Simple Summary
Equine placentitis is associated with abortions, mortality of mares, and foal deaths and produces economic losses for the equine industry. The diagnosis of equine placentitis often occurs long after the establishment of the disease, for which treatments are ineffective. Ultrasound is an excellent tool for detect fetal and placental changes; however, it is not accessible in all cases, and specialized training is required. In this study, we demonstrate that eIL-1β levels in the serum of mares with ultrasonographic signs of placentitis can be used as biomarkers of disease severity and its probable impact on the health and viability of the foal. We did not find significant differences in the activity of proMMP-9 in the serum of mares with placentitis, so new studies will be required to validate proMMP-9 as a biomarker.
Abstract
Equine placentitis is characterized by infection and inflammation of the placenta. Different biomarkers associated with this inflammatory response have been evaluated in experimentally induced equine placentitis, but not in pregnant mares with spontaneous placentitis. The aim of the current study was to determine the concentration of eIL-1β and the activity of proMMP-2 and proMMP-9 in the serum of healthy mares and mares with placentitis on days 240 and 320 of gestation to explore whether these biomarkers are associated with equine maternal placentitis and/or with the birth of an infected or inviable foals. Serum samples were collected from sixteen pregnant English Thoroughbred mares, retrospectively classified as follows: (1) healthy mares with full-term gestation; and (2) mares with ultrasonographic signs of placentitis. The health of each foal was examined at birth, and it was decided to classify the cases into four groups: (1) healthy mares delivering a healthy foals (HM-HF, n = 6); (2) mares with USP delivering a healthy foal (USP-HF, n = 3); (3) mares with USP delivering a live septic foal (USP-LSeF, n = 4); and (4) mares with USP delivering a dead foal (USP-DF, n = 3). eIL-1β was quantified by ELISA, and proMMP-2 and proMMP-9 activity by gelatin zymography electrophoresis. In healthy mares, the serum concentrations of eIL-1β underwent a significant 16.5-fold increase from day 240 to day 320 of gestation. Although similar results were found in the mares with ultrasonographic signs of placentitis that delivered a healthy foal, those delivering a live septic or nonviable foal exhibited much higher concentrations of eIL-1β. proMMP-2 and proMMP-9 activity was not associated with maternal placentitis, foal infection, or death. Hence, the presence of placentitis severe enough to affect the health of the foal can be confirmed or discarded by determining the eIL-1β concentration in mares that have shown ultrasonographic signs of placentitis.
1. Introduction
Equine placentitis is characterized by inflammation of the placenta caused by infection with pathogenic microorganisms [1,2], which can reach the placenta by two pathways. Most commonly, pathogens ascend from the vagina into the uterine lumen before invading placental membranes [3,4,5,6]. Less frequently, pathogens or microbial products are transported from the oral cavity (or other point of entry) to the placenta through the bloodstream [7,8,9,10]. Equine placentitis is linked to premature myometrial activation [11], and consequently, with abortions [12,13,14]. This disease has been associated with the mortality of mares [15] and neonatal foals [16,17], among other conditions.
The causal agents of equine placentitis are Gram-positive (Streptococcus equi subsp. zooepidemicus, Streptococcus equisimilis, Staphylocossus spp., Corynebacterium pseudotuberculosis, and Pseudonocardia spp.), and Gram-negative bacteria (Escherichia coli, Pseudomonas spp., Klebsiela spp., Salmonella abortus equi, and Chlamydia) [2,18,19], virus (gammaherpesvirus and equine herpervirus) [17,20], and fungus (Aspergillus terreus) [21]. Toll-like receptors (TLRs) in placental cells recognize the molecular pattern associated with many of these pathogenic microorganisms [22]. Recently, Hossam El-Sheikh Ali et al. (2021) experimentally induced the development of placentitis in six pregnant Equus caballus mares with S. equi ssp. zooepidemicus and found a higher expression of TLR-2 and TLR-7 in the myometrium and placenta [23,24]. The interaction between TLRs and bacterial pathogens activates the MyD88/IRAK1/TRAF signaling pathway, which activates the nuclear transcription-regulated factor kappa B (NF-ĸB) [25,26]. Finally, NF-ĸB is a critical component of several cytokine signaling pathways [27,28]. Based on the experimental induction of equine placentitis, different biomarkers have been identified that are related to the inflammatory response (IL1β, IL-6, IL-8, and TNFα) [11,29] and to premature activation of myometrial contraction (PGE2, PGE2α, and oxytocin) [5,23,30,31].
Interleukin type 1-beta (IL-β) is a pivotal cytokine in several second messenger signaling pathways in both physiological and pathological processes [32]. IL-1β acts as a modulator during ovulation, in oocyte maturation, during early embryonic development [33,34], and in the activation of the inflammatory response [28,35,36]. It also acts as a modulator of specialized cells of the immune system [11,30] and induces the expression of matrix metalloproteinases (MMPs) [37,38].
In various physiological processes, the expression of MMPs has been associated with cell migration [39,40], angiogenesis [41,42,43], and implantation [44,45], while in several equine pathological conditions, it is linked to an inflammatory response [37,46,47]. Recently, Hossam El-Sheikh Ali et al. (2020), using an experimental model of equine placentitis, found an increase in the expressions of MMP-1 and MMP-8, which was associated with the expressions of TLR-2 and TLR-7 [23,24]. MMPs, are a family of zinc-dependent endopeptidases and produced in several physiological and pathological conditions by a wide variety of cell types, including neutrophils [48,49], macrophages [47], leukocytes [50], bronchial epithelial cells [46,51], and equine endometrial cells [23,52]. These endopeptidases induce the degradation of various structural components of the extracellular matrix, including collagen types I, IV, V, VII, and X, fibronectin, elastin, proteoglycan [53,54], the basement membrane [55], and cell-binding adhesion proteins [56,57].
The substrates degraded by MMPs provide the basis for their classification. Among commonly known are stromelysin-1 (MMP-3), -2 (MMP-10), and -3 (MMP-11); collagenase-1 (MMP-1), -2 (MMP-8), and -3 (MMP-13); gelatin-A (MMP-2) and -B (MMP-9); matrilysin type I (MMP-7) and type II (MMP-26); and membranal type I (MMP-14, -15, -16, and -24) and type II (MMP-23) [58,59,60]. The chromosomal location of all MMPs has been determined in all species with a reference-quality genome [61].
Although there are experimental models of placentitis in mares, the expressions of inflammatory and degradative biomarkers during equine physiological pregnancy and pregnancies with spontaneous placentitis is still unknown. It has been reported that inoculating pregnant mares with Streptococcus equi ssp. zooepidemicus activates the expression of prostaglandins, inflammatory cytokines, at 290 days of gestation [4]. Therefore, it would be important to know if the inflammatory response is activated prior to 290 days of gestation. Thus, the aim of the current contribution was to analyze the concentrations of eIL-1β and the activity of proMMP-2 and proMMP-9 in the serum of mares with and without placentitis at 240 and 320 days of gestation, and to determine if these biomarkers are related to placentitis and foal mortality.
2. Materials and Methods
2.1. Ethics Committee Approval
This protocol was approved by the Ethics Committee on Animal Experimentation at the Universidad Nacional Autónoma de México (SICUAE.MC-2020/2-5).
2.2. Animals and Experimental Design
Figure 1 shows the study design, the classification of mares and the subclassification based on foal health. Sixteen pregnant English Thoroughbred mares (11.7 ± 4.4 years old; weighing 500–600 kg) were included in the study, all underwent medical surveillance throughout the gestation period.
