Serum Ferritin in Obese Dogs: Changes and Comparison with Other Analytes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Samples
2.2. Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- German, A.J. Style over Substance: What Can Parenting Styles Tell Us about Ownership Styles and Obesity in Companion Animals? Br. J. Nutr. 2015, 113 (Suppl. S1), S72–S77. [Google Scholar] [CrossRef] [PubMed]
- Rohlf, V.I.; Toukhsati, S.; Coleman, G.J.; Bennett, P.C. Dog Obesity: Can Dog Caregivers’ (Owners’) Feeding and Exercise Intentions and Behaviors Be Predicted From Attitudes? J. Appl. Anim. Welf. Sci. 2010, 13, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Pegram, C.; Raffan, E.; White, E.; Ashworth, A.H.; Brodbelt, D.C.; Church, D.B.; O’Neill, D.G. Frequency, Breed Predisposition and Demographic Risk Factors for Overweight Status in Dogs in the UK. J. Small Anim. Pract. 2021, 62, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Lindåse, S.; Feltenmark, T.; Krantz, M.; Söder, J. Overweight in Swedish Show Dogs-Prevalence and Association with Performance in Competition. Acta Vet. Scand. 2021, 63, 17. [Google Scholar] [CrossRef]
- Weeth, L.P. Other Risks/Possible Benefits of Obesity. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 843–853. [Google Scholar] [CrossRef]
- Fruh, S.M. Obesity: Risk Factors, Complications, and Strategies for Sustainable Long-Term Weight Management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef]
- Miyai, S.; Hendawy, A.O.; Sato, K. Gene Expression Profile of Peripheral Blood Mononuclear Cells in Mild to Moderate Obesity in Dogs. Vet. Anim. Sci. 2021, 13, 100183. [Google Scholar] [CrossRef]
- Bohn, A.A. Diagnosis of Disorders of Iron Metabolism in Dogs and Cats. Clin. Lab. Med. 2015, 35, 579–590. [Google Scholar] [CrossRef]
- Theil, E.C. Ferritin: At the Crossroads of Iron and Oxygen Metabolism. J. Nutr. 2003, 133, 1549–1553. [Google Scholar] [CrossRef]
- Sharif, K.; Vieira Borba, V.; Zandman-Goddard, G.; Shoenfeld, Y. Eppur Si Muove: Ferritin Is Essential in Modulating Inflammation. Clin. Exp. Immunol. 2018, 191, 149–150. [Google Scholar] [CrossRef]
- Liu, H.D.; Li, W.; Chen, Z.R.; Zhou, M.L.; Zhuang, Z.; Zhang, D.D.; Zhu, L.; Hang, C.H. Increased Expression of Ferritin in Cerebral Cortex after Human Traumatic Brain Injury. Neurol. Sci. 2013, 34, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Huaijantug, S.; Yatmark, P.; Phophug, P.; Worapakdee, M.; Phutrakul, A.; Julapanthong, P.; Chuaychoo, K. Quantitative Ultrasound Elastography and Serum Ferritin Level in Dogs with Liver Tumors. J. Adv. Vet. Anim. Res. 2020, 7, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Jung, I.A.; Kim, S.H.; Cho, W.-K.; Jeong, S.H.; Cho, K.S.; Park, S.H.; Jung, M.H.; Suh, B.-K. Serum Ferritin Level Is Higher in Male Adolescents with Obesity: Results from the Korean National Health and Nutrition Examination Survey 2010. Ann. Pediatr. Endocrinol. Metab. 2013, 18, 141. [Google Scholar] [CrossRef]
