Simple Summary
Practically and economically, predicting pregnancy status is crucial for food animal production. Recently, the expression of interferon-stimulated genes (ISGs) in peripheral blood leukocytes has been examined as a biomarker for predicting the gestational status during maternal recognition in ruminants. Although the ISG levels in the blood cells of cattle have been reported, the practical application of this detection method in embryo-transferred (ET) cows has not been evaluated in detail. The levels of three of the four ISGs in this study were significantly higher in pregnant cows than in non-pregnant cows on day 21 of gestation. The diagnostic performance results showed that the three ISGs are appropriate biomarkers for predicting the gestational status of ET cows. The statistical evaluation of diagnostic accuracy in ET cows indicated that the values of sensitivity, specificity, and accuracy were very high and the positive predictive values of the three ISGs were estimated. The results of this study suggest that ISGs are good biomarkers for gestational status during the peri-implantation period in ET cattle.
Abstract
Pregnancy diagnosis during early gestation is important for cattle reproduction. The expression of interferon-stimulated genes (ISGs) in peripheral blood leukocytes (PBLs) was studied in embryo-transferred (ET) Japanese Black cattle. ISGs in PBLs—ISG15, MX1, MX2, and OAS1—were detected in multiple ovulation ET cattle using a real-time quantitative polymerase chain reaction, and receiver operating characteristic (ROC) curve analysis was performed. Gestational status was predicted using the average ISG levels during the normal estrous cycle (AVE) and the Youden index from the ROC curve analysis as cutoff values. The ISG15, MX1, and MX2 levels were significantly higher in pregnant cattle (n = 10) than in non-pregnant cattle (n = 23) on gestation day 21, whereas the levels of all ISGs were similar between non-pregnant and non-pregnant cattle with late embryonic death (n = 7). ISG15, MX1, and MX2 appropriately predicted the gestational status of ET cows. The statistical evaluation of the diagnostic accuracy in ET cows on day 21 of gestation presented higher values of sensitivity, specificity, accuracy, and positive predictive values of ISG15, MX1, and MX2 using the Youden index than using the AVE. Therefore, ISG15, MX1, and MX2 are excellent biomarkers of gestational status during the peri-implantation period in ET cattle.
1. Introduction
Interferon-tau (IFNT) is a unique molecule derived from trophoblastic cells in ruminants and is specifically produced during the early gestational period [1,2,3]. IFNT exerts an anti-luteolytic effect on the corpus luteum by attenuating the secretion of prostaglandin F2 alpha (PGF2 alpha) in the endometrium, and PGF2 alpha secretion is mediated by the downregulation of oxytocin receptors in uterine epithelial cells [1,2,3]. IFNT not only stimulates endometrial and luteal cells but also other cells, such as hepatocytes and blood cells [3,4,5,6,7]. It recognizes gestation and plays an immunological role in preventing infections and embryo–maternal interactions [8,9]. It plays a crucial role in the regulation of embryonic and endometrial factors during early gestation in ruminants.
Interferon-stimulated genes (ISGs) are induced in response to interferon (IFN) [10]. IFNT, a type I IFN, binds to the cell-surface heterodimeric interferon alpha and beta receptor (IFNAR) 1/IFNAR2 complex [3]. IFNT signaling, through the Janus kinase/signal transducer and the activator of the transcription (JAK/STAT) pathway, involves a transcriptional heterotrimer consisting of phosphorylated STAT1/STAT2 and interferon regulatory factor (IRF9) [3]. Activated interferon regulatory factor 3, which consists of phosphorylated STAT1/STAT2 and IRF9, translocates to the nucleus and promotes gene transcription by binding to IFN-stimulated response elements in the upstream promoter regions of ISGs [11]. It was previously considered that there are several hundred ISGs; however, in recent years, it has been predicted that approximately 10% of the genes in the genome are regulated by IFN [12]. ISG15 ubiquitin-like modifier (ISG15), MX dynamin-like GTPase 1 (MX1), MX2, and 2’–5’ oligoadenylate synthetase 1 (OAS1), which are the focus of the present study, are typical ISGs [11]. ISGs and their related molecules coordinate the establishment of feto–maternal interactions and simultaneously stimulate blood cells. This specific response could be beneficial for predicting the gestational status of cattle during the early peri-implantation period, because IFNT plays specific roles in ruminants [2]. These complex events appear to be closely related to ISG expression in blood cells during maternal recognition.