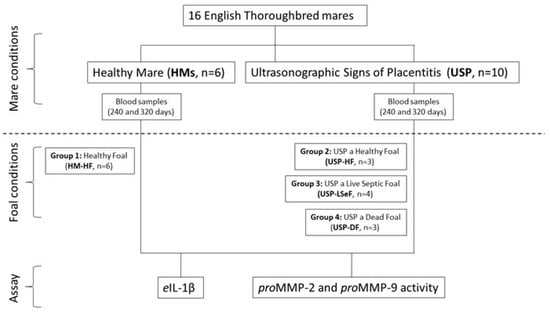
Figure 1.
Schematic diagram of the study design. According to the conditions of the mares, they were classified as healthy mares (HM; n = 6), or mares with ultrasonographic signs of placentitis (USP; n = 10). In each case, the health of the neonatal foal was evaluated, and they were subclassified into four groups: HM-HF (n = 6), USP-HF (n = 3), USP-LSeF (n = 4), and USP-DF (n = 3). The quantification of eIL-1β and the activity of proMMPs was evaluated in the serum from mares at 240 and 320 days of gestation.
According to the medical surveillance and the condition of each placenta recovered after delivery, the mares were assigned to one of two groups: (1) healthy mares with full-term gestation (HMs, n = 6); and (2) mares with ultrasonographic signs of placentitis (USP, n = 10), indicated by a utero-placental thickness greater than 7 mm on day 240 of gestation and/or greater than 10 mm on day 320 [3,62]. In every case, the placentas were recovered immediately after delivery and evaluated for the presence of edema, congestion, necrosis or purulent, local, or generalized exudate [5,63].
In all cases, the health of the foal was also examined at birth to ascertain the presence or absence of sepsis [64]. The foals were classified as healthy foals (HF), or live septic foals (LSeF) if they fulfilled any or all of the following criteria: (1) those who, in the first 24 h of life, presented alterations in their normal behavior, such as discomfort when sleeping, stopping nursing, and diarrhea, or alterations in their general physical examination, taking as normal ranges a heart rate of 80–110 beats/min, respiratory distress with 25–30 breath/min, fever of >38 °C, urinary density of 1.010–1.015, pink mucous membranes, and capillary filling time of 1 s; (2) >1 site of placenta infection based on weight and external evaluation. Dead foals (DF) were those who presented the alterations described above during the first 24 h of birth, underwent treatment, and died; this group included a fetus that was aborted and a foal that was born by placenta previa, alive but weak, and at the general physical examination, its heart frequency was very low, so euthanasia had to be performed [65,66,67].
Considering the health of the mares and that of their foals, it was decided to classify the cases into four groups: (1) healthy mares delivering a healthy foal (HM-HF, n = 6); (2) mares with USP delivering a healthy foal (USP-HF, n = 3); (3) mares with USP delivering a live septic foal (USP-LSeF, n = 4); and (4) mares with USP delivering a dead foal (USP-DF, n = 3), defined by organ dysfunction, subcutaneous edema, and conjunctivitis [15] (Figure 1).
2.3. Blood Samples
Blood samples were obtained from 16 mares at 240 and 320 days of gestation by jugular vein puncture and collected in a Vacutainer tube (BD, Franklin Lakes, NJ, USA). The blood samples were immediately centrifuged at 1500 rpm for 10 min at room temperature. The serum was separated and stored at −70 °C until it was used to measure the concentration of eIL-1β and the activity of proMMP-2 and proMMP-9.
2.4. eIL-1β Assay
The serum levels of eIL-1β were quantified by a specific Douset ELISA assay (DY3340, R&D system, Minneapolis, MN, USA), as previously described [68]. A standard curve was constructed from 125 to 8000 pg/mL. The final concentration of the total protein per sample was expressed in pg.
2.5. Zymography Gel Activity
The activity of proMMP-2 and proMMP-9 was determined by zymography gel, as previously reported in other models of infection [68,69]. SDS-polyacrylamide gels (4%) were co-polymerized with porcine gelatin (1 mg/mL, substrate gel electrophoresis) and loaded with 0.75 µg of protein per slot in non-denaturing loading buffer. The standard for proMMP-2 and proMMP-9 activity was medium from U937 promyelocyte cells (ATCC, CRL-1593.2; Manassas, VA, USA). The samples were diluted with buffer (0.5 M Tris, pH 6.8, 10% glycerol, and 0.1% bromophenol blue). Electrophoresis was carried out at a constant voltage of 25 mA at 4 °C for 90 min. Subsequently, SDS was removed by incubating the sample twice with 2.5% Triton X-100 at room temperature for 15 min under constant agitation. The gels were incubated overnight at 37 °C in activation buffer: 50 mM Tris (pH 7.4), 0.1 M CaCl2, 0.15 M NaCl, and 0.2 mg/mL NaN3. Afterwards, they were stained for 1 h with 1.0% Coomassie brilliant blue R-250 (Sigma Aldrich, St. Louis, MO, USA) in methanol/acetic acid/glycerol/water (10:10:10:70) at room temperature, and then placed in a methanol/acetic acid/water solution (10:10:80) until intense bands were seen. The activity of the lysis bands (proMMP-2 and proMMP-9) was visualized by densitometry with the EpiChemi Darkroom gel documentation system (UVP; CA, USA). The optical density was quantified using NIH ImageJ software and expressed in relative densitometric units.
2.6. Statistical Analysis
All data were analyzed using a Shapiro–Wilk test to assess the normal distribution. The mares, pregnancies, foal features, and MMP activity were analyzed by one-way ANOVA with multiple comparison, followed by Tukey’s test between the HM-HF group and all the other groups (HM-HF vs. USP-HF, HM-HF vs. USP-HF, HM-HF vs. USP-LSeF, and HM-HF vs. USP-DF). eIL-1β concentrations were compared by a non-parametric Mann–Whitney U test. All analyses were conducted using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA). Statistical significance was considered at p < 0.05. All values are expressed as the mean ± standard deviation or the median and interquartile range, depending on the data distribution.
3. Results
The distribution of cases according to the conditions of the mare during pregnancy (HM, or USP) and the status of the foals (HF, LSeF, and DF) are shown in Table 1.

Table 1.
Mares, pregnancies, and foal features.
The average age of the mares was not different among the groups (p = 0.5466). Normal and abnormal pregnancies, and the delivery of healthy and unhealthy foals occurred both in young and old mares. The duration of pregnancy was similar among the groups, except for pregnancies that culminated in a dead foal, which were significantly shorter (321.7 ± 11.9, p = 0.0235) compared to healthy pregnancies resulting in a healthy foal (344.8 ± 5.3). The shortest pregnancy, which culminated in spontaneous abortion on day 308 of gestation, occurred in a mare with USP-DF (p = 0.0235). There were no statistical differences among the groups in the average weight of the foals (p = 0.0699; Table 1), even considering the low weight of the aborted foal (32 kg), which reduced the average in the USP group. No other foal in any group weighed less than 42 kg (p = 0.2854; Table 1).
3.1. Concentration of eIL-1β
The serum concentrations of eIL-1β on days 240 and 320 of gestation for each group of mares are shown in Figure 2 and Figure 3, respectively. On day 240 of gestation, the concentration of eIL-1β was 20.17 ± 3.8 ng/mL in the group of HM-HF and 2.7-fold higher in the USP-HF group; no statistical significance (ns) was found (HM-HF vs. USP-HF, p > 0.9999; ns. Figure 2). Comparing the groups HM-HF and USP-LSeF, the second had a 8.4-fold increase in eIL-1β concentrations (HM vs. USP-LSeF, p = 0.9958), and when compared to those that delivered a dead foal (USP-DF), it showed a 373.4-fold greater level of eIL-1 β (HM vs. USP-DF, p < 0.0001; Figure 2).