- Sun, L.; Franco, O.H.; Hu, F.B.; Cai, L.; Yu, Z.; Li, H.; Ye, X.; Qi, Q.; Wang, J.; Pan, A.; et al. Ferritin Concentrations, Metabolic Syndrome, and Type 2 Diabetes in Middle-Aged and Elderly Chinese. J. Clin. Endocrinol. Metab. 2008, 93, 4690–4696. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, Z.; Tang, Y.; Tan, A.; Gao, Y.; Lu, Z.; Wang, Q.; Chen, Y.; Wu, C.; Zhang, H.; et al. High Serum Ferritin Level Is an Independent Risk Factor for Metabolic Syndrome in a Chinese Male Cohort Population. Diabetol. Metab. Syndr. 2015, 7, 11. [Google Scholar] [CrossRef]
- Vari, I.S.; Balkau, B.; Kettaneh, A.; André, P.; Tichet, J.; Fumeron, F.; Caces, E.; Marre, M.; Grandchamp, B.; Ducimetière, P. Ferritin and Transferrin Are Associated with Metabolic Syndrome Abnormalities and Their Change over Time in a General Population: Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2007, 30, 1795–1801. [Google Scholar] [CrossRef]
- Shim, Y.S.; Kang, M.J.; Oh, Y.J.; Baek, J.W.; Yang, S.; Hwang, I.T. Association of Serum Ferritin with Insulin Resistance, Abdominal Obesity, and Metabolic Syndrome in Korean Adolescent and Adults: The Korean National Health and Nutrition Examination Survey, 2008 to 2011. Medicine 2017, 96, e6179. [Google Scholar] [CrossRef]
- Han, L.; Wang, Y.; Li, J.; Zhang, X.; Bian, C.; Wang, H.; Du, S.; Suo, L. Gender Differences in Associations of Serum Ferritin and Diabetes, Metabolic Syndrome, and Obesity in the China Health and Nutrition Survey. Mol. Nutr. Food Res. 2014, 58, 2189–2195. [Google Scholar] [CrossRef]
- Kim, M.K.; Baek, K.H.; Song, K.H.; Kang, M.I.; Choi, J.H.; Bae, J.C.; Park, C.Y.; Lee, W.Y.; Oh, K.W. Increased Serum Ferritin Predicts the Development of Hypertension among Middle-Aged Men. Am. J. Hypertens. 2012, 25, 492–497. [Google Scholar] [CrossRef]
- Jiang, R.; Manson, J.A.E.; Meigs, J.B.; Ma, J.; Rifai, N.; Hu, F.B. Body Iron Stores in Relation to Risk of Type 2 Diabetes in Apparently Healthy Women. JAMA 2004, 291, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Tvarijonaviciute, A.; Barić-Rafaj, R.; Horvatic, A.; Muñoz-Prieto, A.; Guillemin, N.; Lamy, E.; Tumpa, A.; Ceron, J.J.; Martinez-Subiela, S.; Mrljak, V. Identification of Changes in Serum Analytes and Possible Metabolic Pathways Associated with Canine Obesity-Related Metabolic Dysfunction. Vet. J. 2019, 244, 51–59. [Google Scholar] [CrossRef]
