An Exploratory Study on Spoilage Bacteria and Listeria monocytogenes in Fresh Salmon: Extending Shelf-Life Using Vacuum and Seasonings as Natural Preservatives
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Salmon Fillets
2.2. Microbiological Analysis
2.2.1. Microbiota Dynamics in Salmon Fillets during Storage
2.2.2. Co-Growth of Listeria monocytogenes with Salmon Fillet Microbiota
2.3. In Vitro Antimicrobial Effect of Natural Preservatives (Seasonings) and Mixtures
2.3.1. Susceptibility Testing
2.3.2. Inhibitory Potential of Mixtures of Natural Preservatives (Seasonings)
2.4. Comparative Growth Rate between Commercial Culture Medium and Food Matrix Media
2.5. Statistical Analysis
3. Results
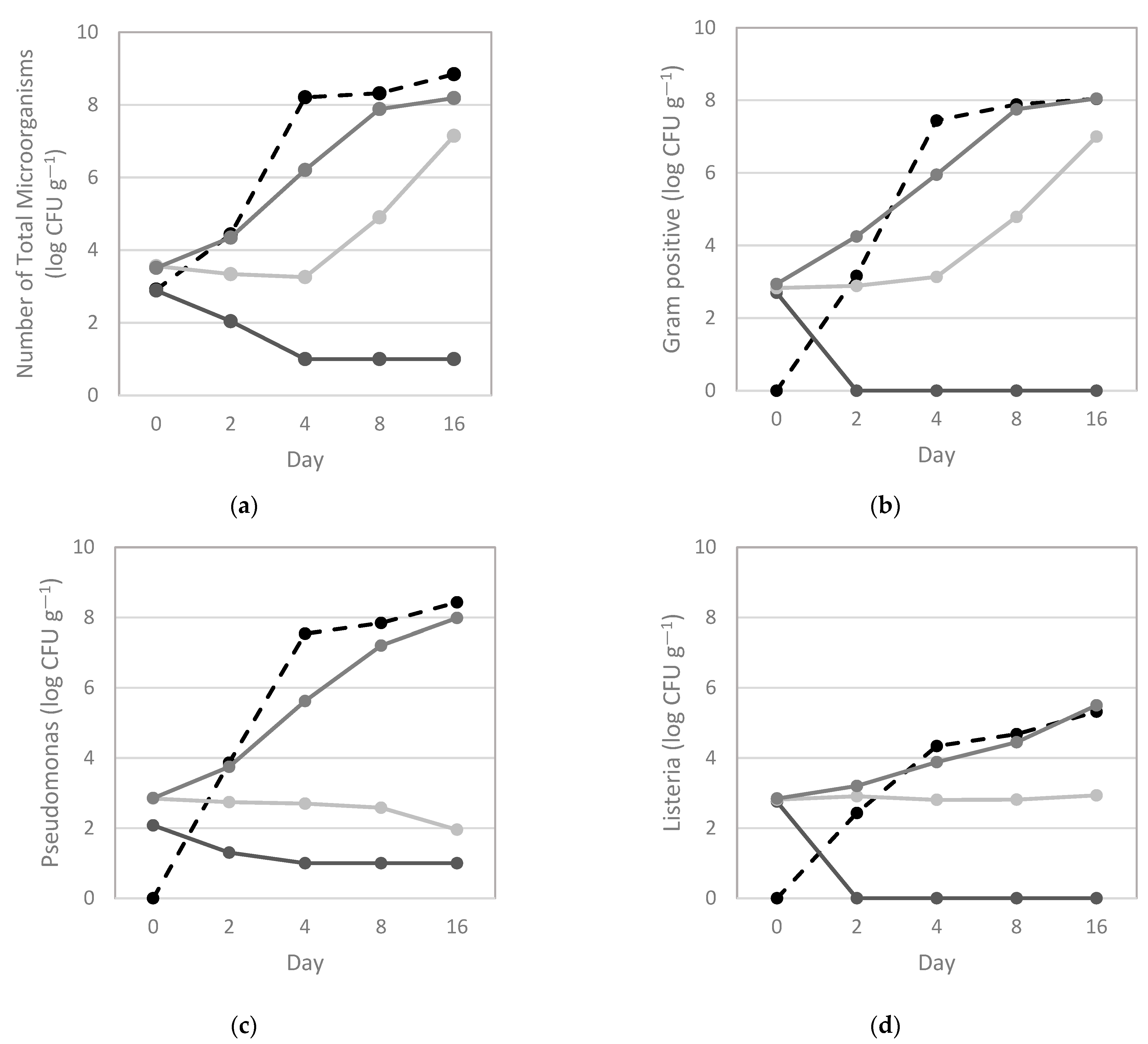
3.1. Characterization of Bacterial Dynamics in Salmon during Storage
3.2. In Vitro Antimicrobial Effect of Different Natural Preservatives (Seasonings) and Mixtures
3.3. Comparative Growth Rate between Commercial and Salmon Media
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Isolate | Bacterial Species | Isolate | Bacterial Species |
|---|---|---|---|
| E2M1P/V2 | Arthrobacter alpinus | E2M3/A1 | Pseudomonas fragi |
| E1M2P/V6 | Brochothrix thermosphacta | E2M5/A1 | Pseudomonas fragi |
| M5AN2 | Brochothrix thermosphacta | E2M5P/A1 | Pseudomonas fragi |
| E2M2/A4 | Brochothrix thermosphacta | E2M1P/V4 | Pseudomonas fragi |
