Changes of Enterocyte Morphology and Enterocyte: Goblet Cell Ratios in Dogs with Protein-Losing and Non-Protein-Losing Chronic Enteropathies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dogs Inclusion
2.2. Morphometrical Study of the Enterocytes and Goblet Cell-to-Enterocyte Ratio
2.3. Laboratory and Clinical Data
2.4. Statistical Analysis
3. Results
3.1. Animal Charactheristics
3.2. Height and Width of the Enterocytes, and Goblet Cell-to-Enterocyte Ratio
3.3. Clinical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allenspach, K.; Wieland, B.; Gröne, A.; Gaschen, F. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 2007, 21, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Washabau, R.J.; Day, M.J.; Willard, M.D.; Hall, E.J.; Jergens, A.E.; Mansell, J.; Minami, T.; Bilzer, T.W.; Group, W.I.G.S. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J. Vet. Intern. Med. 2010, 24, 10–26. [Google Scholar] [PubMed]
- Dandrieux, J. Inflammatory bowel disease versus chronic enteropathy in dogs: Are they one and the same? J. Small Anim. Pract. 2016, 57, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.E.; Heilmann, R.M. Canine chronic enteropathy—Current state-of-the-art and emerging concepts. Front. Vet. Sci. 2022, 9, 923013. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, K.; Culverwell, C.; Chan, D. Long-term outcome in dogs with chronic enteropathies: 203 cases. Vet. Rec. 2016, 178, 368. [Google Scholar] [CrossRef]
- Allenspach, K.; Iennarella-Servantez, C. Canine protein losing enteropathies and systemic complications. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 111–122. [Google Scholar] [CrossRef]
- Craven, M.D.; Washabau, R.J. Comparative pathophysiology and management of protein-losing enteropathy. J. Vet. Intern. Med. 2019, 33, 383–402. [Google Scholar] [CrossRef]
- Dossin, O.; Lavoué, R. Protein-losing enteropathies in dogs. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 399–418. [Google Scholar] [CrossRef]
- Day, M.; Bilzer, T.; Mansell, J.; Wilcock, B.; Hall, E.; Jergens, A.; Minami, T.; Willard, M.; Washabau, R. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J. Comp. Pathol. 2008, 138, S1–S43. [Google Scholar] [CrossRef]
- Wennogle, S.; Priestnall, S.; Webb, C. Histopathologic characteristics of intestinal biopsy samples from dogs with chronic inflammatory enteropathy with and without hypoalbuminemia. J. Vet. Intern. Med. 2017, 31, 371–376. [Google Scholar] [CrossRef]