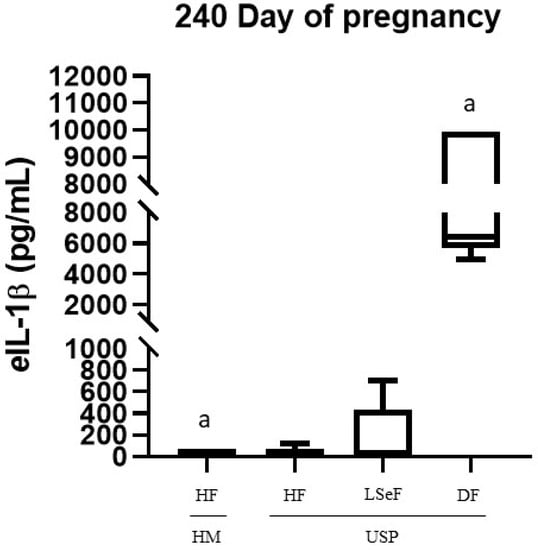
Figure 2.
Concentration of eIL-1β in the serum of mares at 240 days of pregnancy. Healthy mares (HM; n = 6), and mares with ultrasonographic signs of placentitis (USP; n = 10). Healthy foals (HF), live septic foals (LSeF), and dead foals (DF). The data are presented as boxes representing the median (central line) with the interquartile range (25th and 75th percentiles), and the whiskers represent the extreme points. Each assay was performed in duplicate. a p < 0.00001.
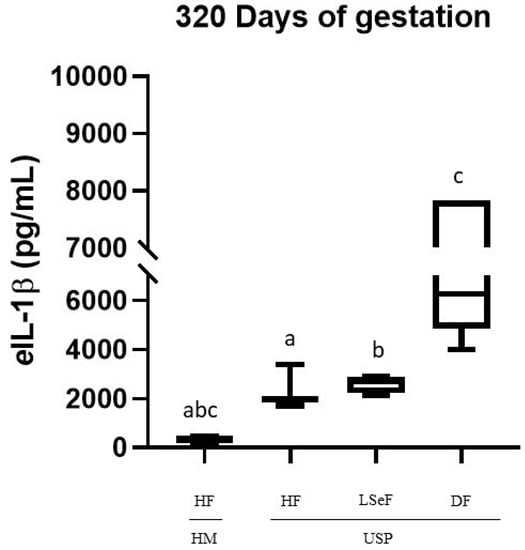
Figure 3.
Concentrations of eIL-1β in the serum of mares at 320 days of pregnancy. Healthy mares (HM; n = 6), and mares with ultrasonographic signs of placentitis (USP; n = 10). Healthy foals (HF), live septic foals (LSeF), and dead foals (DF). The data are presented as boxes representing the median (central line) with the interquartile range (25th and 75th percentiles), and the whiskers represent the extreme points. Each assay was performed in duplicate. a p = 0.0002, b p = 0.0006, c p < 0.0001.
When comparing USP mares according to the foal data (HF, LSeF, and DF), we found statistically significant differences between USP-HF and USP-DF (p < 0.0001). In contrast, we did not find significant differences between USP-LSeF and USP-HF (p = 0.9981; Figure 2).
On day 320 of pregnancy, the concentration of eIL-1β was significantly different between the groups of HM-HF and USP-HF (p = 0.0002), and between mares in the USP group that delivered a live septic foal (HM vs. USP-LSeF, p = 0.0006) and those who delivered a dead foal (HM vs. USP-DF, p < 0.0001). All showed significantly high levels of eIL-1β (Figure 3).
When comparing the USP mares according to the foal data (HF, LSeF, and DF), we found statistically significant differences between USP-LSeF and USP-DF (p < 0.0001; Figure 2). However, no statistically significant difference was found between USP-HF and USP-LSeF (p = 0.7795).
According to these results, the pathogenic process of spontaneous placentitis in mares appears to activate an inflammatory response mediated by eIL-1β, as has been demonstrated in the experimental models of induced placentitis [29]. In those models, extracellular MMPs are activated in the next phase of the inflammatory response [68,69], which is why the lytic activity profile of proMMP-2 and proMMP-9 was determined in the serum of mares in the next step.
3.2. proMMP-2 Activity at 240 and 320 Days of Gestation
3.2.1. proMMP-2 Activity at 240 Days of Gestation
On day 240 of pregnancy, the level of proMMP-2 was 924.4 ± 233.2 (relative densitometric units) in the HM-HF group, and 1.1-fold lower in mares in the USP-HF group, (MH-HF vs. USP-HF, p = 0.9587; Figure 4B). Comparing the HM-HF to the USP group that delivered a live septic foal showed a 1.2-fold increment (HM-HF vs. USP-LSeF, p = 0.6713), while comparing the HM-HF group to mares in the USP group that delivered a dead foal showed a 1.1-fold increment (HM-HF vs. USP-DF, p = 0.8681; Figure 4B).
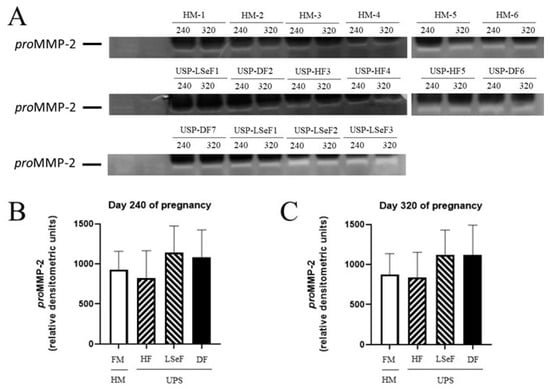
Figure 4.
Activity of proMMP-2 in the serum of mares at 240 and 320 days of pregnancy. The activity band of the electrophoretic mobility marker U937 (lanes 1 and 2) resulting from gel zymography is portrayed for the three groups of mares: healthy mares (HM-HF, lanes 1–6), and mares with ultrasonographic signs of placentitis (USP, lanes 1–10). The product of gestation indicates a healthy foal (HF), live septic foal (LSeF), or dead foal (DF) (A). The optical density of each lysis band was determined at 240 (B) and 320 days of gestation (C). Data are expressed as the mean ± standard deviation in relative densitometric units.
3.2.2. proMMP-2 Activity at 320 Days of Gestation
On day 320 of pregnancy, the level of proMMP-2 was 876.7 ± 257.4 (relative densitometric units) in HM-HF group and 1.04-fold lower in the USP group that delivered a healthy foal (HM vs. USP-HF, p = 0.9979, ns; Figure 4C). When the HM-HF group was compared to mares of the USP group, an increase of 1.2-fold was found (HM vs. USP-LSeF, p = 0.5961). When the HM-HF group was compared to mares in the USP group that delivered a dead foal, 1.27-fold increase was found (HM-HF vs. USP-DF, p = 0.6690; Figure 4C).
3.2.3. proMMP-9 Activity at 240 Days of Gestation
On day 240 of pregnancy, the level of proMMP-9 activity was 573.2 ± 209.6 (relative D.O units) in the HM-HF group and 1.06-fold lower in mares with the USP group that delivered a healthy foal (HF vs. USP-HF, p = 0.9999; Figure 5B). Comparing the HM-HF to the USP group that delivered a live septic foal, an increment of 1.4-fold was found (HM-HF vs. USP-LSeF, p = 0.7143), and when the HM-HF group was compared to the USP group that delivered a dead foal, a 1.12-fold increase was found, (HM-HF vs. USP-DF, p = 0.9999).