| E2M1/V2 | Buttiauxella gaviniae | E1M1/A1 | Pseudomonas gessardi |
| E1M1/V2 | Carnobacterium maltaromaticum | E1M1/A4 | Pseudomonas gessardi |
| E1M2/V1 | Carnobacterium maltaromaticum | E1M1P/A2 | Pseudomonas gessardi |
| E1M2/V4 | Carnobacterium maltaromaticum | E1M2/A1 | Pseudomonas gessardi |
| E1M4A/V2 | Carnobacterium maltaromaticum | E1M1/V4 | Pseudomonas jessinii |
| M5VA2 | Carnobacterium maltaromaticum | E1M3/V1 | Pseudomonas jessinii |
| M5AN3 | Carnobacterium maltaromaticum | E2M4/A3 | Pseudomonas poae/P. trivialis |
| E2M1/V1 | Carnobacterium maltaromaticum | E1M2/A2 | Pseudomonas psychrophila |
| E2M2/V1 | Carnobacterium maltaromaticum | E1M3P/A2 | Pseudomonas psychrophila |
| E2M2/V3 | Carnobacterium maltaromaticum | E2M2/A1 | Pseudomonas psychrophila |
| E2M2P/V1 | Carnobacterium maltaromaticum | E2M2P/A1 | Pseudomonas versuta/P. fragi |
| E2M3/V2 | Carnobacterium maltaromaticum | E1M4/V3 | Pseudomonas weihenstephanensis |
| E2M5/V3 | Carnobacterium maltaromaticum | E1M3P/V3 | Psychrobacter cibarius |
| E2M6/V1 | Carnobacterium maltaromaticum | E2M4P/A2 | Psychrobacter cibarius |
| E1M1/V1 | Escherichia coli | E2M1P/A1 | Psychrobacter cibarius |
| E2M4/V1 | Moraxella osloensis | E2M4P/V3 | Psychrobacter maritimus |
| E2M3/V1 | Paenibacillus cineris/P. rhizosphaerae | E2M1/A5 | Psychrobacter nivimaris |