Predicting pregnancy status is essential for animal food production, both practically and economically. Various methods of gestational prediction, including rectal palpation, ultrasonography, blood progesterone level measurement, and estrus detection, have been used to address this critical issue in cattle [13,14,15]. The detection of return to estrus on days 18–32 post-artificial insemination (AI) is the easiest method for identifying non-pregnant cattle [13]; however, this method is not sufficiently accurate for pregnancy diagnosis owing to its low efficiency (<50%) [16]. Diagnosis based on the rectal palpation of the ovary and uterus is the most widely used method, and it is performed correctly as early as 35 days post-AI [13,14]. Recently, portable brightness (B)-mode ultrasonography has been reported to be a widespread method, and it has been used to correctly detect early pregnancy 27 days post-AI under real-world conditions [13,17]. Moreover, indirect methods, such as the measurement of progesterone concentrations and pregnancy-associated glycoprotein (PAG) levels in milk and blood, have been developed [13,14,15]. Profiling progesterone concentrations on days 18–24 post-AI could be an effective method for identifying non-pregnant cattle [13]. However, non-pregnant cattle with an extended luteal phase are diagnosed as pregnant. Thus, progesterone tests are inadequate for the identification of pregnant cattle [13]. PAGs are secreted by the binucleated trophoblast cells in the placenta, and their levels increase from 15 to 35 days of gestation in cattle [13,18]. Assay kits for milk and blood PAG levels have recently been commercialized. The PAG levels in milk and blood are reliable indicators for pregnancy diagnosis approximately 26–30 days post-AI [13,14].
In the above-mentioned studies, correct diagnosis was made after the period of normal return to estrus, and thus, early rebreeding opportunities were probably lost in non-pregnant cattle. Therefore, establishing pregnancy diagnosis methods before the end of the estrous cycle is required to improve reproductive efficiency. In addition, the detection and prediction of pregnancy loss (late embryonic death or abortion) after a single early pregnancy diagnosis are difficult; thus, multiple examinations are required to confirm pregnancy status. However, IFNT is a specific molecule that is difficult to use as a gestation prediction signal during the early implantation periods; that being said, it plays a role later on [1,2,19]. Recently, ISG expression in peripheral blood leukocytes (PBLs) has been examined as a biomarker for predicting the gestational status during maternal recognition in ruminants [20,21,22]. The prediction efficiency of ISGs, specifically ISG15 and MX2, for the establishment of gestation has been confirmed as reliable biomarkers in cattle [21,23,24,25,26].
Embryo transfer is an assisted reproductive technology that is distinct from AI and has been developed and used in the food and livestock industries [14,27]. Embryo transfer is a method used to promote livestock improvement and produce animals with a high market value; however, the conception rate remains to be improved because only around half of embryo transfers succeed in practice [28,29,30]. In embryo transfer, as in AI, early pregnancy diagnosis, especially the identification of non-pregnant cattle, increases the chance of insemination and shortens the calving interval, consequently improving reproductive efficiency. ISG15 expression in PBLs, a biomarker for predicting gestational status during maternal recognition, has been reported to increase in embryo transferred (ET) cows [31,32]. However, the practical applications of this detection method have not yet been evaluated in detail.
Therefore, in this study, we aimed to clarify the expression patterns of four ISGs [ISG15, MX1, MX2, and OAS1] in the PBLs of multiple ovulation embryo transfer (MOET) cattle on day 21 after estrus (14 days after embryo transfer) and to verify a method for predicting pregnancy status in ET Japanese Black (JB) cattle during the peri-implantation period.
2. Materials and Methods
2.1. Animals
The Iwate University Laboratory Animal Care and Use Committee approved the experimental and feeding conditions of the cattle used in this study (approval numbers: A201244, A201434, and A201701). Twenty-two heifers and eighteen parous JB cattle (n = 40) from an experimental farm were used in this study. Bovine blastocysts were produced on day 7 using the MOET technique [33]. Transferable embryos, identified according to the International Embryo Transfer Society manual as either code 1 or 2 single blastocysts on day 7 after insemination, were transferred to the uterine horn ipsilateral to the corpus luteum horn of each synchronized recipient on day 7 after estrus, as previously reported [34]. Recipient estrous behavior was observed at least twice daily, in the morning and evening. Peripheral blood was collected on day 21 of gestation (14 days after embryo transfer). The pregnancy status was confirmed on days 30 and 60 of gestation using ultrasonographic detection (UD). In the present study, three different categories of cows, namely, 10 pregnant ET cows, 23 non-pregnant cows, and 7 cows that were non-pregnant with late embryonic death, were included from the experimental farm. In pregnant ET cows, the conceptus was observed using UD at approximately 30 days (23 days after embryo transfer) and 60 days of gestation. Conversely, in non-pregnant ET cows, no conceptuses were observed at approximately 30 and 60 days. Pregnancy loss in cows in the present study occurred after a conceptus was observed on day 30 of gestation. Thus, the conceptus was observed on day 30 but not on day 60 in the late embryonic death group.