- Allenspach, K.A.; Mochel, J.P.; Du, Y.; Priestnall, S.L.; Moore, F.; Slayter, M.; Rodrigues, A.; Ackermann, M.; Krockenberger, M.; Mansell, J. Correlating gastrointestinal histopathologic changes to clinical disease activity in dogs with idiopathic inflammatory bowel disease. Vet. Pathol. 2019, 56, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.; Evans, R.; Ackermann, M.; Hostetter, J.; Willard, M.; Mansell, J.; Bilzer, T.; Wilcock, B.; Washabau, R.; Hall, E. Design of a simplified histopathologic model for gastrointestinal inflammation in dogs. Vet. Pathol. 2014, 51, 946–950. [Google Scholar] [CrossRef]
- Casamian-Sorrosal, D.; Willard, M.; Murray, J.; Hall, E.; Taylor, S.; Day, M. Comparison of histopathologic findings in biopsies from the duodenum and ileum of dogs with enteropathy. J. Vet. Intern. Med. 2010, 24, 80–83. [Google Scholar] [CrossRef]
- García-Sancho, M.; Sainz, Á.; Villaescusa, A.; Rodríguez, A.; Rodríguez-Franco, F. White spots on the mucosal surface of the duodenum in dogs with lymphocytic plasmacytic enteritis. J. Vet. Sci. 2011, 12, 165–169. [Google Scholar] [CrossRef]
- Pérez-Merino, E.M.; Cristóbal-Verdejo, I.; Duque-Carrasco, F.J.; Espadas-González, L.; Pastor-Sirvent, N.; Usón-Casaús, J.M. Relationship between serum cobalamin concentration and endoscopic ileal appearance and histology in dogs with chronic inflammatory enteropathy. J. Vet. Intern. Med. 2022, 36, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Cerquetella, M.; Antonelli, E.; Pengo, G.; Magi, G.E.; Villanacci, V.; Rostami-Nejad, M.; Spaterna, A.; Bassotti, G. The importance of histologic parameters of lacteal involvement in cases of canine lymphoplasmacytic enteritis. Gastroenterol. Hepatol. Bed. Bench. 2015, 8, 33. [Google Scholar] [PubMed]
- Jergens, A.E.; Schreiner, C.A.; Frank, D.E.; Niyo, Y.; Ahrens, F.E.; Eckersall, P.; Benson, T.J.; Evans, R. A scoring index for disease activity in canine inflammatory bowel disease. J. Vet. Intern. Med. 2003, 17, 291–297. [Google Scholar] [CrossRef]
- Bao, F.; Green, P.H.; Bhagat, G. An update on celiac disease histopathology and the road ahead. Arch. Pathol. Lab. Med. 2012, 136, 735–745. [Google Scholar] [CrossRef]
- Abtahi, S.; Sailer, A.; Roland, J.T.; Haest, X.; Chanez-Paredes, S.D.; Ahmad, K.; Sadiq, K.; Iqbal, N.T.; Ali, S.A.; Turner, J.R. Intestinal Epithelial Digestive, Transport, and Barrier Protein Expression Is Increased in Environmental Enteric Dysfunction. Lab. Investig. 2023, 103, 100036. [Google Scholar] [CrossRef]
- Capuano, M.; Iaffaldano, L.; Tinto, N.; Montanaro, D.; Capobianco, V.; Izzo, V.; Tucci, F.; Troncone, G.; Greco, L.; Sacchetti, L. MicroRNA-449a overexpression, reduced NOTCH1 signals and scarce goblet cells characterize the small intestine of celiac patients. PLoS ONE 2011, 6, e29094. [Google Scholar] [CrossRef]