| E2M1/A4 | Pseudomonas antarctica | E2M5P/V2 | Psychrobacter sp. |
| E1M1P/A3 | Pseudomonas fragi | E1M1P/V2 | Shewanella baltica |
| E1M2P/A1 | Pseudomonas fragi | E1M1P/V3 | Shewanella baltica |
| M5VA4 | Pseudomonas fragi | M5AE1 | Shewanella putrefaciens |
| Mixture | OEO (%) | Garlic Powder (mg mL−1) | NaCl (%) | Lemon Juice (%) |
|---|---|---|---|---|
| Mixture 1 | 0.002 | 0.08 | 5.00 | 2.500 |
| Mixture 2 | 0.002 | 3.13 | 2.50 | 0.625 |
| Mixture 3 | 0.005 | 0.06 | 0.25 | 0.250 |
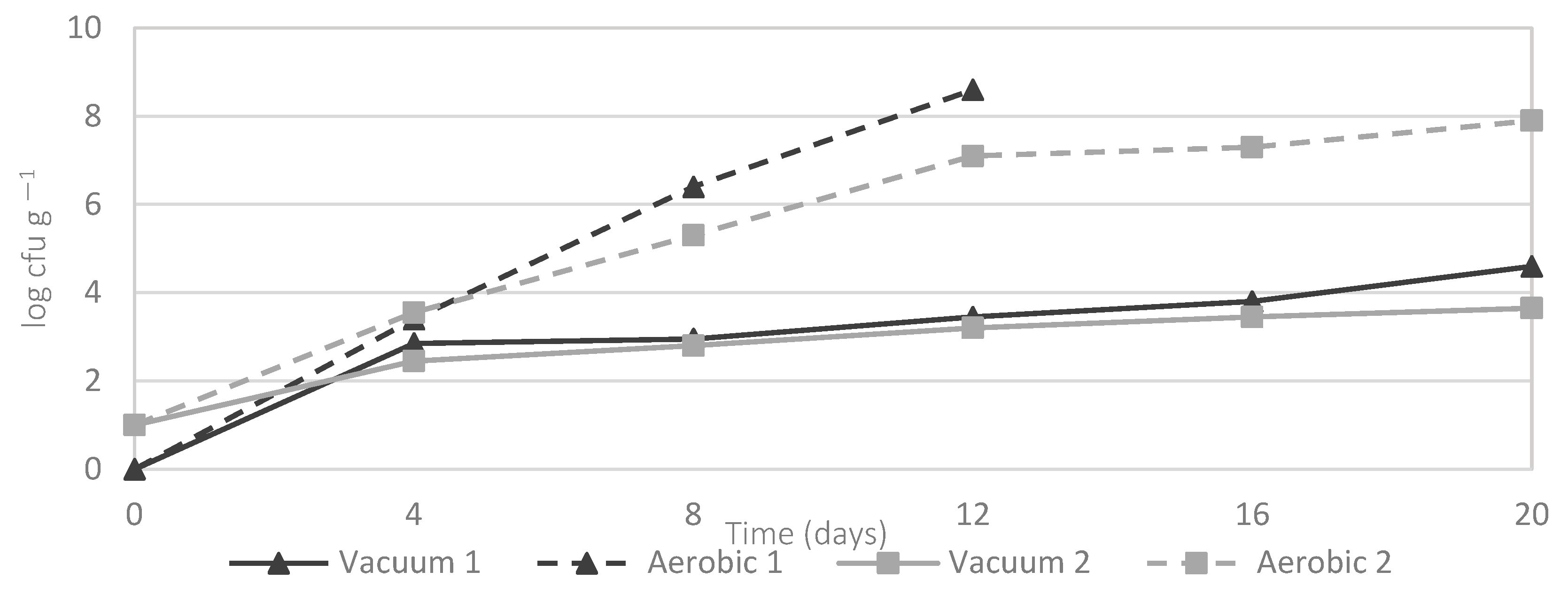
| Bacterial Species | Aerobic 1 | Vacuum 1 | Aerobic 2 | Vacuum 2 |
|---|---|---|---|---|
| Pseudomonas spp. | 8 | 4 | 7 | 1 |
| Psychrobacter spp. | 0 | 1 | 3 | 3 |
| Brochothrix thermosphacta | 0 | 2 | 0 | 1 |
| Carnobacterium maltaromaticum | 0 | 6 | 0 | 8 |
| Shewanella spp. | 0 | 3 | 0 | 0 |
| Escherichia coli | 0 | 1 | 0 | 0 |
| Buttiauxella gaviniae | 0 | 0 | 0 | 1 |
| Arthrobacter alpinus | 0 | 0 | 0 | 1 |
| Paenibacillus cineris | 0 | 0 | 0 | 1 |
| Moraxella spp. | 0 | 0 | 0 | 1 |
References
- European Commission. Facts and Figures on the Common Fisheries Policy. Available online: https://oceans-and-fisheries.ec.europa.eu/facts-and-figures/facts-and-figures-common-fisheries-policy/consumption_en#:~:text=Fisheriesandaquacultureproductsare,therestoftheworld (accessed on 9 May 2023).