- Mutlu-Türkoǧlu, Ü.; Öztezcan, S.; Telci, A.; Orhan, Y.; Aykaç-Toker, G.; Sivas, A.; Uysal, M. An Increase in Lipoprotein Oxidation and Endogenous Lipid Peroxides in Serum of Obese Women. Clin. Exp. Med. 2003, 2, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Bajnok, L.; Seres, I.; Varga, Z.; Jeges, S.; Peti, A.; Karanyi, Z.; Juhasz, A.; Csongradi, E.; Mezosi, E.; Nagy, E.V.; et al. Relationship of Serum Resistin Level to Traits of Metabolic Syndrome and Serum Paraoxonase 1 Activity in a Population with a Broad Range of Body Mass Index. Exp. Clin. Endocrinol. Diabetes 2008, 116, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Kota, S.K.; Meher, L.K.; Kota, S.K.; Jammula, S.; Krishna, S.V.S.; Modi, K.D. Implications of Serum Paraoxonase Activity in Obesity, Diabetes Mellitus, and Dyslipidemia. Indian J. Endocrinol. Metab. 2013, 17, 402. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Tecles, F.; Cerón, J.J. Relationship between Serum Butyrylcholinesterase and Obesity in Dogs: A Preliminary Report. Vet. J. 2010, 186, 197–200. [Google Scholar] [CrossRef]
- Laflamme, D. Development and Validation of a Body Condition Score System for Dogs. Canine Pract. 1997, 22, 10–15. [Google Scholar]
- Tvarijonaviciute, A.; Ceron, J.J.; Holden, S.L.; Cuthbertson, D.J.; Biourge, V.; Morris, P.J.; German, A.J. Obesity-Related Metabolic Dysfunction in Dogs: A Comparison with Human Metabolic Syndrome. BMC Vet. Res. 2012, 8, 147. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Tecles, F.; Caldin, M.; Tasca, S.; Cerón, J. Validation of Spectrophotometric Assays for Serum Paraoxonase Type-1 Measurement in Dogs. Am. J. Vet. Res. 2012, 73, 34–41. [Google Scholar] [CrossRef]
- Erel, O. A Novel Automated Direct Measurement Method for Total Antioxidant Capacity Using a New Generation, More Stable ABTS Radical Cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Erel, O. A New Automated Colorimetric Method for Measuring Total Oxidant Status. Clin. Biochem. 2005, 38, 1103–11111. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.P.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Hernández-Ruiz, J.; Pardo-Marin, L.; Segarra, S.; Cerón, J.J. Changes in Serum Biomarkers of Oxidative Stress after Treatment for Canine Leishmaniosis in Sick Dogs. Comp. Immunol. Microbiol. Infect. Dis. 2016, 49, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Subiela, S.; Cerón, J.J. Validation of Commercial Assays for the Determination of Haptoglobin, C-Reactive Protein and Serum Amyloid A in Dogs. Arch. Med. Vet. 2005, 37, 60–67. [Google Scholar]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory Links between Obesity and Metabolic Disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Després, J.P.; Lemieux, I. Abdominal Obesity and Metabolic Syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Cerón, J.J.; Eckersall, P.D.; Martínez-Subiela, S. Acute Phase Proteins in Dogs and Cats: Current Knowledge and Future Perspectives. Vet. Clin. Pathol. 2005, 34, 85–99. [Google Scholar] [CrossRef]