- Harvey, N.D. How old is my dog? Identification of rational age groupings in pet dogs based upon normative age-linked processes. Front. Vet. Sci. 2021, 8, 643085. [Google Scholar] [CrossRef] [PubMed]
- Meyerholz, D.K.; Beck, A.P. Principles and approaches for reproducible scoring of tissue stains in research. Lab. Investig. 2018, 98, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Stricker, S.; Müller, M.; Zimmer, K.-P.; Jacob, R. Altered Posttranslational Modification of Microtubules Contributes to Disturbed Enterocyte Morphology in Celiac Disease. Int. J. Mol. Sci. 2023, 24, 2635. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.N.; Heal, C.J. Evolutionary developments in interpreting the gluten-induced mucosal celiac lesion: An Archimedian heuristic. Nutrients 2017, 9, 213. [Google Scholar] [CrossRef]
- Rostami, K.; Ensari, A.; Marsh, M.N.; Srivastava, A.; Villanacci, V.; Carroccio, A.; Asadzadeh Aghdaei, H.; Bai, J.C.; Bassotti, G.; Becheanu, G. Gluten induces subtle histological changes in duodenal mucosa of patients with non-coeliac gluten sensitivity: A multicentre study. Nutrients 2022, 14, 2487. [Google Scholar] [CrossRef] [PubMed]
- Sbarbati, A.; Valletta, E.; Bertini, M.; Cipolli, M.; Morroni, M.; Pinelli, L.; Tatò, L. Gluten sensitivity and ‘normal’ histology: Is the intestinal mucosa really normal? Dig. Liver Dis. 2003, 35, 768–773. [Google Scholar] [CrossRef]
- Martucci, S.; Fraser, J.S.; Biagi, F.; Corazza, G.R.; Ciclitira, P.J.; Ellis, H.J. Characterizing one of the DQ2 candidate epitopes in coeliac disease: A-gliadin 51–70 toxicity assessed using an organ culture system. Eur. J. Gastroenterol. Hepatol. 2003, 15, 1293–1298. [Google Scholar] [CrossRef]
- Shidrawi, R.; Day, P.; Przemioslo, R.; Ellis, H.; Nelufer, J.; Ciclitira, P. In vitro toxicity of gluten peptides in coeliac disease assessed by organ culture. Scand. J. Gastroenterol. 1995, 30, 758–763. [Google Scholar] [CrossRef]
- Volkmann, M.; Steiner, J.; Fosgate, G.T.; Zentek, J.; Hartmann, S.; Kohn, B. Chronic diarrhea in dogs–retrospective study in 136 cases. J. Vet. Intern. Med. 2017, 31, 1043–1055. [Google Scholar] [CrossRef]
- Walker, D.; Knuchel-Takano, A.; McCutchan, A.; Chang, Y.M.; Downes, C.; Miller, S.; Stevens, K.; Verheyen, K.; Phillips, A.; Miah, S. A comprehensive pathological survey of duodenal biopsies from dogs with diet-responsive chronic enteropathy. J. Vet. Intern. Med. 2013, 27, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Pengo, G.; Caldin, M.; Piccionello, A.P.; Steiner, J.M.; Cohen, N.D.; Jergens, A.E.; Suchodolski, J.S. Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL# 3 strains in dogs with idiopathic inflammatory bowel disease. PLoS ONE 2014, 9, e94699. [Google Scholar]