- Hicks, D. Seafood Safety and Quality: The Consumer’s Role. Foods 2016, 5, 71. [Google Scholar] [CrossRef]
- FAO. Food Loss and Waste in Fish Value Chains. Available online: https://www.fao.org/flw-in-fish-value-chains/resources/articles/the-importance-of-markets-in-reducing-food-loss-and-waste-flw-in-the-aquatic-food-value-chains/en/ (accessed on 9 May 2023).
- Nie, X.; Zhang, R.; Cheng, L.; Zhu, W.; Li, S.; Chen, X. Mechanisms underlying the deterioration of fish quality after harvest and methods of preservation. Food Control 2022, 135, 108805. [Google Scholar] [CrossRef]
- Hassoun, A.; Emir Çoban, Ö. Essential oils for antimicrobial and antioxidant applications in fish and other seafood products. Trends Food Sci. Technol. 2017, 68, 26–36. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations Food Wastage Footprint. Impacts on Natural Resources. Available online: https://www.fao.org/3/i3347e/i3347e.pdf (accessed on 8 May 2023).
- Davidson, P.M.; Cekmer, H.B.; Monu, E.A.; Techathuvanan, C. The use of natural antimicrobials in food. In Handbook of Natural Antimicrobials for Food Safety and Quality; Elsevier: Philadelphia, PA, USA, 2015; pp. 1–27. [Google Scholar]
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food Safety through Natural Antimicrobials. Antibiotics 2019, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Bahurmiz, O.M.; Ahmad, R.; Ismail, N.; Adzitey, F.; Sulaiman, S.-F. Antimicrobial Activity of Selected Essential Oils on Pseudomonas Species Associated with Spoilage of Fish with Emphasis on Cinnamon Essential Oil. J. Aquat. Food Prod. Technol. 2020, 29, 789–800. [Google Scholar] [CrossRef]
- Erkmen, O.; Bozoglu, F.T. Food Preservation by Combination of Techniques (Hurdle Technology). In Food Microbiology: Principles into Practice; Wiley: Hoboken, NJ, USA, 2016; pp. 166–180. [Google Scholar]
- Giacometti, F.; Shirzad-Aski, H.; Ferreira, S. Antimicrobials and Food-Related Stresses as Selective Factors for Antibiotic Resistance along the Farm to Fork Continuum. Antibiotics 2021, 10, 671. [Google Scholar] [CrossRef]
- Noseda, B.; Vermeulen, A.; Ragaert, P.; Devlieghere, F. Packaging of Fish and Fishery Products. In Seafood Processing; John Wiley & Sons, Ltd: Chichester, UK, 2013; pp. 237–261. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Bouhdid, S.; Abrini, J.; Zhiri, A.; Espuny, M.J.; Manresa, A. Investigation of functional and morphological changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Origanum compactum essential oil. J. Appl. Microbiol. 2009, 106, 1558–1568. [Google Scholar] [CrossRef]
