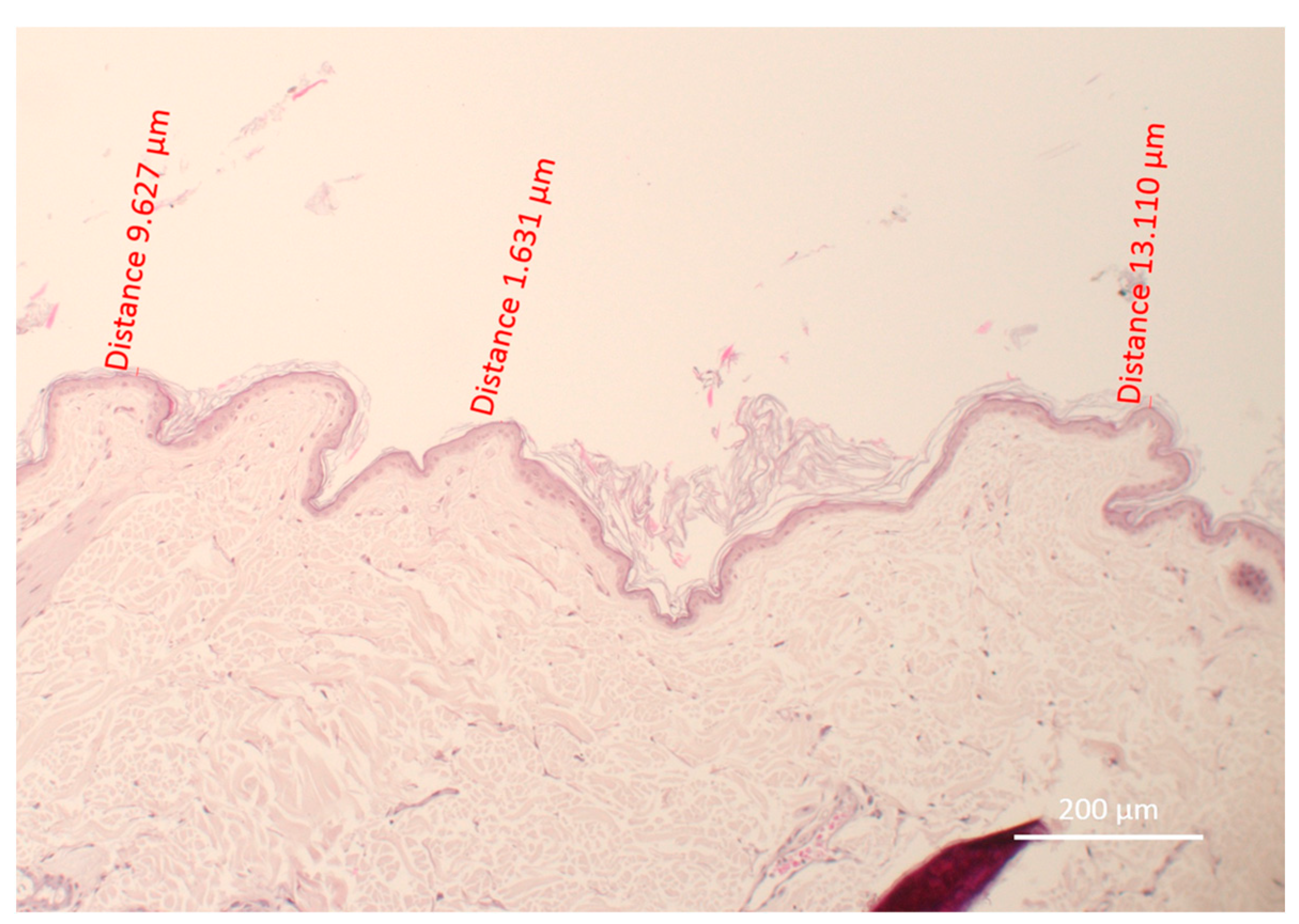

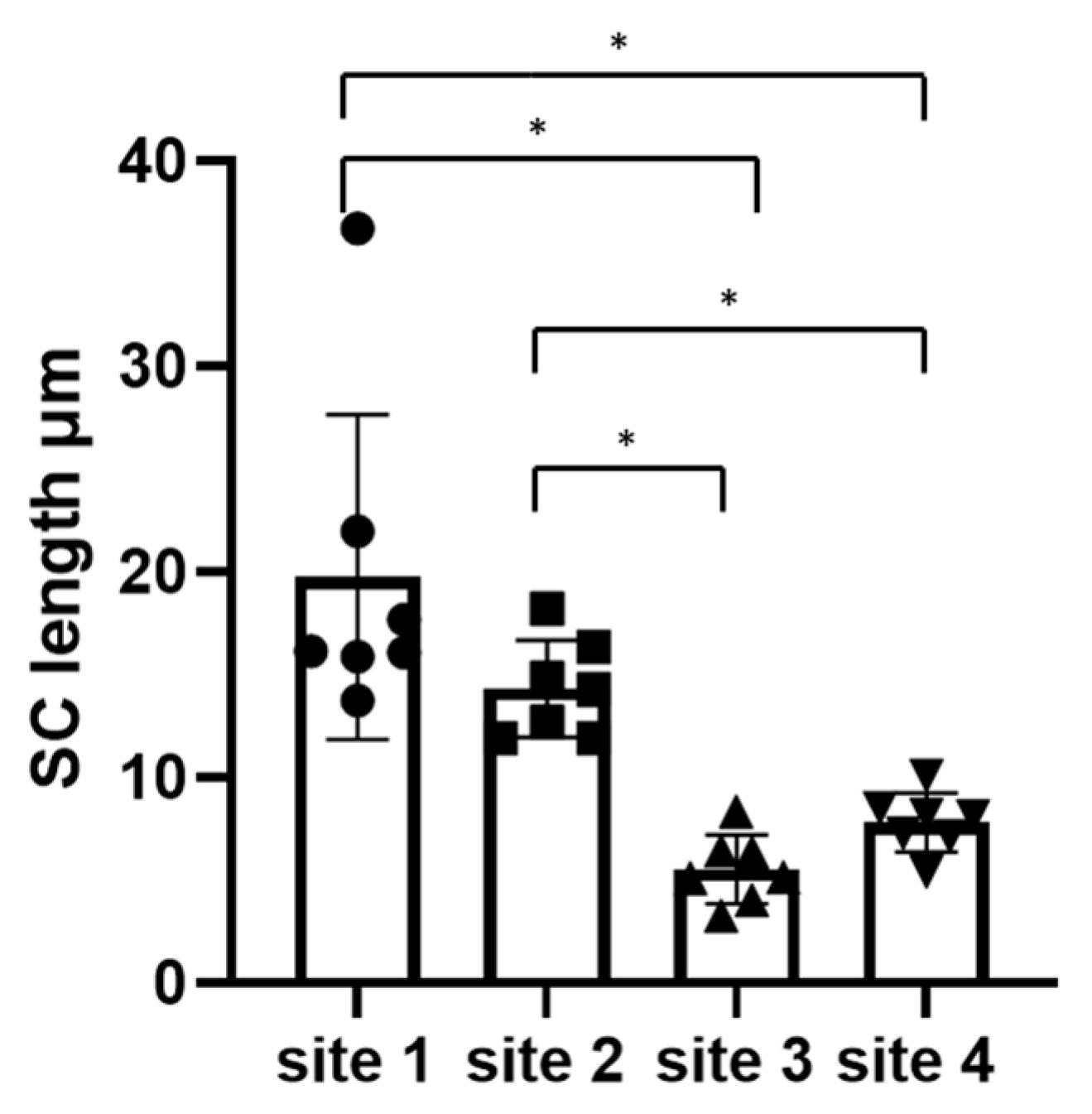
| Thickness (µm) | Dog 1 | Dog 2 | Dog 3 | Dog 4 | Dog 5 | Dog 6 | Dog 7 | Mean |
|---|---|---|---|---|---|---|---|---|
| Site 1 | 15.89 | 21.98 | 36.72 | 16.08 | 13.74 | 17.68 | 16.15 | 19.7485714 |
| Site 2 | 11.91 | 18.22 | 16.36 | 14.85 | 14.28 | 11.93 | 12.72 | 14.3242857 |
| Site 3 | 6.38 | 3.29 | 5.1 | 4.06 | 6.44 | 8.34 | 5.15 | 5.53714286 |
| Site 4 (number of D-squames®) | 8.19 (29) | 5.4 (50) | 7.28 (42) | 8.12 (46) | 8.53 (42) | 10.09 (41) | 7.16 (29) | 7.82428571 (39.85714) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosca, M.; Legain, M.; Noël, G.; Idée, A.; Pin, D. Macroscopic Skin Examination Can Determine the Number of Strips Necessary to Study the stratum corneum in Dogs. Vet. Sci. 2023, 10, 547. https://doi.org/10.3390/vetsci10090547
Mosca M, Legain M, Noël G, Idée A, Pin D. Macroscopic Skin Examination Can Determine the Number of Strips Necessary to Study the stratum corneum in Dogs. Veterinary Sciences. 2023; 10(9):547. https://doi.org/10.3390/vetsci10090547
Chicago/Turabian StyleMosca, Marion, Mélanie Legain, Guillaume Noël, Adrien Idée, and Didier Pin. 2023. "Macroscopic Skin Examination Can Determine the Number of Strips Necessary to Study the stratum corneum in Dogs" Veterinary Sciences 10, no. 9: 547. https://doi.org/10.3390/vetsci10090547
APA StyleMosca, M., Legain, M., Noël, G., Idée, A., & Pin, D. (2023). Macroscopic Skin Examination Can Determine the Number of Strips Necessary to Study the stratum corneum in Dogs. Veterinary Sciences, 10(9), 547. https://doi.org/10.3390/vetsci10090547