- Zeissig, S.; Bürgel, N.; Günzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007, 56, 61–72. [Google Scholar] [CrossRef]
- Ridyard, A.E.; Brown, J.K.; Rhind, S.M.; Else, R.W.; Simpson, J.W.; Miller, H.R. Apical junction complex protein expression in the canine colon: Differential expression of claudin-2 in the colonic mucosa in dogs with idiopathic colitis. J. Histochem. Cytochem. 2007, 55, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- German, A.; Hall, E.; Day, M. Analysis of leucocyte subsets in the canine intestine. J. Comp. Pathol. 1999, 120, 129–145. [Google Scholar] [CrossRef] [PubMed]
- German, A.; Hall, E.; Kelly, D.; Watson, A.; Day, M. An immunohistochemical study of histiocytic ulcerative colitis in boxer dogs. J. Comp. Pathol. 2000, 122, 163–175. [Google Scholar] [CrossRef]
- Crabtree, J.; Heatley, R.; Losowsky, M. Glycoprotein synthesis and secretion by cultured small intestinal mucosa in coeliac disease. Gut 1989, 30, 1339–1343. [Google Scholar] [CrossRef]
- Jones, C.; Jablonski, S.A.; Petroff, B.K.; Langlois, D.K. Relationship between serum magnesium, calcium, and parathyroid concentrations in dogs with abnormally low serum 25-hydroxyvitamin D concentration and chronic or protein-losing enteropathy. J. Vet. Intern. Med. 2023, 37, 101–109. [Google Scholar] [CrossRef]
- Goodwin, L.; Goggs, R.; Chan, D.; Allenspach, K. Hypercoagulability in dogs with protein-losing enteropathy. J. Vet. Intern. Med. 2011, 25, 273–277. [Google Scholar] [CrossRef]
- Batt, R.; Hall, E.; McLean, L.; Simpson, K. Small intestinal bacterial overgrowth and enhanced intestinal permeability in healthy beagles. Am. J. Vet. Res. 1992, 53, 1935–1940. [Google Scholar]
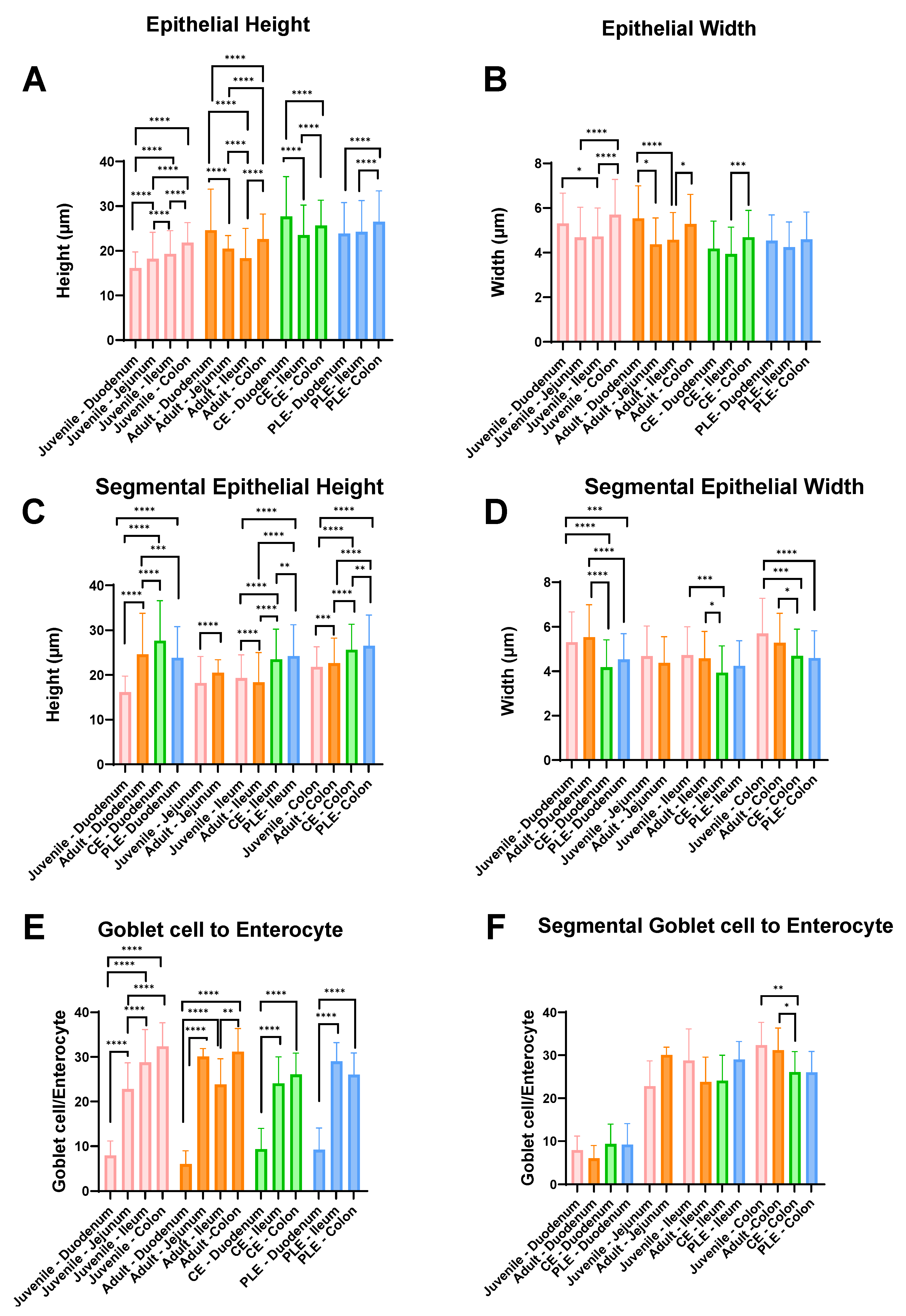
| Groups | Healthy Dogs (Mean ± SD) | CE Dogs (Mean ± SD) | ||||||
|---|---|---|---|---|---|---|---|---|
| Juvenile | Adult | CE | PLE | |||||
| Length (µm) | Height | Width | Height | Width | Height | Width | Height | Width |
| Duodenum | 16.2 ± 3.6 | 5.3 ± 1.4 | 24.6 ± 9.2 | 5.5 ± 1.5 | 27.7 ± 8.9 | 4.2 ± 1.2 | 23.9 ± 7.0 | 4.5 ± 1.2 |
| Jejunum | 18.2 ± 5.9 | 4.7 ± 1.4 | 20.5 ± 2.9 | 4.4 ± 1.2 | NA | NA | NA | NA |
| Ileum | 19.3 ± 5.2 | 4.7 ± 1.3 | 18.3 ± 6.7 | 4.6 ± 1.2 | 23.5 ± 6.7 | 3.9 ± 1.2 | 24.3 ± 7.0 | 4.2 ± 1.1 |
| Colon | 21.8 ± 4.5 | 5.7 ± 1.6 | 22.6 ± 5.6 | 5.3 ± 1.3 | 25.7 ± 5.7 | 4.7 ± 1.2 | 26.5 ± 6.9 | 4.6 ± 1.2 |
| GC to E (%) | Juvenile | Adult | CE | PLE | ||||
| Duodenum | 7.9 ± 3.3 | 6.0 ± 3.0 | 9.4 ± 4.6 | 9.2 ± 4.9 | ||||
| Jejunum | 22.8 ± 5.9 | 30.1 ± 1.8 | NA | NA | ||||