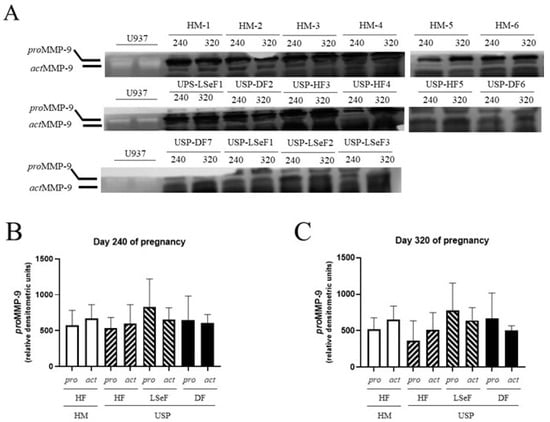
Figure 5.
Activity of proMMP-9 and actMMP-9 in the serum of mares at 240 and 320 days of pregnancy. The activity band of the electrophoretic mobility marker U937 (lanes 1 and 2) resulting from gel zymography is shown for the three groups of mares: healthy mares (HM-Hf, lanes 1–6), and mares with ultrasonographic signs of placentitis (USP, lanes 1–10). The product of gestation is indicated as a healthy foal (HF), live septic foal (LSeF), or dead foal (DF) (A). The optical density of each lysis band was determined at 240 (B) and 320 days of gestation (C). Data are expressed as the mean ± standard deviation in relative densitometric units (The original pictures can be found in Figure S1).
3.2.4. actMMP-9 Activity at 240 Days of Gestation
On day 240 of pregnancy, the activity of the active isoform (act) of MMP-9 was 671.9 ± 191.1 (relative D.O units) in the HM-HF group (Figure 5B) and 1.12-fold lower in the USP group that delivered a healthy foal (HM-HF vs. USP-HF, p = 0.9819; Figure 5B). Mares with USP delivering a live septic foal and those delivering a dead foal showed 1.19-fold (HM-HF vs. USP-LSeF, p = 0.9838) and 1.10-fold (HM-HF vs. USP-DF, p = 0.9883) higher activity of actMMP-9 (Figure 5B).
3.2.5. proMMP-9 Activity at 320 Days of Gestation
On day 320 of pregnancy, the activity of proMMP-9 was 517.2 ± 160.3 (relative D.O units) in the HM-HF group (Figure 5B) and 1.41-fold lower in mares in the USP group that delivered a healthy foal (HM-HF vs. USP-HF, p = 0.9460; Figure 5C). Comparing the group of HM-HF to the USP group that delivered a live septic foal and those that delivered a dead foal showed 1.5-fold (HM-HF vs. USP-LSeF, p = 0.9186) and 1.28-fold (HM-HF vs. USP-DF, p = 0.9486) higher proMMP-9 activity (Figure 5C).
3.2.6. actMMP-9 Activity at 320 Days of Gestation
On day 320 of pregnancy, the activity of actMMP-9 was 655.5 ± 182.2 (relative D.O units) in the HM-HF group (Figure 5C), and 1.28-fold lower in the USP group that delivered a healthy foal (HM-HF vs. USP-HF, p = 0.7800; Figure 5C). Comparing HM-HF mares to mares in the USP group that delivered a live septic foal and those that delivered a dead foal exhibited 1.29-fold (HM-HF vs. USP-LSeF, p = 0.9341) and 1.29-fold (HM-HF vs. USP-DF, p = 0.7649) decreased actMMP-9 activity (Figure 5C).
4. Discussion
Equine placentitis is one of the main causes of abortion [70,71], mare mortality, premature activation of labor, and foal mortality [2,63]. The models of equine placentitis involving the inoculation of pathogenic bacteria (Streptococcus equi subspecies zooepidemicus, Escherichia coli, and/or beta-hemolytic Streptococcus dysgalactiae) [4,29,72,73,74] have demonstrated that infection-induced inflammation activates a complex signaling network, which leads to the production of IL-1β, IL-6, TNFα [29,30], IL-8 [2,75], and prostaglandins E2 and F2 [31]. Additionally, the induced inflammation increases the level of degradative metalloproteinases (MMPs) in the amniotic fluid [76,77] and the expression of genes involved in placental regulation (PLAC8, PAPPA, and LGALS1) [78,79].
Several studies have been carried out on the role of inflammatory molecules in preterm and term labor in humans [80,81], but there is little information on the activation of these inflammatory biomarkers during normal equine gestation or as result of spontaneous placentitis. Hence, the aim of the current contribution was to evaluate inflammatory biomarkers during gestation in mares with and without placentitis. Three biomarkers were measured in mare serum at 240 and 320 days of gestation: the proinflammatory eIL-1β and the activity of collagenolytic proMMP-2 and proMMP-9. In healthy mares, the concentration of eIL-1β underwent a significant 16.5-fold increase from day 240 to day 320 of gestation. In mares with USP, the concentration of eIL-1β showed a similar change during the pregnancies, leading to the delivery of a healthy foal.
4.1. The Inflammatory Response and the Activation of Labor
With experimental models of equine and human placentitis, it has been established that the inflammatory response is mediated by eIL-1β or IL-1 β [29,68]. The current study reveals that this is similar in spontaneous equine placentitis. eIL-1β modulates the onset of labor in mares by inducing placental inflammation through the expression of TNFα [80,82] and chemotactic cytokines (IL-6, IL-8) [81]. These molecules amplify the inflammatory response by recruiting professional antigen-presenting cells of the immune system [83]. The inflammatory response stimulates the synthesis of prostaglandin (PGE) and oxytocin, which, together, initiate uterine contractions [84].
In the next phase of the inflammatory response, as evidenced in several models of infection, extracellular MMPs are activated [68,69]. These proteins favor a key step that involves uterine contractions and the rupture of the chorionic and amniotic membranes (Figure 6).
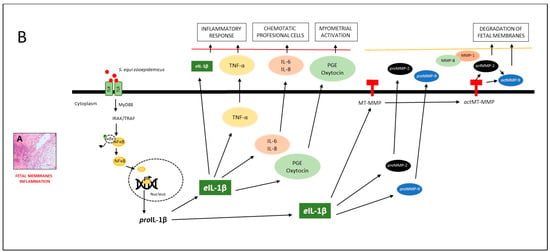
Figure 6.
Model of the effect of eIL-1β and proMMP-9 in mares with placentitis. (A) Histological section of equine placental inflammation is illustrated. (B) According to the model, TOLL-like receptors (TLR-2 and TLR-7) [23,24] recognize different pathogen-associated molecular patterns (PAMPs), such as flagellin, lipoproteins, LPS, DNA, and RNA [22]. They then induce the phosphorylation of IRAK/TRAF, which [24] activates the nuclear factor kappa-light-chain-enhancer of activated B cells (NFĸB), facilitating the transcription of eIL-1β [26]. The latter has been associated with the inflammatory response (TNFα) [27,28] and chemotactic cytokines (IL-6, IL-8). These molecules amplify the inflammatory response by recruiting professional antigen-presenting cells of the immune system [46,47,50]. The inflammatory response stimulates the synthesis of prostaglandin (PGE) and oxytocin, which, together, initiate uterine contractions. In the next phase of the inflammatory response, eIL-1β triggers the activation of proMMP-2 and proMMP-9 [40], leading to the degradation of different structures of the fetal membranes. This results in the weakening of the tensile strength of these membranes, and therefore, the onset of premature labor [49,52].