- Wood, I.S.; De Heredia, F.P.; Wang, B.; Trayhurn, P. Cellular Hypoxia and Adipose Tissue Dysfunction in Obesity. Proc. Nutr. Soc. 2009, 68, 370–377. [Google Scholar] [CrossRef]
- Hämäläinen, P.; Saltevo, J.; Kautiainen, H.; Mäntyselkä, P.; Vanhala, M. Erythropoietin, Ferritin, Haptoglobin, Hemoglobin and Transferrin Receptor in Metabolic Syndrome: A Case Control Study. Cardiovasc. Diabetol. 2012, 11, 116. [Google Scholar] [CrossRef]
- German, A.J.; Hervera, M.; Hunter, L.; Holden, S.L.; Morris, P.J.; Biourge, V.; Trayhurn, P. Improvement in Insulin Resistance and Reduction in Plasma Inflammatory Adipokines after Weight Loss in Obese Dogs. Domest. Anim. Endocrinol. 2009, 37, 214–226. [Google Scholar] [CrossRef]
- Veiga, A.P.M.; Price, C.A.; de Oliveira, S.T.; Dos Santos, A.P.; Campos, R.; Barbosa, P.R.; González, F.H.D. Association of Canine Obesity with Reduced Serum Levels of C-Reactive Protein. J. Vet. Intern. Med. 2008, 228, 224–228. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Tecles, F.; Martínez-Subiela, S.; Cerón, J.J. Effect of Weight Loss on Inflammatory Biomarkers in Obese Dogs. Vet. J. 2012, 193, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Tvarijonaviciute, A.; Martinez, S.; Gutierrez, A.; Ceron, J.J.; Tecles, F. Serum Acute Phase Proteins Concentrations in Dogs during Experimentally Short-Term Induced Overweight. A Preliminary Study. Res. Vet. Sci. 2011, 90, 31–34. [Google Scholar] [CrossRef]
- Caldin, M.; Tasca, S.; Carli, E.; Bianchini, S.; Furlanello, T.; Martinez-Subiela, S.; Cerón, J.J. Serum Acute Phase Protein Concentrations in Dogs with Hyperadrenocorticism with and without Concurrent Inflammatory Conditions. Vet. Clin. Pathol. 2009, 38, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Tvarijonaviciute, A.; Caldin, M.; Martinez-Subiela, S.; Tecles, F.; Pastor, J.; Ceron, J.J. Serum Paraoxonase 1 and Butyrylcholinesterase in Dogs with Hyperadrenocorticism. Vet. J. 2015, 203, 262–263. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, A.; Dhand, N.K.; McCann, T.; Knottenbelt, C.M.; Tebb, A.J.; Evans, H.; Eckersall, P.D.; Ramsey, I.K. Monitoring the Response of Canine Hyperadrenocorticism to Trilostane Treatment by Assessment of Acute Phase Protein Concentrations. J. Small Anim. Pract. 2010, 51, 204–209. [Google Scholar] [CrossRef]
- Nieman, L.K.; Biller, B.M.K.; Findling, J.W.; Newell-Price, J.; Savage, M.O.; Stewart, P.M.; Montori, V.M.; Edwards, H. The Diagnosis of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1526. [Google Scholar] [CrossRef]