| Ileum | 28.8 ± 7.4 | 23.8 ± 5.7 | 24.1 ± 5.9 | 29.0 ± 4.2 | ||||
| Colon | 32.4 ± 5.3 | 31.2 ± 5.2 | 26.1 ± 4.8 | 26.0 ± 4.9 | ||||
| CCECAI vs. | r | Correlation | (+/−) | p-Value |
|---|---|---|---|---|
| Hematocrit (%) | −0.540 | Moderate | Negative | 0.0001 |
| Hgb (g/dL) | −0.499 | Moderate | Negative | 0.0008 |
| RBC (×106/µL) | −0.462 | Moderate | Negative | 0.0027 |
| Colonocyte mean width (µm) | −0.483 | Moderate | Negative | 0.0044 |
| Magnesium (mg/dL) | −0.434 | Moderate | Negative | 0.0023 |
| Calcium (mg/dL) | −0.410 | Moderate | Negative | 0.0034 |
| Albumin (g/dL) | −0.384 | Moderate | Negative | 0.0077 |
| BCS | −0.295 | Low | Negative | 0.0275 |
| CIBDAI score | 0.947 | High | Positive | <0.0001 |
| Parameters | CE (Mean ± SD) | PLE (Mean ± SD) | p-Value | q-Value |
|---|---|---|---|---|
| Cholesterol (mg/dL) | 197 ± 84.49 | 122.6 ± 49.22 | 0.001 | 0.020 |
| Albumin (g/dL) | 3.22 ± 0.52 | 1.47 ± 0.25 | <0.001 | <0.001 |
| Total protein (g/dL) | 5.99 ± 0.54 | 3.38 ± 0.59 | <0.001 | <0.001 |
| Calcium (g/dL) | 10.15 ± 0.88 | 7.85 ± 1.51 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Regañón, D.; Gabriel, V.; Livania, V.; Liu, D.; Ahmed, B.H.; Lincoln, A.; Wickham, H.; Ralston, A.; Merodio, M.M.; Sahoo, D.K.; et al. Changes of Enterocyte Morphology and Enterocyte: Goblet Cell Ratios in Dogs with Protein-Losing and Non-Protein-Losing Chronic Enteropathies. Vet. Sci. 2023, 10, 417. https://doi.org/10.3390/vetsci10070417
Díaz-Regañón D, Gabriel V, Livania V, Liu D, Ahmed BH, Lincoln A, Wickham H, Ralston A, Merodio MM, Sahoo DK, et al. Changes of Enterocyte Morphology and Enterocyte: Goblet Cell Ratios in Dogs with Protein-Losing and Non-Protein-Losing Chronic Enteropathies. Veterinary Sciences. 2023; 10(7):417. https://doi.org/10.3390/vetsci10070417
Chicago/Turabian StyleDíaz-Regañón, David, Vojtech Gabriel, Vanessa Livania, Dongjie Liu, Basant H. Ahmed, Addison Lincoln, Hannah Wickham, Abigail Ralston, Maria M. Merodio, Dipak K. Sahoo, and et al. 2023. "Changes of Enterocyte Morphology and Enterocyte: Goblet Cell Ratios in Dogs with Protein-Losing and Non-Protein-Losing Chronic Enteropathies" Veterinary Sciences 10, no. 7: 417. https://doi.org/10.3390/vetsci10070417
APA StyleDíaz-Regañón, D., Gabriel, V., Livania, V., Liu, D., Ahmed, B. H., Lincoln, A., Wickham, H., Ralston, A., Merodio, M. M., Sahoo, D. K., Zdyrski, C., Meyerholz, D. K., Mochel, J. P., & Allenspach, K. (2023). Changes of Enterocyte Morphology and Enterocyte: Goblet Cell Ratios in Dogs with Protein-Losing and Non-Protein-Losing Chronic Enteropathies. Veterinary Sciences, 10(7), 417. https://doi.org/10.3390/vetsci10070417