2.2. Sample Collection, RNA Extraction, and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Blood collection, RNA extraction, and RT-qPCR were performed as previously described [26]. Approximately 3 mL of peripheral blood was collected in PAXgene blood RNA tubes (Qiagen, Hilden, Germany) and incubated at room temperature for 2 h. RNA was extracted using an RNA extraction kit (PAXgene Blood RNA Kit; Qiagen). RNA samples were evaluated using a NanoDrop spectrophotometer (ND-1000; Thermo Fisher Scientific, Waltham, MA, USA) and treated with DNase (TURBO DNA-free Kit; Ambion, Austin, TX, USA) to remove contaminating genomic DNA. Reverse transcription was performed using 1 µg of total RNA with random primers and a high-capacity reverse transcription kit (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s instructions. The reverse transcription cycle was performed at 25 °C for 10 min, 37 °C for 120 min, and 85 °C for 5 s in a thermal cycler. PCR analysis was performed using the Power SYBR Green PCR Master Mix (Thermo Fisher Scientific) on the ABI7300 real-time PCR system (Applied Biosystems), as previously described [24]. The primers used to amplify each gene are listed in Table 1 [24]. The amplification conditions were as follows: initial sample incubation at 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min, with the collection of fluorescence signals at the end of each cycle. The melting curve for detecting the SYBR Green-based objective amplicon was confirmed at 65 to 95 °C in 0.5 °C increments. To quantify the concentration of each mRNA, standard curves were generated via the serial dilution of a plasmid containing the corresponding cDNA, as previously described [22]. For standard plasmid preparation, PCR fragments were subcloned into the pGEM-T Easy Vector System (Promega, Madison, WI, USA), according to the manufacturer’s instructions, and the sequences of the cloned plasmid containing cDNA were analyzed using the ABI PRISM 3100-Avant Genetic Analyzer (Applied Biosystems). In addition, the mRNA expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene for the normalization of internal expression in the samples. The expression of beta-actin and ribosomal protein L27, in addition to that of GAPDH, was also examined to evaluate their use as reference genes for RT-qPCR. The results showed no significant differences in their expression. Quantitative PCR was performed according to the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines [35].

Table 1.
Primer sequences for RT-qPCR.
2.3. Estimation of Threshold Values
The average values (AVE) of normalized ISG expression levels during the estrous cycle in JB cattle have been previously reported [26] as follows: ISG15, 0.174; MX1, 0.065; MX2, 0.098; and OAS1, 0.351. ISG expression levels on day 21 of gestation in ET cows were used to construct a receiver operating characteristic (ROC) curve. According to the RT-qPCR experiments in the present study, the reference cDNA for ISG15, MX1, MX2, and OAS1 and cDNA sample synthesized from PBL RNA were measured simultaneously to allow comparison with AVE values obtained in previous studies. The Youden index and area under the ROC curve (AUC) were estimated using JMP software (SAS Institute Inc., Cary, NC, USA). The AVE and Youden index were used as threshold values to predict the accuracy of pregnancy diagnosis in ET cows on day 21 of gestation. The results were as follows: true positive (TP), where the pregnancy status was determined as positive using the cutoff value of AVE or the Youden index and with the use of UD at around 60 days of gestation; false positive (FP), where the pregnancy status was determined as positive using the cutoff value of AVE or the Youden index but negative using UD; true negative (TN), where the pregnancy status was determined as negative using both cutoff value of AVE or the Youden index and UD; and false negative (FN), where the pregnancy status was determined as negative using the cutoff value of AVE or the Youden index but positive using UD. The sensitivity (TP/(TP + FN) × 100 (probability of a diagnosis of pregnancy among cows that were actually pregnant)), specificity )TN/(TN + FP) × 100 (probability that cows were diagnosed as non-pregnant)), positive predictive value (PPV; TP/(TP + FP) × 100), and negative predictive value (NPV; TN/(TN + FN) × 100) were calculated. The accuracy of pregnancy determination, (TP + TN)/(TP + FP + TN + FN) × 100, was evaluated by calculating the diagnostic accuracy.
2.4. Statistical Analysis
Gene expression was measured using RT-qPCR and analyzed using Kruskal–Wallis test, followed by Steel–Dwass test, using JMP 7 software. Results with p < 0.05 were considered significant.