- Pedrós-Garrido, S.; Clemente, I.; Calanche, J.B.; Condón-Abanto, S.; Beltrán, J.A.; Lyng, J.G.; Brunton, N.; Bolton, D.; Whyte, P. Antimicrobial activity of natural compounds against listeria spp. and their effects on sensory attributes in salmon (Salmo salar) and cod (Gadus morhua). Food Control 2020, 107, 106768. [Google Scholar]
- Hadjicharalambous, C.; Grispoldi, L.; Goga, B.C. Quantitative risk assessment of Listeria monocytogenes in a traditional RTE product. EFSA J. 2019, 17, e170906. [Google Scholar]
- European Centre for Disease Prevention and Control and European Food Safety Authority. Multi-country outbreak of Listeria monocytogenes clonal complex 8 infections linked to consumption of cold-smoked fish products. EFSA Support. Publ. 2019, 16, 1665E. [Google Scholar]
- Møretrø, T.; Moen, B.; Heir, E.; Hansen, A.Å.; Langsrud, S. Contamination of salmon fillets and processing plants with spoilage bacteria. Int. J. Food Microbiol. 2016, 237, 98–108. [Google Scholar] [CrossRef]
- Pavan, M.E.; Franco, R.J.; Rodriguez, J.M.; Gadaleta, P.; Abbott, S.L.; Janda, J.M.; Zorzópulos, J. Phylogenetic relationships of the genus Kluyvera: Transfer of Enterobacter intermedius Izard et al. 1980 to the genus Kluyvera as Kluyvera intermedia comb. nov. and reclassification of Kluyvera cochleae as a later synonym of K. intermedia. Int. J. Syst. Evol. Microbiol. 2005, 55, 437–442. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Ramires, T.; Kleinubing, N.R.; Iglesias, M.A.; Vitola, H.R.S.; Núncio, A.S.P.; Kroning, I.S.; Moreira, G.M.S.G.; Fiorentini, Â.M.; da Silva, W.P. Genetic diversity, biofilm and virulence characteristics of Listeria monocytogenes in salmon sushi. Food Res. Int. 2021, 140, 109871. [Google Scholar] [CrossRef]
- Bergis, H.; Bonanno, L.; Asséré, A.; Lombard, B. Eurl Lm Technical Guidance Document on Challenge Tests and Durability Studies for Assessing Shelf-Life of Ready-to-Eat Foods Related to Listeria monocytogenes. Available online: https://food.ec.europa.eu/system/files/2021-07/biosafety_fh_mc_tech-guide-doc_listeria-in-rte-foods_en_0.pdf (accessed on 23 May 2023).