Thus, the activity profiles of proMMP-2 and proMMP-9 were determined in this study, as reported in other models of infection. No association was found between spontaneous equine placentitis and the serum concentrations of these metalloproteinases (Figure 5).
The alterations in proMMP-2 and proMMP-9 observed in experimental models of infection in human placental tissues were measured in the tissues themselves [68]. In contrast, alterations identified during labor after normal or high-risk pregnancies in mares were examined in amniotic fluid [77]. Consequently, the lack of a significant difference between groups in the concentrations of proMMP-2 and proMMP-9 in maternal serum in this study does not rule out possible alterations at the level of tissues or fetal fluids.
In human pregnancies, IL-1β is one of the proinflammatory molecules upregulated to prepare for term labor [85]. Our data suggest the existence of a similar process in mares, as evidenced by the robust 16.5-fold increase in the concentration of eIL-1β between days 240 and 320 in normal pregnancies (healthy mares). The expression of this cytokine was exacerbated by the degree of inflammation in the mares with placentitis that later did not give birth to a healthy foal. This is evidenced by the much higher concentrations of eIL-1β (at 240 and 320 days of pregnancy) in these animals compared to the levels found in healthy mares and in those with USB that delivered healthy foals (Figure 2 and Figure 3).
Interestingly, the concentration of eIL-1β was not significantly different between healthy mares (that delivered a healthy foal) and mares with USP that also delivered healthy foals. This suggests that ultrasonography may lead to false positives or may be able to detect slight inflammation that does not affect the fetus. On the other hand, there was a significant difference between the level of eIL-1β during pregnancy when comparing healthy mares to those with USP that delivered a live septic neonatal foal, indicating the presence of clinically significant inflammation in the latter despite the lack of macroscopic damage to the placenta collected at the time of delivery. This suggests that the concentrations of eIL-1β could be used to determine whether or not a mare with USP is at risk of delivering a septic or dead foal.
4.2. The Inflammatory Response to the Infectious Process
A high secretion of eIL-1β was detected after the experimental induction of equine placentitis and in cases of mares with retained placenta.
Both were associated with elevated expressions of IL-6, IL-8, and TNFα at the fetal–maternal interface [2,29,30,75,82]. The activation of this inflammatory pathway affects the immune tolerance of the fetus, triggering premature birth with a low probability of survival [80].
LeBlanc et al. (2012) infected pregnant mares with 1 × 108 CFU/mL of Streptococcus equi subspecies zooepidemicus, finding increases in the concentrations of eIL-1β, IL-6, TNFα, and PGE2, and PGF2α in the allantoic fluid. These changes were associated with the premature activation of labor, leading to abortions in 87.5% of the mares by day 309 of gestation [29]. Coinciding with the aforementioned study, the upregulation of eIL-1β found in this study in most mares with USP at days 240 (Figure 2) and 320 of gestation (Figure 3) was associated with foal infection and mortality (Table 1).
Recently, Fedorka et al. (2019) intracervically inoculated S. zooepidemicus to pregnant mares and found significant increases in the concentrations of eIL-1β, eIL-6, and eIL-10 in the amniotic fluid, but not in the serum of mares or neonatal foals [2]. In the current contribution, we found an elevated concentration of eIL-1β in the serum of mares with USP that delivered a live septic or dead foal, but not in those that delivered a healthy foal (Figure 2 and Figure 3).
Our results suggest that the quantification of eIL-1β and proMMPs in the serum of pregnant mares offers a non-invasive alternative that will help veterinarians to confirm the diagnosis of placentitis, reducing possible complications for both the pregnant mare and the foal. The determination of these biomarkers is fast, low-cost, and does not require specialized personnel or expensive equipment.
5. Conclusions
In this study, we demonstrated that eIL-1β levels in the serum of mares with ultrasonographic signs of placentitis can be used as biomarkers of disease severity and its probable impact on the heath and viability of the foal.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci10090532/s1, Figure S1: The original pictures of Figure 5A.
Author Contributions
Conceptualization, H.F.-H.; ELISA methodology, M.M.M.-V. and I.L.-P.; activity of proMMPs methodology M.M.M.-V. and R.J.A.-G.; monitored and provided veterinary care to the mares throughout their pregnancy, carried out ultrasonographic evaluations, and collected blood samples E.M.-S. and S.H.-V.; examined the health of the foals and the condition of each placenta after delivery A.M.B.; data curation, M.M.M.-V., L.Z. and H.F.-H.; formal analysis, M.M.M.-V., R.A.-M., L.Z., J.L.-C., J.S.L.-C. and H.F.-H.; writing—original draft preparation, H.F.-H.; writing—review and editing, A.M.B., L.Z. and H.F.-H. All authors have read and agreed to the published version of the manuscript.
Funding
The current project was supported by a grant (PAPIIT IN-226816, assigned to AMB) from the Universidad Nacional de Autónoma de México (UNAM), México City, México. The authors IL-P, RJA-G, RA-M, SH-V, AM-B, L-Z, JL-C, JSL-C, and HF-H paid for the publication of the article. UNAM was not involved in any stage of the study, and therefore, has no conflict of interest with the contents of the manuscript.
Institutional Review Board Statement
This protocol was approved by the Ethics Committee on Animal Experimentation at the Universidad Nacional Autónoma de México (SICUAE.MC-2020/2-5 assigned to HF-H).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All the relevant information from the study is described in the manuscript.