3. Results
3.1. Classical ISG Expression in ET Cattle and ROC Curve Analysis
The expression of ISG15, MX1, and MX2 was significantly higher in pregnant cattle than in non-pregnant cattle on day 21 of gestation; however, no differences were observed in OAS1 expression (Table 2). The expression of most ISGs was similar between the non-pregnant and late embryonic death groups.

Table 2.
Expression of interferon-stimulated genes (ISGs) in peripheral blood leukocytes (PBLs) of pregnant and non-pregnant embryo-transferred Japanese Black (JB) cattle on day 21 of gestation (14 days after embryo transfer).
To determine a more suitable predictive marker for embryonic status, specifically in ET cattle, ISG expression levels in PBL were estimated and evaluated using the ROC curve analysis (Figure 1 and Table 3).
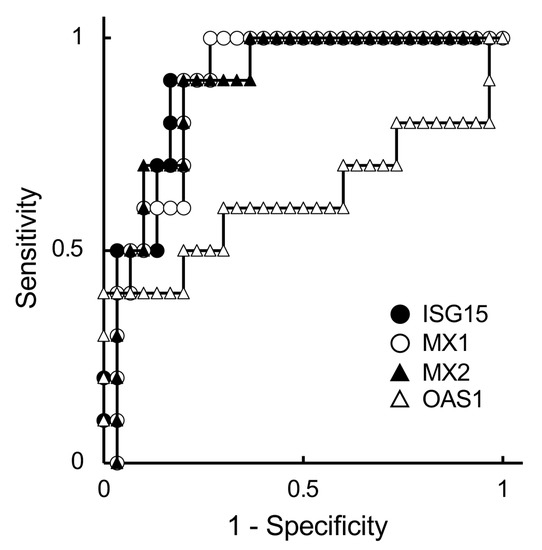
Figure 1.
Receiver operating characteristic (ROC) curves of expression of ISGs in embryo-transferred JB cattle on day 21 of gestation. The expressions of ISG15 (closed circle), MX1 (open circle), MX2 (closed triangle), and OAS1 (open triangle) were analyzed using ROC curves. The horizontal and vertical axes are 1-specificity and sensitivity, respectively. Therefore, the upper-left corner is the ideal point with 100% sensitivity and specificity.

Table 3.
Area under the curve (AUC) and the Youden index cutoff values estimated using receiver operating characteristic analysis in embryo-transferred JB cattle on day 21 of gestation (14 days after embryo transfer).
We used the values of the non-pregnant and non-pregnant with late embryonic death groups for ROC curve analysis because the ISG levels were not different between these groups (Table 2). The ROC curve showed that all ISG levels, except the OAS1 level, appropriately predicted the gestational status on day 21 (Figure 1). The AUC values of ISGs were categorized as high or nearly high (Table 3).
3.2. Prediction of Gestational Statuses during the Peri-Implantation Period in ET Cattle
The gestational status during the peri-implantation period in ET cattle was predicted using conventional cutoff values (AVE) for ISGs, which were estimated from a previous study [26], and the Youden index cutoff values were estimated using the ROC curve analysis (Table 3). When applying the AVE values of the ISGs to predict gestational status on day 21 of gestation, the sensitivity was relatively higher (60.0–100%), but the specificity and accuracy values (10.0–66.6%) were lower than those determined using the Youden index (Table 4). For ISG15, MX1, and MX2, the sensitivity, specificity, and accuracy of the Youden index were 80% or higher. Each NPV showed high values estimated using AVE and the Youden index methods.

Table 4.
Predicted values on day 21 of gestation (14 days after embryo transfer) estimated using AVE and the Youden index in JB cattle.
4. Discussion
Indicators of gestational prediction are practically and economically crucial for domestic cattle reproduction. We established the accuracy of ISG expression in PBLs as predictive evidence in artificially inseminated Holstein and JB cattle [25,26] and applied this method to JB cows in the current study. First, the expression of ISGs in PBLs was examined to determine the suitability of the practical application of blood ISG levels as indicators of gestation prediction during the peri-implantation phase. Ten pregnant, twenty-three non-pregnant, and seven non-pregnant cows with late embryonic death were used to predict the gestational status of ET cows using ISG values. The fertility rates of the flock were low but presented a convenient model for late embryonic loss and/or death.