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Liu, Y.; Wang, X.; Liu, B.; Yuan, S.; Qin, X.; Dong, Q. Microrisk Lab: An Online Freeware for Predictive Microbiology. Foodborne Pathog. Dis. 2021, 18, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Dolea, D.; Rizo, A.; Fuentes, A.; Barat, J.; Fernández-Segovia, I. Effect of thyme and oregano essential oils on the shelf life of salmon and seaweed burgers. Food Sci. Technol. Int. 2018, 24, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.B. Micro-Organisms in Foods 2: Sampling for Microbiological Analysis; Principles and Specific Applications. Technometrics 1977, 19, 221. [Google Scholar] [CrossRef]
- Fidalgo, L.G.; Pinto, C.A.; Delgadillo, I.; Saraiva, J.A. Hyperbaric Storage of Vacuum-Packaged Fresh Atlantic Salmon (Salmo salar) Loins by Evaluation of Spoilage Microbiota and Inoculated Surrogate-Pathogenic Microorganisms. Food Eng. Rev. 2021, 13, 651–659. [Google Scholar] [CrossRef]
- Amanatidou, A. Effect of combined application of high pressure treatment and modified atmospheres on the shelf life of fresh Atlantic salmon. Innov. Food Sci. Emerg. Technol. 2000, 1, 87–98. [Google Scholar] [CrossRef]
- Macé, S.; Cornet, J.; Chevalier, F.; Cardinal, M.; Pilet, M.-F.; Dousset, X.; Joffraud, J.-J. Characterisation of the spoilage microbiota in raw salmon (Salmo salar) steaks stored under vacuum or modified atmosphere packaging combining conventional methods and PCR–TTGE. Food Microbiol. 2012, 30, 164–172. [Google Scholar] [CrossRef]
- Leisner, J.J.; Laursen, B.G.; Prévost, H.; Drider, D.; Dalgaard, P. Carnobacterium: Positive and negative effects in the environment and in foods. FEMS Microbiol. Rev. 2007, 31, 592–613. [Google Scholar] [CrossRef] [PubMed]
- Duffes, F.; Leroi, F.; Boyaval, P.; Dousset, X. Inhibition of Listeria monocytogenes by Carnobacterium spp. strains in a simulated cold smoked fish system stored at 4 °C. Int. J. Food Microbiol. 1999, 47, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Brillet, A.; Pilet, M.F.; Prevost, H.; Drider, D. Evidence on inhibition of Listeria monocytogenes by divercin V41 action. Lett. Appl. Microbiol. 2003, 36, 288–292. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, F.B.; de Souza, V.M.; Thomaz, M.R.S.; Fernandes, L.P.; de Oliveira, W.P.; De Martinis, E.C.P. Use of Carnobacterium maltaromaticum cultures and hydroalcoholic extract of Lippia sidoides Cham. against Listeria monocytogenes in fish model systems. Int. J. Food Microbiol. 2011, 146, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. Proposal to Enable the Use of a New Food Additive, Carnobacterium divergens M35, as an Antimicrobial Preservative in Sliced Ready-to-Eat Cold-Smoked Salmon and Trout. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/public-involvement-partnerships/proposal-use-new-food-additive-carnobacterium-divergens-antimicrobial-preservative-sliced-ready-cold-smoked-salmon-sliced/consultation.html (accessed on 9 May 2023).