Acknowledgments
This paper is derived from the Master of Sciences thesis of María Margarita Morales-Vázquez (number 308241178) at the Programa de Maestría en Ciencias de la Producción y de la Salud Animal, Universidad Nacional Autónoma de México. Morales-Vázquez was supported by a scholarship from the Consejo Nacional de Ciencia y Tecnología (CONACyT), México.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Donahue, J.M.; Williams, N.M. Emergent causes of placentitis and abortion. Vet. Clin. N. Am. Equine Pract. 2000, 16, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Fedorka, C.E.; Ball, B.A.; Scoggin, K.E.; Loux, S.C.; Troedsson, M.H.T.; Adams, A.A. The feto-maternal immune response to equine placentitis. Am. J. Reprod. Immunol. 2019, 82, e13179. [Google Scholar] [CrossRef] [PubMed]
- Cummins, C.; Carrington, S.; Fitzpatrick, E.; Duggan, V. Ascending placentitis in the mare: A review. Ir. Vet. J. 2008, 61, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.B.; Ball, B.A.; Loux, S.C.; Boakari, Y.L.; Scoggin, K.E.; El-Sheikh Ali, H.; Cogliati, B.; Esteller-Vico, A. Uterine cervix as a fundamental part of the pathogenesis of pregnancy loss associated with ascending placentitis in mares. Theriogenology 2020, 145, 167–175. [Google Scholar] [CrossRef]
- LeBlanc, M. Ascending placentitis in the mare: An update. Reprod. Domest. Anim. 2010, 45 (Suppl. S2), 28–34. [Google Scholar] [CrossRef]
- Peric, A.; Weiss, J.; Vulliemoz, N.; Baud, D.; Stojanov, M. Bacterial Colonization of the Female Upper Genital Tract. Int. J. Mol. Sci. 2019, 20, 3405. [Google Scholar] [CrossRef]
- Chopra, A.; Radhakrishnan, R.; Sharma, M. Porphyromonas gingivalis and adverse pregnancy outcomes: A review on its intricate pathogenic mechanisms. Crit. Rev. Microbiol. 2020, 46, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Constant, O.; Maarifi, G.; Blanchet, F.P.; Van de Perre, P.; Simonin, Y.; Salinas, S. Role of Dendritic Cells in Viral Brain Infections. Front. Immunol. 2022, 13, 862053. [Google Scholar] [CrossRef] [PubMed]
- Layman, Q.D.; Rezabek, G.B.; Ramachandran, A.; Love, B.C.; Confer, A.W. A retrospective study of equine actinobacillosis cases: 1999–2011. J. Vet. Diagn. Investig. 2014, 26, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, S. Specific immune response of mares and their newborn foals to Actinobacillus spp. present in the oral cavity. Acta Vet. Scand. 2001, 42, 237–242. [Google Scholar] [CrossRef]
- El-Sheikh Ali, H.; Legacki, E.L.; Loux, S.C.; Esteller-Vico, A.; Dini, P.; Scoggin, K.E.; Conley, A.J.; Stanley, S.D.; Ball, B.A. Equine placentitis is associated with a downregulation in myometrial progestin signalingdagger. Biol. Reprod. 2019, 101, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Kahler, A.; McGonnell, I.M.; Smart, H.; Kowalski, A.A.; Smith, K.C.; Wathes, D.C.; Mestre, A.M. Fetal morphological features and abnormalities associated with equine early pregnancy loss. Equine Vet. J. 2021, 53, 530–541. [Google Scholar] [CrossRef] [PubMed]
- LeCuyer, T.E.; Rink, A.; Bradway, D.S.; Evermann, J.F.; Nicola, A.V.; Baszler, T.; Haldorson, G.J. Abortion in a Mediterranean miniature donkey (Equus asinus) associated with a gammaherpesvirus similar to Equid herpesvirus 7. J. Vet. Diagn. Investig. 2015, 27, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Murase, H.; Miyazawa, M.; Harada, T.; Ozawa, M.; Sato, F.; Hada, T. Aborted fetal sizes of Thoroughbred horses in Hidaka, Japan, between 2005 and 2015. J. Equine Sci. 2017, 28, 47–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abraham, M.; Bauquier, J. Causes of equine perinatal mortality. Vet. J. 2021, 273, 105675. [Google Scholar] [CrossRef] [PubMed]
- Grandolfo, E.; Parisi, A.; Ricci, A.; Lorusso, E.; de Siena, R.; Trotta, A.; Buonavoglia, D.; Martella, V.; Corrente, M. High mortality in foals associated with Salmonella enterica subsp. enterica Abortusequi infection in Italy. J. Vet. Diagn. Investig. 2018, 30, 483–485. [Google Scholar] [CrossRef]
- Marenzoni, M.L.; Bietta, A.; Lepri, E.; Proietti, P.C.; Cordioli, P.; Canelli, E.; Stefanetti, V.; Coletti, M.; Timoney, P.J.; Passamonti, F. Role of equine herpesviruses as co-infecting agents in cases of abortion, placental disease and neonatal foal mortality. Vet. Res. Commun. 2013, 37, 311–317. [Google Scholar] [CrossRef]
- Baumann, S.; Gurtner, C.; Marti, H.; Borel, N. Detection of Chlamydia species in 2 cases of equine abortion in Switzerland: A retrospective study from 2000 to 2018. J. Vet. Diagn. Investig. 2020, 32, 542–548. [Google Scholar] [CrossRef]
- Ryan, P.L.; Christiansen, D.L.; Hopper, R.M.; Walters, F.K.; Moulton, K.; Curbelo, J.; Greene, J.M.; Willard, S.T. HORSE SPECIES SYMPOSIUM: A novel approach to monitoring pathogen progression during uterine and placental infection in the mare using bioluminescence imaging technology and lux-modified bacteria1,2. J. Anim. Sci. 2011, 89, 1541–1551. [Google Scholar] [CrossRef][Green Version]
- Marenzoni, M.L.; Stefanetti, V.; Danzetta, M.L.; Timoney, P.J. Gammaherpesvirus infections in equids: A review. Vet. Med. Res. Rep. 2015, 6, 91–101. [Google Scholar] [CrossRef][Green Version]
- Orellana-Guerrero, D.; Renaudin, C.; Edwards, L.; Rose, E.; Aleman, M.; Moore, P.F.; Dujovne, G. Fungal Placentitis Caused by Aspergillus terreus in a Mare: Case Report. J. Equine Vet. Sci. 2019, 83, 102799. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.L.; Hopkins, L.J.; Gangloff, M.; Bryant, C.E. The molecular basis for recognition of bacterial ligands at equine TLR2, TLR1 and TLR6. Vet. Res. 2013, 44, 50. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh Ali, H.; Boakari, Y.L.; Loux, S.C.; Dini, P.; Scoggin, K.E.; Esteller-Vico, A.; Kalbfleisch, T.; Ball, B.A. Transcriptomic analysis reveals the key regulators and molecular mechanisms underlying myometrial activation during equine placentitis†. Biol. Reprod. 2020, 102, 1306–1325. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh Ali, H.; Dini, P.; Scoggin, K.; Loux, S.; Fedorka, C.; Boakari, Y.; Norris, J.; Esteller-Vico, A.; Kalbfleisch, T.; Ball, B. Transcriptomic analysis of equine placenta reveals key regulators and pathways involved in ascending placentitis†. Biol. Reprod. 2020, 104, 638–656. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh Ali, H.; Loux, S.C.; Kennedy, L.; Scoggin, K.E.; Dini, P.; Fedorka, C.E.; Kalbfleisch, T.S.; Esteller-Vico, A.; Horohov, D.W.; Erol, E.; et al. Transcriptomic analysis of equine chori-oallantois reveals immune networks and molecular mechanisms involved in nocardioform placentitis. Vet. Res. 2021, 52, 103. [Google Scholar] [CrossRef]
- Figueiredo, M.D.; Vandenplas, M.L.; Hurley, D.J.; Moore, J.N. Differential induction of MyD88- and TRIF-dependent pathways in equine monocytes by Toll-like receptor agonists. Vet. Immunol. Immunopathol. 2009, 127, 125–134. [Google Scholar] [CrossRef]