On day 21 of pregnancy, the levels of ISG15, MX1, and MX2 in PBLs were considerably higher in the pregnant group than in the non-pregnant group, similar to those reported by previous studies on AI [25]. Therefore, the reliability of ISG cutoff values was assessed in ET cows to predict the gestational status during the peri-implantation interval. The Youden index has been used to estimate the cutoff values for different physiological and pathological statuses using ROC curves [29,30]. The use of previously reported AVE ISG cutoff values [19] and the reliability of this technique were confirmed using ROC and the Youden index analyses in the present study, and the application of AVE cutoff values confirmed the credibility of the method. Using the AVE in ET JB cattle on day 21 of gestation, high sensitivity and NPV were observed, but the specificity, accuracy, and PPV were low. The Youden index cutoff method had higher specificity and accuracy than the AVE estimation. The AUC, obtained using the ROC curve analysis, indicated the accuracy of these predictions with high AUC values [36,37]. The values of ISG15, MX1, and MX2 on day 21 were approximately 0.9 in ET cows and were almost the same as those in previous studies on AI cattle [26]. In previous studies, the differences in ISG level, specifically ISG15 and MX2, between pregnant and non-pregnant cattle implied that they could be prominent indicators for predicting the gestation status in artificially inseminated Holstein and JB cattle [25,26]. However, MX1 is not a suitable indicator when applying the AVE cutoff value for prediction because of its low specificity, precision, and PPV. However, by applying the Youden index to the cutoff value, MX1 can also be used as an appropriate indicator, equivalent to ISG15 and MX2. Therefore, ISG15, MX1, and MX2 may be suitable indicators of pregnancy in ET cattle.
Interestingly, similar blood ISG levels were observed in the non-pregnant and late embryonic death groups in the present study. These results pose a simple question: why was the gene expression different between pregnant and non-pregnant cattle with late embryonic death on day 21 of gestation? This question arises because both groups would have had a conceptus around day 21. The ISG levels may reflect embryonic morphological changes during the implantation process [38,39]. The ISG levels in the non-pregnant and late embryonic death groups were similar in ET cattle on day 21, which may indicate the embryo development status. Embryos at approximately 17–19 days of gestation significantly change their shapes and activities, and the expanded fetal membrane covers the entire endometrial surface during the critical period of implantation [3,38,40]. The discrepancy in ISG level between the pregnant and non-pregnant late embryonic death groups suggests insufficient IFNT secretion by the embryos in the late embryonic death group.
In domestic cattle reproduction, the true fertility rate is crucial for farmers who expect their cows to become pregnant as soon as possible after calving. Although various new technologies and strategies, such as superovulation, in vitro fertilization, and embryo transfer, have been applied to improve lifetime production in the cattle industry over the last several decades, embryo loss or death after fertilization remains a major issue, especially at 3–4 weeks [19]. Novel and cutting-edge techniques, such as embryo transfer, would be helpful in improving fertility in not only cattle but also other mammalian species, including humans, and their successful management should be considered. The results of the current study showed that the conceptus was observed using ultrasonography at around 30 days in both pregnant and late embryonic death groups. However, the ISG levels of the late embryonic death group on day 21 were lower than those of the pregnant group. It is important to determine whether embryos are dead, alive, or developing well. This means that some indicators are needed to determine the embryonic conditions. We expect that ISG detection methods in PBL will be useful in solving challenging issues. Unlike positive prediction, the negative prediction of gestational status in embryo transfer was reliable and ISGs were detectable in PBLs 14 days after embryo transfer. The application of ISG expression in PBLs during the peri-implantation period will provide accurate information under these circumstances. However, the sample size in the current study was small and none of the data on different days of the peri-implantation period, except for day 21 in ET cattle, were sufficient for gestational prediction. Further studies using more animals around peri-implantation are necessary to confirm the reliability of this theory regarding high gene expression in ET cattle.
There are several practical methods for evaluating gestational status during early gestation, including serum progesterone and pregnancy-associated protein B measurements, and transrectal palpation. However, the prediction of gestational status in ET cattle is still difficult during the implantation period [14,41,42]. Specific gene expression in PBLs may be a reliable predictive method for determining the pregnancy status. Although this procedure may be reliable because the ISG prediction method has been established and used in cattle and other ruminants, we do not have sufficient information to identify PBLs that respond to ISG15, MX1, MX2, and/or OAS1 [22]. The association between the patterns of gene expression in PBLs and indicators of different physiological circumstances is clear [24,25,43,44]. Further studies on the specificity of each gene are required to understand the precise responses to particular ISG molecules and the detailed roles of ISGs in the development of early stage embryos.
5. Conclusions
Classical ISGs, specifically ISG15, MX1, and MX2, are excellent biomarkers for predicting the non-pregnancy status during the peri-implantation period in ET JB cattle. These biomarkers may be reliable for the negative prediction of gestation during embryo transfer but are less reliable for establishing positive predictions. In addition, the expression of ISGs in the PBLs provides early gestational information during the recognition of gestation, regardless of the conception method in cattle.