- Brillet, A.; Pilet, M.-F.; Prevost, H.; Cardinal, M.; Leroi, F. Effect of inoculation of Carnobacterium divergens V41, a biopreservative strain against Listeria monocytogenes risk, on the microbiological, chemical and sensory quality of cold-smoked salmon. Int. J. Food Microbiol. 2005, 104, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Aymerich, T.; Rodríguez, M.; Garriga, M.; Bover-Cid, S. Assessment of the bioprotective potential of lactic acid bacteria against Listeria monocytogenes on vacuum-packed cold-smoked salmon stored at 8 °C. Food Microbiol. 2019, 83, 64–70. [Google Scholar] [CrossRef]
- Zilelidou, E.A.; Skandamis, P.N. Growth, detection and virulence of Listeria monocytogenes in the presence of other microorganisms: Microbial interactions from species to strain level. Int. J. Food Microbiol. 2018, 277, 10–25. [Google Scholar] [CrossRef]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; Nørrung, B.; et al. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, e05134. [Google Scholar]
- Paparella, A.; Taccogna, L.; Aguzzi, I.; Chaves-López, C.; Serio, A.; Marsilio, F.; Suzzi, G. Flow cytometric assessment of the antimicrobial activity of essential oils against Listeria monocytogenes. Food Control 2008, 19, 1174–1182. [Google Scholar] [CrossRef]
- Ibrahim, F.; Vesterlund, S. Lactococcus lactis ssp. lactis as Protective Culture in Vacuum-Packed Raw Salmon (Salmo salar). J. Aquat. Food Prod. Technol. 2014, 23, 601–607. [Google Scholar] [CrossRef]
- Brunner, S.R.; Varga, J.F.A.; Dixon, B. Antimicrobial Peptides of Salmonid Fish: From Form to Function. Biology 2020, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Lakshmanaperumalsamy, P.; Chandramohan, D. Fish flesh agar medium—A suitable experimental medium for the detection of spoilage bacteria. Antonie Van Leeuwenhoek 1985, 51, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, A.J.; Chajęcka-Wierzchowska, W.; Zadernowska, A. Ceviche-Natural Preservative: Possibility of Microbiota Survival and Effect on L. monocytogenes. Foods 2022, 11, 860. [Google Scholar] [CrossRef]
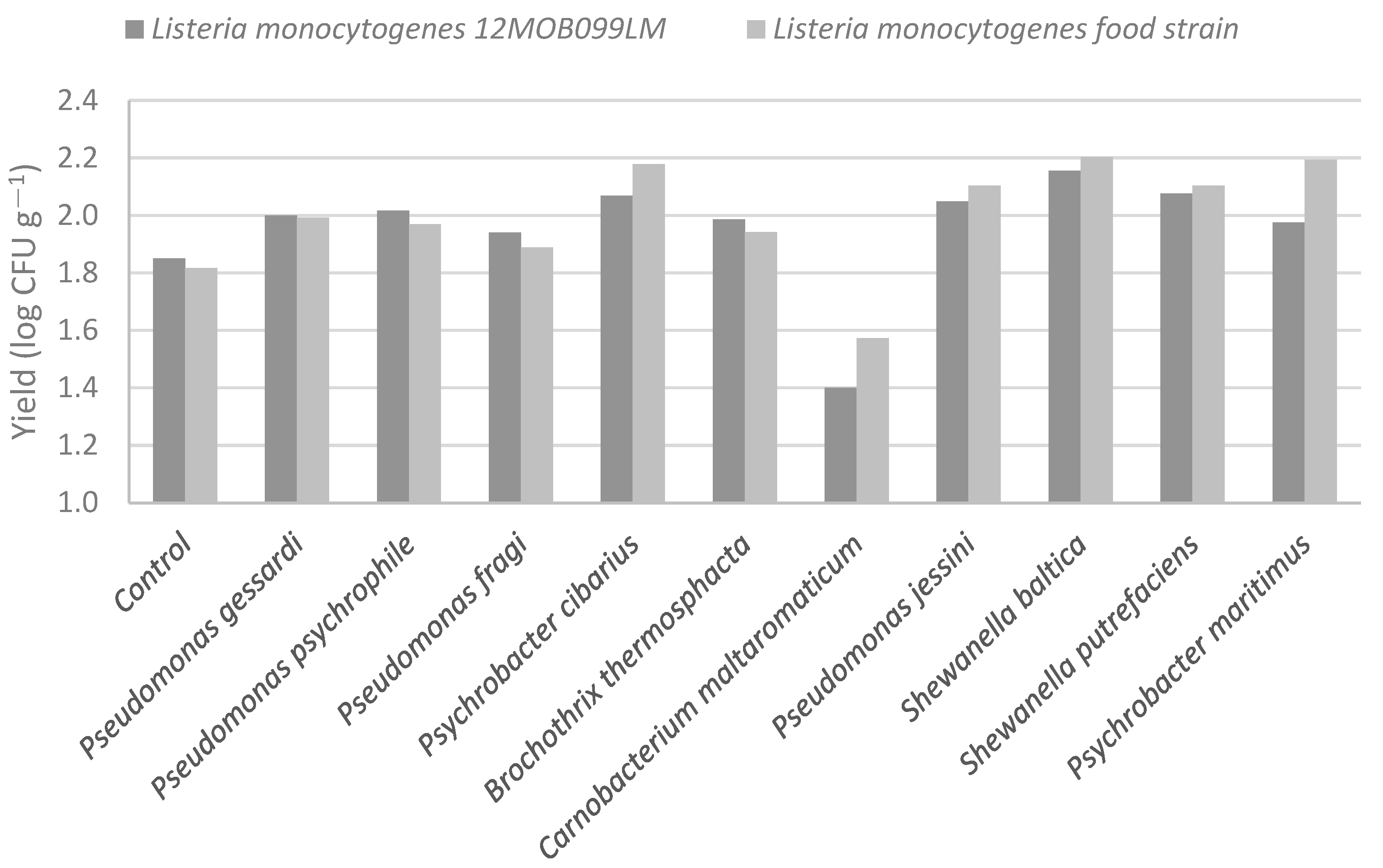
| Bacterial Species | NaCl (%) | Oregano Essential Oil (%) | Lemon Juice (%) | Garlic Powder (mg mL−1) | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Pseudomonas gessardi | 5 | 10 | 0.03 | >1 | 2.5 | 2.5 | 12.5 | 25 |