- Domino, M.; Jasinski, T.; Kautz, E.; Juszczuk-Kubiak, E.; Ferreira-Dias, G.; Zabielski, R.; Sady, M.; Gajewski, Z. Expression of genes involved in the NF-kappaB-dependent pathway of the fibrosis in the mare endometrium. Theriogenology 2020, 147, 18–24. [Google Scholar] [CrossRef]
- Siemieniuch, M.J.; Szóstek, A.Z.; Gajos, K.; Kozdrowski, R.; Nowak, M.; Okuda, K. Type of Inflammation Differentially Affects Expression of Interleukin 1β and 6, Tumor Necrosis Factor-α and Toll-Like Receptors in Subclinical Endometritis in Mares. PLoS ONE 2016, 11, e0154934. [Google Scholar] [CrossRef]
- Leblanc, M.M.; Giguère, S.; Lester, G.D.; Brauer, K.; Paccamonti, D.L. Relationship between infection, inflammation and premature parturition in mares with experimentally induced placentitis. Equine Vet. J. 2012, 44, 8–14. [Google Scholar] [CrossRef]
- Lyle, S.K. Immunology of infective preterm delivery in the mare. Equine Vet. J. 2014, 46, 661–668. [Google Scholar] [CrossRef]
- McGlothlin, J.A.; Lester, G.D.; Hansen, P.J.; Thomas, M.; Pablo, L.; Hawkins, D.L.; LeBlanc, M.M. Alteration in uterine contractility in mares with experimentally induced placentitis. Reproduction 2004, 127, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Pinteaux, E.; Abdulaal, W.H.; Mufazalov, I.A.; Humphreys, N.E.; Simonsen-Jackson, M.; Francis, S.; Müller, W.; Waisman, A. Cell-specific conditional deletion of interleukin-1 (IL-1) ligands and its receptors: A new toolbox to study the role of IL-1 in health and disease. J. Mol. Med. 2020, 98, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, M.; Duchamp, G.; Gérard, N. In vivo effect of interleukin-1beta and interleukin-1RA on oocyte cytoplasmic maturation, ovulation, and early embryonic development in the mare. Reprod. Biol. Endocrinol. 2005, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, L.; Brayboy, L. Macrophages: An indispensable piece of ovarian health. Biol. Reprod. 2021, 104, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, T.; Zdrojkowski, Ł.; Kautz, E.; Juszczuk-Kubiak, E.; Ferreira-Dias, G.; Domino, M. The NF-κB-signalling pathway in mare′s endometrium infiltrated with the inflammatory cells. Reprod. Domest. Anim. 2022, 57, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Katila, T.; Ferreira-Dias, G. Evolution of the Concepts of Endometrosis, Post Breeding Endometritis, and Susceptibility of Mares. Animals 2022, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.K.; Richter, I.G.; Ahrens, T.; Merle, R.; Alalwani, A.; Lilge, S.; Purschke, K.; Barnewitz, D.; Gehlen, H. MMP-9 Concentration in Peritoneal Fluid Is a Valuable Biomarker Associated with Endotoxemia in Equine Colic. Mediat. Inflamm. 2021, 2021, 9501478. [Google Scholar] [CrossRef]
- Jaworska, J.; Ropka-Molik, K.; Piórkowska, K.; Szmatoła, T.; Kowalczyk-Zięba, I.; Wocławek-Potocka, I.; Siemieniuch, M. Transcriptome Profiling of the Retained Fetal Membranes—An Insight in the Possible Pathogenesis of the Disease. Animals 2021, 11, 675. [Google Scholar] [CrossRef]
- Read, J.E.; Cabrera-Sharp, V.; Offord, V.; Mirczuk, S.M.; Allen, S.P.; Fowkes, R.C.; de Mestre, A.M. Dynamic changes in gene expression and signalling during trophoblast development in the horse. Reproduction 2018, 156, 313–330. [Google Scholar] [CrossRef]
- Vagnoni, K.E.; Ginther, O.J.; Lunn, D.P. Metalloproteinase Activity has a Role in Equine Chorionic Girdle Cell Invasion1. Biol. Reprod. 1995, 53, 800–805. [Google Scholar] [CrossRef]
- El-Sheikh Ali, H.; Scoggin, K.E.; Ruby, R.; Loynachan, A.; Boakari, Y.; Fernandes, C.; Dini, P.; Fedorka, C.E.; Loux, S.C.; Esteller-Vico, A.; et al. Equine cervical remodeling during placentitis and the prepartum period: A transcriptomic approach. Reproduction 2021, 161, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Safari, Z.; Soudi, S.; Jafarzadeh, N.; Hosseini, A.Z.; Vojoudi, E.; Sadeghizadeh, M. Promotion of angiogenesis by M13 phage and RGD peptide in vitro and in vivo. Sci. Rep. 2019, 9, 11182. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; He, Y.; Li, L.; Mao, W.; Chen, X.; Ni, H.; Dong, Y.; Lyu, F. Exosomal MMP2 derived from mature osteoblasts promotes angiogenesis of endothelial cells via VEGF/Erk1/2 signaling pathway. Exp. Cell Res. 2019, 383, 111541. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Han, C.-H.; Hu, F.-F.; Wang, Y.-B.; Cao, Y.-J. The correlation analysis of human embryonic MMP-9 secretion and embryo quality. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2354–2358. [Google Scholar] [PubMed]
- Morales-Hernández, F.V.; Bautista-Bautista, G.; Acuña-González, R.J.; Vázquez-Cárdenas, P.; López-Canales, J.S.; Lozano-Cuenca, J.; Osorio-Caballero, M.; Flores-Herrera, H. Differential proMMP-2 and proMMP-9 secretion in human pre-implantation embryos at day 5 of development. Acta Biochim. Pol. 2022, 69, 683–689. [Google Scholar] [CrossRef]
- Barton, A.K.; Shety, T.; Bondzio, A.; Einspanier, R.; Gehlen, H. Metalloproteinases and their inhibitors are influenced by inhalative glucocorticoid therapy in combination with environmental dust reduction in equine recurrent airway obstruction. BMC Vet. Res. 2016, 12, 282. [Google Scholar] [CrossRef] [PubMed]
- Clutterbuck, A.L.; Harris, P.; Allaway, D.; Mobasheri, A. Matrix metalloproteinases in inflammatory pathologies of the horse. Vet. J. 2010, 183, 27–38. [Google Scholar] [CrossRef]
- Bradley, L.M.; Douglass, M.F.; Chatterjee, D.; Akira, S.; Baaten, B.J.G. Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus-induced toll-like receptor signaling. PLoS Pathog. 2012, 8, e1002641. [Google Scholar] [CrossRef] [PubMed]
- Rossi, H.S.; Koho, N.M.; Ilves, M.; Rajamäki, M.M.; Mykkänen, A.K. Expression of extracellular matrix metalloproteinase inducer and matrix metalloproteinase-2 and -9 in horses with chronic airway inflammation. Am. J. Vet. Res. 2017, 78, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Loftus, J.P.; Johnson, P.J.; Belknap, J.K.; Pettigrew, A.; Black, S.J. Leukocyte-derived and endogenous matrix metalloproteinases in the lamellae of horses with naturally acquired and experimentally induced laminitis. Vet. Immunol. Immunopathol. 2009, 129, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Raulo, S.M.; Sorsa, T.; Tervahartiala, T.; Pirila, E.; Maisi, P. MMP-9 as a marker of inflammation in tracheal epithelial lining fluid (TELF) and in bronchoalveolar fluid (BALF) of COPD horses. Equine Vet. J. 2001, 33, 128–136. [Google Scholar] [CrossRef]
- Szóstek-Mioduchowska, A.; Baclawska, A.; Okuda, K.; Skarzynski, D. Effect of proinflammatory cytokines on endometrial collagen and metallopeptidase expression during the course of equine endometrosis. Cytokine 2019, 123, 154767. [Google Scholar] [CrossRef] [PubMed]
- Fugler, L.A.; Eades, S.C.; Moore, R.M.; Koch, C.E.; Keowen, M.L. Plasma matrix metalloproteinase activity in horses after intravenous infusion of lipopolysaccharide and treatment with matrix metalloproteinase inhibitors. Am. J. Vet. Res. 2013, 74, 473–480. [Google Scholar] [CrossRef]