Author Contributions
Conceptualization, H.Y., K.K. and K.H.; methodology, H.Y., K.K. and K.H.; formal analysis, K.K.; investigation, H.Y. and K.K.; resources, T.-i.H., K.I. (Kosuke Iga), H.M., T.Y., Y.H., K.I. (Kei Imai), T.I.-O. and T.T.; data curation, H.Y., K.K. and K.H.; writing—original draft preparation, H.Y., K.K. and K.H.; writing—review and editing, H.Y., T.K., K.K. and K.H.; visualization, K.K.; supervision, K.K. and K.H.; project administration, K.K. and K.H.; funding acquisition, K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Program on Innovative Technologies for Animal Breeding, Reproduction, and Vaccine Development of the MINISTRY OF AGRICULTURE, FORESTRY, AND FISHERIES of Japan, grant number REP-1003.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of IWATE UNIVERSITY (approval numbers: A201244, A201434, and A201701)” for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Fumiyo Taguchi and Mariko Sato for extracting RNA from bovine blood.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martal, J.; Chêne, N.; Camous, S.; Huynh, L.; Lantier, F.; Hermier, P.; L’Haridon, R.; Charpigny, G.; Charlier, M.; Chaouat, G. Recent developments and potentialities for reducing embryo mortality in ruminants: The role of IFN-τ and other cytokines in early pregnancy. Reprod. Fertil. Dev. 1997, 9, 355–380. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.M. Conceptus inferteons and maternal recognition of pregnancy. Biol. Reprod. 1989, 40, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.R.; Sinedino, L.D.P.; Spencer, T.E. Paracrine and endocrine actions of interferon tau (IFNT). Reproduction 2017, 154, F45–F59. [Google Scholar] [CrossRef] [PubMed]
- Shirasuna, K.; Matsumoto, H.; Matsuyama, S.; Kimura, K.; Bollwein, H.; Miyamoto, A. Possible role of interferon tau on the bovine corpus luteum and neutrophils during the early pregnancy. Reproduction 2015, 150, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Wijma, R.; Stangaferro, M.L.; Kamat, M.M.; Vasudevan, S.; Ott, T.L.; Giordano, J.O. Embryo mortality around the period of maintenance of the corpus luteum causes alterations to the ovarian function of lactating dairy cows. Biol. Reprod. 2016, 95, 112. [Google Scholar] [CrossRef]
- Ruhmann, B.; Giller, K.; Hankele, A.K.; Ulbrich, S.E.; Schmicke, M. Interferon-τ induced gene expression in bovine hepatocytes during early pregnancy. Theriogenology 2017, 104, 198–204. [Google Scholar] [CrossRef]
- Alhussien, M.N.; Kamboj, A.; Aljader, M.A.; Panda, B.S.K.; Yadav, M.L.; Sharma, L.; Mohammed, S.; Sheikh, A.A.; Lotfan, M.; Kapila, R.; et al. Effect of tropical thermal stress on peri-implantation immune responses in cows. Theriogenology 2018, 114, 149–158. [Google Scholar] [CrossRef]
- Forde, N.; Bazer, F.W.; Spencer, T.E.; Lonergan, P. ‘Conceptualizing’ the endometrium: Identification of conceptus-derived proteins during early pregnancy in cattle1. Biol. Reprod. 2015, 92, 156. [Google Scholar] [CrossRef]
- Bazer, F.W.; Thatcher, W.W. Chronicling the discovery of interferon tau. Reproduction 2017, 154, F11–F20. [Google Scholar] [CrossRef]
- Au-Yeung, N.; Horvath, C.M. Transcriptional and chromatin regulation in interferon and innate antiviral gene expression. Cytokine Growth Factor Rev. 2018, 44, 11–17. [Google Scholar] [CrossRef]
- Williams, B.R.G.; Williams, B.R.G. Transcriptional regulation of interferon-stimulated genes. Eur. J. Biochem. 1991, 200, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W. Interferon-stimulated genes: What do they all do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef]
- Fricke, P.M.; Ricci, A.; Giordano, J.O.; Carvalho, P.D. Methods for and implementation of pregnancy diagnosis in dairy cows. Vet. Clin. North Am.-Food Anim. Pract. 2016, 32, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.S.; Britt, J.H. A 100-year review: Practical female reproductive management. J. Dairy Sci. 2017, 100, 10292–10313. [Google Scholar] [CrossRef] [PubMed]