- Mosing, M.; German, A.J.; Holden, S.L.; MacFarlane, P.; Biourge, V.; Morris, P.J.; Iff, I. Oxygenation and Ventilation Characteristics in Obese Sedated Dogs before and after Weight Loss: A Clinical Trial. Vet. J. 2013, 198, 367–371. [Google Scholar] [CrossRef]
- Qian, X.; Droste, S.K.; Lightman, S.L.; Reul, J.M.H.M.; Linthorst, A.C.E. Circadian and Ultradian Rhythms of Free Glucocorticoid Hormone Are Highly Synchronized between the Blood, the Subcutaneous Tissue, and the Brain. Endocrinology 2012, 153, 4346. [Google Scholar] [CrossRef]
- Lightman, S.L.; Conway-Campbell, B.L. The Crucial Role of Pulsatile Activity of the HPA Axis for Continuous Dynamic Equilibration. Nat. Rev. Neurosci. 2010, 11, 710–718. [Google Scholar] [CrossRef]
| Variable | Lean/Normal Weight (CG, n = 55) | Overweight/Obese (OG, n = 37) | P |
|---|---|---|---|
| Sex (female/male) | 32/23 | 23/21 | 0.203 |
| Sterilised, % | 40 | 73 | 0.002 |
| Age (Years) * | 8 (6.6–11) | 10 (7–10.5) | 0.183 |
| Body weight (Kg) * | 5.525 (4.1–10.7) | 8.4 (6.375–12.05) | 0.027 |
| BCS * | 5 (4–5) | 6 (6–7) | 0.000 |
| Tartar (%) * | 55 (26.25–80) | 65 (50–80) | 0.449 |
| Gingiva (0/1/2/3) | 6/16/22/11 | 3/12/18/4 | 0.572 |
| Meals per day (1/2/3/>3) | 3/22/12/18 | 4/17/6/10 | 0.494 |
| Supplements (Yes/No) | 49/5 | 30/4 | 0.706 |
| Snacks (Yes/No) | 28/27 | 26/11 | 0.064 |
| Variable | Lean/Normal Weight (CG, n = 55) | Overweight/Obese (OG, n = 37) | P (CG vs. OG) | Reference |
|---|---|---|---|---|
| RBC (106/µL) | 7.28 (6.57–7.66) | 7.23 (6.79–7.68) | 0.605 | 5.69–8.56 |
| Haemoglobin (g/dL) | 17.3 (15.4–18.1) | 17.4 (15.75–18.45) | 0.251 | 13.7–20.6 |
| Haematocrit (%) | 49.4 (46–52) | 51.9 (46.9–54.5) | 0.052 | 37–58 |
| MCV (fL) | 69.6 (67.6–71) | 71.4 (69.45–74.15) | 0.001 | 61.7–74.1 |
| MCH (pg) | 23.7 (22.9–24.6) | 24.2 (23.25–25.3) | 0.263 | 21.4–25.5 |
| MCHC (g/dL) | 33.4 (32.4–34) | 32.6 (32.1–33.1) | 0.001 | 33.2–36.8 |
| RDW (%) | 11.5 (11.2–12) | 11.8 (11.4–12.25) | 0.091 | 11.3–15.1 |
| WBC (103/µL) | 6.72 (5.77–8.12) | 6.94 (5.89–8.615) | 0.558 | 5.2–14 |
| Segmented (103/µL) | 3.75 (3.148–4.79) | 4.35 (3.66–5.36) | 0.055 | 3.1–11 |
| Lymphocytes (103/µL) | 2.09 (1.6–2.625) | 1.8 (1.45–2.45) | 0.119 | 1–3.8 |
| Monocytes (103/µL) | 0.37 (0.28–0.525) | 0.45 (0.275–0.525) | 0.421 | 0.2–0.8 |
| Eosinophiles (103/µL) | 0.325 (0.185–0.475) | 0.28 (0.17–0.42) | 0.305 | 0.1–1.4 |
| Basophiles (103/µL) | 0.03 (0.01–0.04) | 0.02 (0.0125–0.04) | 0.897 | 0–0.03 |
| Platelets (103/µL) | 347 (284.8–427) | 345 (257–372) | 0.382 | 175–588 |
| MPV (fL) | 10.2 (9.325–12.13) | 10.8 (9.8–12.1) | 0.214 | 8.6–15.8 |
| PCT (%) | 0.36 (0.29–0.4275) | 0.35 (0.29–0.44) | 0.732 | 0.2–0.8 |