| Pseudomonas psychrophile | 5 | 10 | 0.03 | 0.5 | 2.5 | 5 | 12.5 | 12.5 |
| Pseudomonas fragi | 5 | 10 | 0.03 | >1 | 2.5 | 5 | 6.25 | 12.5 |
| Psychrobacter cibarius | 10 | 10 | 0.004 | 0.004 | 0.625 | 1.25 | 3.13 | 12.5 |
| Brochothrix thermosphacta | 10 | 10 | 0.016 | 0.03 | 2.5 | 2.5 | 3.13 | 3.13 |
| Carnobacterium maltaromaticum | 10 | 10 | 0.016 | 0.3 | 2.5 | 2.5 | 12.5 | 3.13 |
| Pseudomonas jessini | 5 | 10 | 0.016 | >1 | 2.5 | 2.5 | 12.5 | 25 |
| Shewanella baltica | 5 | >10 | 0.004 | 0.008 | 1.25 | 2.5 | 6.25 | 12.5 |
| Shewanella putrefaciens | 5 | >10 | 0.004 | 0.008 | 1.25 | 2.5 | 6.25 | 12.5 |
| Psychrobacter maritimus | 5 | >10 | 0.004 | 0.016 | 1.25 | 2.5 | 6.25 | 12.5 |
| Listeria monocytogenes 12MOB099LM | 10 | >10 | 0.13 | 0.13 | 5 | 10 | 12.5 | 25 |
| Listeria monocytogenes (food strain) | >10 | >10 | 0.06 | 0.25 | 2.5 | 10 | 12.5 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemos, M.-L.; Prata, J.C.; Rodrigues, I.C.; Martins-Costa, S.; Archer, B.; Machado, J.; Dilão, R.; Vaz-Pires, P.; Martins da Costa, P. An Exploratory Study on Spoilage Bacteria and Listeria monocytogenes in Fresh Salmon: Extending Shelf-Life Using Vacuum and Seasonings as Natural Preservatives. Vet. Sci. 2023, 10, 423. https://doi.org/10.3390/vetsci10070423
Lemos M-L, Prata JC, Rodrigues IC, Martins-Costa S, Archer B, Machado J, Dilão R, Vaz-Pires P, Martins da Costa P. An Exploratory Study on Spoilage Bacteria and Listeria monocytogenes in Fresh Salmon: Extending Shelf-Life Using Vacuum and Seasonings as Natural Preservatives. Veterinary Sciences. 2023; 10(7):423. https://doi.org/10.3390/vetsci10070423
Chicago/Turabian StyleLemos, Maria-Leonor, Joana C. Prata, Inês C. Rodrigues, Sofia Martins-Costa, Bernardo Archer, Jorge Machado, Rui Dilão, Paulo Vaz-Pires, and Paulo Martins da Costa. 2023. "An Exploratory Study on Spoilage Bacteria and Listeria monocytogenes in Fresh Salmon: Extending Shelf-Life Using Vacuum and Seasonings as Natural Preservatives" Veterinary Sciences 10, no. 7: 423. https://doi.org/10.3390/vetsci10070423
APA StyleLemos, M.-L., Prata, J. C., Rodrigues, I. C., Martins-Costa, S., Archer, B., Machado, J., Dilão, R., Vaz-Pires, P., & Martins da Costa, P. (2023). An Exploratory Study on Spoilage Bacteria and Listeria monocytogenes in Fresh Salmon: Extending Shelf-Life Using Vacuum and Seasonings as Natural Preservatives. Veterinary Sciences, 10(7), 423. https://doi.org/10.3390/vetsci10070423