- Ott, T.L. Symposium review: Immunological detection of the bovine conceptus during early pregnancy. J. Dairy Sci. 2019, 102, 3766–3777. [Google Scholar] [CrossRef] [PubMed]
- Senger, P.L. The estrus detection problem: New concepts, technologies, and possibilities. J. Dairy Sci. 1994, 77, 2745–2753. [Google Scholar] [CrossRef]
- Quintela, L.A.; Barrio, M.; Peña, A.I.; Becerra, J.J.; Cainzos, J.; Herradón, P.G.; Díaz, C. Use of ultrasound in the reproductive management of dairy cattle. Reprod. Domest. Anim. 2012, 47, 34–44. [Google Scholar] [CrossRef]
- Filho, R.V.O.; Franco, G.A.; Reese, S.T.; Dantas, F.G.; Fontes, P.L.P.; Cooke, R.F.; Rhinehart, J.D.; Thompson, K.W.; Pohler, K.G. Using pregnancy associated glycoproteins (PAG) for pregnancy detection at day 24 of gestation in beef cattle. Theriogenology 2020, 141, 128–133. [Google Scholar] [CrossRef]
- Hansen, P.J. The incompletely fulfilled promise of embryo transfer in cattle—Why aren’t pregnancy rates greater and what can we do about it? J. Anim. Sci. 2020, 98, skaa288. [Google Scholar] [CrossRef]
- Gifford, C.; Racicot, K.; Clark, D.S.; Austin, K.J.; Hansen, T.R.; Lucy, M.C.; Davies, C.J.; Ott, T.L. Regulation of interferon-stimulated genes in peripheral blood leukocytes in pregnant and bred, nonpregnant dairy cows. J. Dairy Sci. 2007, 90, 274–280. [Google Scholar] [CrossRef]
- Green, J.C.; Okamura, C.S.; Poock, S.E.; Lucy, M.C. Measurement of interferon-tau (IFN-τ) stimulated gene expression in blood leukocytes for pregnancy diagnosis within 18-20d after insemination in dairy cattle. Anim. Reprod. Sci. 2010, 121, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Kizaki, K.; Shichijo-Kizaki, A.; Furusawa, T.; Takahashi, T.; Hosoe, M.; Hashizume, K. Differential neutrophil gene expression in early bovine pregnancy. Reprod. Biol. Endocrinol. 2013, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.L.; Dalton, J.C.; Ott, T.L.; Racicot, K.E.; Chebel, R.C. Correlation between reproductive status and steady-state messenger ribonucleic acid levels of the myxovirus resistance gene, MX2, in peripheral blood leukocytes of dairy heifers. J. Anim. Sci. 2007, 85, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Toji, N.; Shigeno, S.; Kizaki, K.; Koshi, K.; Matsuda, H.; Hashiyada, Y.; Imai, K.; Takahashi, T.; Ishiguro-Oonuma, T.; Hashizume, K. Evaluation of interferon-stimulated genes in peripheral blood granulocytes as sensitive responders to bovine early conceptus signals. Vet. J. 2017, 229, 37–44. [Google Scholar] [CrossRef]
- Yoshino, H.; Toji, N.; Sasaki, K.; Koshi, K.; Yamagishi, N.; Takahashi, T.; Ishiguro-Oonuma, T.; Matsuda, H.; Yamanouchi, T.; Hashiyada, Y.; et al. A predictive threshold value for the diagnosis of early pregnancy in cows using interferon-stimulated genes in granulocytes. Theriogenology 2018, 107, 188–193. [Google Scholar] [CrossRef]
- Yoshino, H.; Kizaki, K.; Iga, K.; Hirata, T.; Matsuda, H.; Yamanouchi, T.; Hashiyada, Y.; Toji, N.; Ishiguro-Oonuma, T.; Takahashi, T.; et al. Use of a prediction method for early pregnancy status utilizing receiver operating characteristic curve analysis of peripheral blood leukocyte interferon-stimulated genes in Japanese-black cattle. Anim. Reprod. Sci. 2020, 214, 106283. [Google Scholar] [CrossRef]
- Hasler, J.F. Forty years of embryo transfer in cattle: A review focusing on the journal Theriogenology, the growth of the industry in north america, and personal reminisces. Theriogenology 2014, 81, 152–169. [Google Scholar] [CrossRef]
- Hashiyada, Y.; Okada, M.; Imai, K. Transition of the pregnancy rate of bisected bovine embryos after co-transfer with trophoblastic vesicles prepared from in vivo-cultured in vitro-fertilized embryos. J. Reprod. Dev. 2005, 51, 749–756. [Google Scholar] [CrossRef]