| MPC (g/dL) | 21.55 (20.8–22.18) | 21.65 (20.25–22.6) | 0.590 | 21–100 |
| PMDW (pg) | 0.715 (0.6525–0.8) | 0.8 (0.7–0.85) | 0.088 | 0.64–1.07 |
| Variable | Lean/Normal Weight (CG, n = 55) | Overweight/Obese (OG, n = 37) | P (CG vs. OG) | Reference |
|---|---|---|---|---|
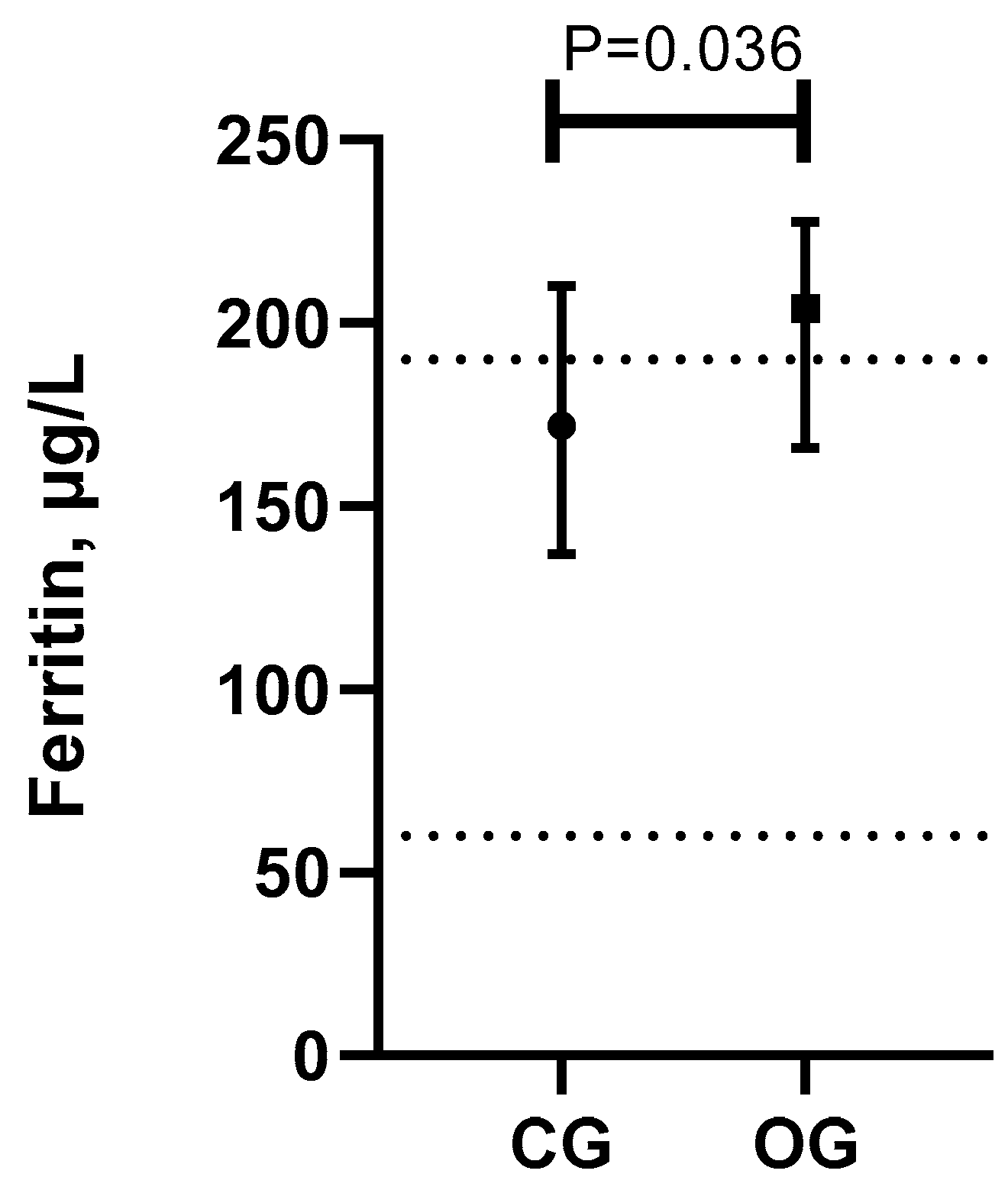
| Ferritin (µg/L) | 172 (137–210) | 204 (166–227.5) a | 0.036 | 60–190 |
| Total proteins (g/dL) | 6.37 (6.06–6.76) | 6.89 (6.655–7.37) | <0.001 | 5.4–7.2 |
| Albumin (g/dL) | 3.1 (2.9–3.4) | 3.3 (3.1–3.65) | 0.007 | 2.5–3.6 |
| Globulins (g/dL) | 3.3 (2.8–3.88) | 3.5 (3.3–3.95) | 0.028 | 2.6–3.8 |
| CRP (µg/mL) | 2.1 (1.6–4.8) | 2.1 (1.45–4.3) | 0.313 | 0–12 |
| Haptoglobin (g/L) | 3 (2.1–3.79) | 3.6 (2.885–4.25) a | 0.031 | 0–3 |
| Fe (µg/dL) | 134.8 (99.25–175.6) | 153.6 (105.2–186.4) | 0.456 | 81–198 |
| UIBC (µg/dL) | 254 (210.4–312.1) | 274.7 (219.4–362) | 0.386 | 148–311 |
| TIBC (µg/dL) | 373.7 (345.8–462.6) | 452.8 (391.9–517) a | 0.019 | 305–436 |
| Ferritin saturation (%) | 35.6 (23.93–43.84) | 35.3 (25.01–46.37) | 0.963 | 23.1–51.5 |
| CK (IU/L) | 104 (67–161) | 99 (72.5–131) | 0.962 | 30–360 |
| AST (IU/L) | 29 (22–34) | 28 (21–35.5) | 0.845 | 0–50 |
| ALT (IU/L) | 48 (36–70) | 58 (38–89.5) a | 0.205 | 0–50 |
| ALP (IU/L) | 79 (53–111) | 112 (66.5–190) | 0.037 | 25–190 |
| gGT (IU/L) | 4.8 (3.175–6.675) | 3.9 (1.25–5.6) | 0.133 | 1–6.5 |
| Total bilirubin (mg/dL) | 0.14 (0.1–0.18) | 0.12 (0.11–0.16) | 0.795 | 0.06–0.24 |
| BchE (µmol/mL.min) | 4.36 (3.5–5.6) | 5.2 (4.25–8.05) a | 0.005 | 3–5 |
| PON-1 (IU/mL) | 3.5 (3.1–3.9) | 3.6 (3.15–4.15) | 0.558 | 3–4.3 |
| Cholesterol (mg/dL) | 239 (207–282) | 239 (212–340.5) | 0.659 | 120–300 |
| Triglycerides (mg/dL) | 64 (55–89) | 103 (69.5–185.5) | <0.001 | 30–200 |
| Amylase (IU/L) | 607 (448–795) | 648 (517.5–791) | 0.608 | 250–1300 |
| Urea (mg/dL) | 38.2 (31.3–47.9) | 36.6 (30.9–46.3) | 0.994 | 20–50 |
| Creatinine (mg/dL) | 0.9 (0.8–1) | 0.9 (0.795–1.03) | 0.864 | 0.5–1.5 |
| Glucose (mg/dL) | 101 (95–108) | 96 (85.5–108.8) | 0.052 | 70–110 |
| Calcium (mg/dL) | 9.91 (9.54–10.43) | 10.76 (10.15–11.11) | <0.001 | 9.6–11.7 |
| Phosphorus (mg/dL) | 3.6 (3.22–4.2) | 3.9 (3.65–4.45) | 0.066 | 2.6–4 |
| Ca/P | 2.75 (2.3–3.225) | 2.7 (2.4–2.9) | 0.884 |
| Ferritin vs. | All Dogs (n = 92) | Lean/Normal Weight (CG, n = 55) | Overweight/Obese (OG, n = 37) |
|---|---|---|---|
| RBC | 0.017 | 0.084 | −0.159 |
| Haemoglobin | 0.081 | 0.027 | 0.074 |
| Haematocrit | 0.065 | 0.032 | −0.169 |
| MCV | 0.094 | −0.238 | 0.448 |
| MCH | 0.148 | −0.017 | 0.457 |
| MCHC | 0.173 | 0.273 | 0.306 |
| RDW | 0.033 | 0.180 | −0.557 |
| WBC | −0.001 | 0.184 | −0.410 |
| Segmented | 0.110 | 0.216 | −0.339 |
| Lymphocytes | −0.159 | −0.086 | −0.165 |