- Woessner, J.F. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991, 5, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Kargozaran, H.; Yuan, S.Y.; Breslin, J.W.; Watson, K.D.; Gaudreault, N.; Breen, A.; Wu, M.H. A role for endothelial-derived matrix metalloproteinase-2 in breast cancer cell transmigration across the endothelial-basement membrane barrier. Clin. Exp. Metastasis 2007, 24, 495–502. [Google Scholar] [CrossRef]
- Allport, J.R.; Lim, Y.C.; Shipley, J.M.; Senior, R.M.; Shapiro, S.D.; Matsuyoshi, N.; Vestweber, D.; Luscinskas, F.W. Neutrophils from MMP-9- or neutrophil elastase-deficient mice show no defect in transendothelial migration under flow in vitro. J. Leukoc. Biol. 2002, 71, 821–828. [Google Scholar] [CrossRef]
- Nawrocki-Raby, B.; Gilles, C.; Polette, M.; Martinella-Catusse, C.; Bonnet, N.; Puchelle, E.; Foidart, J.-M.; van Roy, F.; Birembaut, P. E-Cadherin mediates mmp down-regulation in highly invasive bronchial tumor cells. Am. J. Pathol. 2003, 163, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta BBA Mol. Cell Res. 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Renaudin, C.; Troedsson, M.; Gillis, C.; King, V.; Bodena, A. Ultrasonographic evaluation of the equine placenta by transrectal and transabdominal approach in the normal pregnant mare. Theriogenology 1997, 47, 559–573. [Google Scholar] [CrossRef]
- McAfoos, J.L.; Ellerbrock, R.E.; Canisso, I.F. Fetal Death Associated With Premature Mammary Gland Development and Lactation in a Mare Treated With Weekly Injections of Long-Acting Progesterone. J. Equine Vet. Sci. 2019, 81, 102783. [Google Scholar] [CrossRef] [PubMed]
- Borba, L.D.A.; Nogueira, C.E.W.; Bruhn, F.R.P.; da Silva, G.C.; Feijó, L.S.; Canisso, I.F.; Curcio, B.D.R. Peripheral blood markers of sepsis in foals born from mares with experimentally induced ascending placentitis. Vet. Rec. 2020, 187, 29. [Google Scholar] [CrossRef] [PubMed]
- Koterba, A.M.; Brewer, B.D.; Tarplee, F.A. Clinical and clinicopathological characteristics of the septicaemic neonatal foal: Review of 38 cases. Equine Vet. J. 1984, 16, 376–382. [Google Scholar] [CrossRef]
- Taylor, S. A review of equine sepsis. Equine Vet. Educ. 2015, 27, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.M.; Ruby, R.E.; Dembek, K.A.; Barr, B.S.; Reuss, S.M.; Magdesian, K.G.; Olsen, E.; Burns, T.; Slovis, N.M.; Wilkins, P.A. Evaluation of updated sepsis scoring systems and systemic inflammatory response syndrome criteria and their association with sepsis in equine neonates. J. Vet. Intern. Med. 2018, 32, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Flores-Herrera, H.; García-López, G.; Díaz, N.; Molina-Hernández, A.; Osorio-Caballero, M.; Soriano-Becerril, D.; Zaga-Clavellina, V. An experimental mixed bacterial infection induced differential secretion of proinflammatory cytokines (IL-1β, TNFα) and proMMP-9 in human fetal membranes. Placenta 2012, 33, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Nissinen, L.; Kähäri, V.-M. Matrix metalloproteinases in inflammation. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Takechi, M.; Uchida-Fujii, E.; Miyazawa, K.; Nukada, T.; Niwa, H. Ten cases of Mycobacterium avium subsp. hominissuis infections linked to equine abortions in Japan, 2018–2019. Vet. Med. Sci. 2021, 7, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.M.; Foote, A.K.; Smith, K.C.; Verheyen, K.L.; Mestre, A.M. Incidence and causes of pregnancy loss after Day 70 of gestation in Thoroughbreds. Equine Vet. J. 2021, 53, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.B.; Donahue, J.M.; Giles, R.C.; Petrites-Murphy, M.B., Jr.; Poonacha, K.B.; Roberts, A.W.; Smith, B.J.; Tramontin, R.R.; Tuttle, P.A.; Swerczek, T.W. Etiology and pathology of equine placentitis. J. Vet. Diagn. Investig. 1993, 5, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Loux, S.; Ball, B. The proteome of fetal fluids in mares with experimentally-induced placentitis. Placenta 2018, 64, 71–78. [Google Scholar] [CrossRef]
- Pinho, M.D.; Erol, E.; Ribeiro-Gonçalves, B.; Mendes, C.I.; Carriço, J.A.; Matos, S.C.; Preziuso, S.; Luebke-Becker, A.; Wieler, L.H.; Melo-Cristino, J.; et al. Beta-hemolytic Streptococcus dysgalactiae strains isolated from horses are a genetically distinct population within the Streptococcus dysgalactiae taxon. Sci. Rep. 2016, 6, 31736. [Google Scholar] [CrossRef]
- Macpherson, M.L.; Giguere, S.; Pozor, M.A.; Burden, C.A.; Berghaus, L.J.; Berghaus, R.D.; Varner, J.C.; Hayna, J.T.; SuBenson, M.; Randell, S.A.; et al. Evidence for anti-inflammatory effects of firocoxib administered to mares with experimentally induced placentitis. Am. J. Reprod. Immunol. 2021, 86, e13396. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.K.; Shety, T.; Klier, J.; Geis, S.; Einspanier, R.; Gehlen, H. Metalloproteinases and their Inhibitors under the Course of Immunostimulation by CPG-ODN and Specific Antigen Inhalation in Equine Asthma. Mediat. Inflamm. 2019, 2019, 7845623. [Google Scholar] [CrossRef]
- Ellero, N.; Lanci, A.; Ferlizza, E.; Andreani, G.; Mariella, J.; Isani, G.; Castagnetti, C. Activities of matrix metalloproteinase-2 and -9 in amniotic fluid at parturition in mares with normal and high-risk pregnancy. Theriogenology 2021, 172, 116–122. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh Ali, H.; Scoggin, K.; Linhares Boakari, Y.; Dini, P.; Loux, S.; Fedorka, C.; Esteller-Vico, A.; Ball, B. Kinetics of placenta-specific 8 (PLAC8) in equine placenta during pregnancy and placentitis. Theriogenology 2021, 160, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Aubone, A.M.P.; Bisiau, C.M.; McCue, P.M.; Bouma, G.J. Presence of Clock genes in equine full-term placenta. J. Anim. Sci. 2020, 98, skaa094. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Taylor, B.D. Exploring Inflammatory Mediators in Fetal and Maternal Compartments During Human Parturition. Obstet. Gynecol. 2019, 134, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.M.; Bryant, A.H.; Rees, A.; Down, B.; Jones, R.H.; Thornton, C.A. Production and regulation of interleukin-1 family cytokines at the materno-fetal interface. Cytokine 2017, 99, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, J.; Janowski, T. Expression of proinflammatory cytokines IL-1β, IL-6 and TNFα in the retained placenta of mares. Theriogenology 2019, 126, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Naskou, M.C.; Norton, N.A.; Copland, I.B.; Galipeau, J.; Peroni, J.F. Innate immune responses of equine monocytes cultured in equine platelet lysate. Vet. Immunol. Immunopathol. 2018, 195, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.J.; Fortier, M.A.; Bernal, A.L. Prostaglandin pathway gene expression in human placenta, amnion and choriodecidua is differentially affected by preterm and term labour and by uterine inflammation. BMC Pregnancy Childbirth 2014, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Hadley, E.E.; Richardson, L.S.; Torloni, M.R.; Menon, R. Gestational tissue inflammatory biomarkers at term labor: A systematic review of literature. Am. J. Reprod. Immunol. 2018, 79, e12776. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).