- Geary, T.W.; Burns, G.W.; Moraes, J.G.N.; Moss, J.I.; Denicol, A.C.; Dobbs, K.B.; Ortega, M.S.; Hansen, P.J.; Wehrman, M.E.; Neibergs, H.; et al. Identification of beef heifers with superior uterine capacity for pregnancy. Biol. Reprod. 2016, 95, 47. [Google Scholar] [CrossRef]
- Ealy, A.D.; Wooldridge, L.K.; McCoski, S.R. Board invited review: Post-transfer consequences of in vitro-produced embryos in cattle. J. Anim. Sci. 2019, 97, 2555–2568. [Google Scholar] [CrossRef]
- Matsuyama, S.; Kojima, T.; Kato, S.; Kimura, K. Relationship between quantity of IFNT estimated by IFN-stimulated gene expression in peripheral blood mononuclear cells and bovine embryonic mortality after AI or ET. Reprod. Biol. Endocrinol. 2012, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, H.; Moriyasu, S.; Kageyama, S.; Sawai, K.; Takahashi, H.; Geshi, M.; Fujii, T.; Koyama, T.; Koyama, K.; Miyamoto, A.; et al. Enhancement of maternal recognition of pregnancy with parthenogenetic embryos in bovine embryo transfer. Theriogenology 2014, 81, 1108–1115. [Google Scholar] [CrossRef]
- Hiraizumi, S.; Nishinomiya, H.; Oikawa, T.; Sakagami, N.; Sano, F.; Nishino, O.; Kurahara, T.; Nishimoto, N.; Ishiyama, O.; Hasegawa, Y.; et al. Superovulatory response in Japanese Black cows receiving a single subcutaneous porcine follicle–stimulating hormone treatment or six intramuscular treatments over three days. Theriogenology 2015, 83, 466–473. [Google Scholar] [CrossRef]
- Sugimura, S.; Akai, T.; Somfai, T.; Hirayama, M.; Aikawa, Y.; Ohtake, M.; Hattori, H.; Kobayashi, S.; Hashiyada, Y.; Konishi, K.; et al. Time-lapse cinematography-compatible polystyrene-based microwell culture system: A novel tool for tracking the development of individual bovine embryos. Biol. Reprod. 2010, 83, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.; Benes, V.; Garson, J.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Perkins, N.J.; Liu, A.; Bondell, H. Optimal Cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology 2005, 16, 73–81. [Google Scholar] [CrossRef]
- Brooks, K.; Burns, G.; Spencer, T.E. Conceptus elongation in ruminants: Roles of progesterone, prostaglandin, interferon tau and cortisol. J. Anim. Sci. Biotechnol. 2014, 5, 53. [Google Scholar] [CrossRef]
- Romero, J.J.; Antoniazzi, A.Q.; Nett, T.M.; Ashley, R.L.; Webb, B.T.; Smirnova, N.P.; Bott, R.C.; Bruemmer, J.E.; Bazer, F.W.; Anthony, R.V.; et al. Temporal Release, paracrine and endocrine actions of ovine conceptus-derived interferon-tau during early pregnancy. Biol. Reprod. 2015, 93, 146. [Google Scholar] [CrossRef]
- Spencer, T.E.; Forde, N.; Lonergan, P. The role of progesterone and conceptus-derived factors in uterine biology during early pregnancy in ruminants. J. Dairy Sci. 2016, 99, 5941–5950. [Google Scholar] [CrossRef]
- Balhara, A.K.; Gupta, M.; Singh, S.; Mohanty, A.K.; Singh, I. Early pregnancy diagnosis in bovines: Current status and future directions. Sci. World J. 2013, 2013, 958540. [Google Scholar] [CrossRef] [PubMed]
- Reese, S.T.; Pereira, M.C.; Vasconcelos, J.L.M.; Smith, M.F.; Green, J.A.; Geary, T.W.; Peres, R.F.G.; Perry, G.A.; Pohler, K.G. Markers of pregnancy: How early can we detect pregnancies in cattle using pregnancy-associated glycoproteins (PAGs) and microRNAs? Anim. Reprod. 2016, 13, 200–208. [Google Scholar] [CrossRef]
- Sasaki, K.; Yamagishi, N.; Kizaki, K.; Devkota, B.; Hashizume, K. Microarray-based gene expression profiling of peripheral blood mononuclear cells in dairy cows with experimental hypocalcemia and milk fever. J. Dairy Sci. 2014, 97, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Kizaki, K.; Kageyama, T.; Toji, N.; Koshi, K.; Sasaki, K.; Yamagishi, N.; Ishiguro-Oonuma, T.; Takahashi, T.; Hashizume, K. Gene expression profiles in bovine granulocytes reflect the aberration of liver functions. Anim. Sci. J. 2020, 91, e13324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).