| Monocytes | −0.018 | 0.090 | −0.310 |
| Eosinophiles | −0.102 | 0.096 | −0.492 |
| Basophiles | −0.117 | −0.035 | −0.310 |
| Platelets | −0.210 | −0.157 | −0.251 |
| MPV | −0.019 | 0.033 | −0.285 |
| PCT | −0.150 | −0.007 | −0.369 |
| MPC | 0.156 | 0.095 | 0.299 |
| PCDW | 0.038 | 0.302 | −0.473 |
| MPM | −0.005 | 0.044 | −0.187 |
| PMDW | 0.033 | 0.082 | −0.159 |
| Total proteins | 0.150 | 0.094 | −0.044 |
| Albumin | −0.036 | −0.066 | −0.226 |
| Globulins | 0.080 | 0.079 | −0.146 |
| CRP | −0.018 | 0.118 | −0.155 |
| Haptoglobin | −0.080 | −0.037 | −0.368 |
| Fe | 0.117 | 0.028 | 0.016 |
| UIBC | −0.065 | −0.083 | −0.156 |
| TIBC | 0.082 | −0.014 | −0.177 |
| Ferritin saturation | 0.090 | 0.005 | 0.122 |
| CK | 0.102 | 0.146 | −0.114 |
| AST | 0.225 | 0.187 | 0.187 |
| ALT | 0.161 | 0.141 | 0.126 |
| FAL | 0.136 | −0.018 | 0.295 |
| gGT | −0.270 | −0.303 | −0.008 |
| Total bilirubin | 0.132 | 0.034 | 0.331 |
| BChE | 0.192 | 0.081 | 0.084 |
| PON-1 | 0.048 | 0.045 | 0.169 |
| Cholesterol | 0.024 | −0.076 | 0.271 |
| Triglycerides | 0.154 | 0.130 | −0.040 |
| Amylase | 0.164 | 0.204 | 0.081 |
| Urea | 0.099 | 0.020 | 0.352 |
| Creatinine | 0.152 | 0.147 | 0.166 |
| Glucose | −0.202 | −0.001 | −0.207 |
| Calcium | 0.043 | −0.051 | −0.087 |
| Phosphorus | −0.068 | −0.139 | 0.004 |
| Ca/P | 0.080 | 0.055 | −0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco-Martínez, L.; Pardo-Marín, L.; Sánchez-Mateos, L.; Muñoz-Prieto, A.; García-Martínez, J.D.; Cerón, J.J.; Martínez-Subiela, S.; Rubio, C.P.; Tvarijonaviciute, A. Serum Ferritin in Obese Dogs: Changes and Comparison with Other Analytes. Vet. Sci. 2023, 10, 457. https://doi.org/10.3390/vetsci10070457
Franco-Martínez L, Pardo-Marín L, Sánchez-Mateos L, Muñoz-Prieto A, García-Martínez JD, Cerón JJ, Martínez-Subiela S, Rubio CP, Tvarijonaviciute A. Serum Ferritin in Obese Dogs: Changes and Comparison with Other Analytes. Veterinary Sciences. 2023; 10(7):457. https://doi.org/10.3390/vetsci10070457
Chicago/Turabian StyleFranco-Martínez, Lorena, Luis Pardo-Marín, Laura Sánchez-Mateos, Alberto Muñoz-Prieto, Juan Diego García-Martínez, José J. Cerón, Silvia Martínez-Subiela, Camila P. Rubio, and Asta Tvarijonaviciute. 2023. "Serum Ferritin in Obese Dogs: Changes and Comparison with Other Analytes" Veterinary Sciences 10, no. 7: 457. https://doi.org/10.3390/vetsci10070457
APA StyleFranco-Martínez, L., Pardo-Marín, L., Sánchez-Mateos, L., Muñoz-Prieto, A., García-Martínez, J. D., Cerón, J. J., Martínez-Subiela, S., Rubio, C. P., & Tvarijonaviciute, A. (2023). Serum Ferritin in Obese Dogs: Changes and Comparison with Other Analytes. Veterinary Sciences, 10(7), 457. https://doi.org/10.3390/vetsci10070457










