Development of Yorkshire Terrier Dentition
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Study Design
2.3. Data Handling
2.4. Statistical Modelling
3. Results
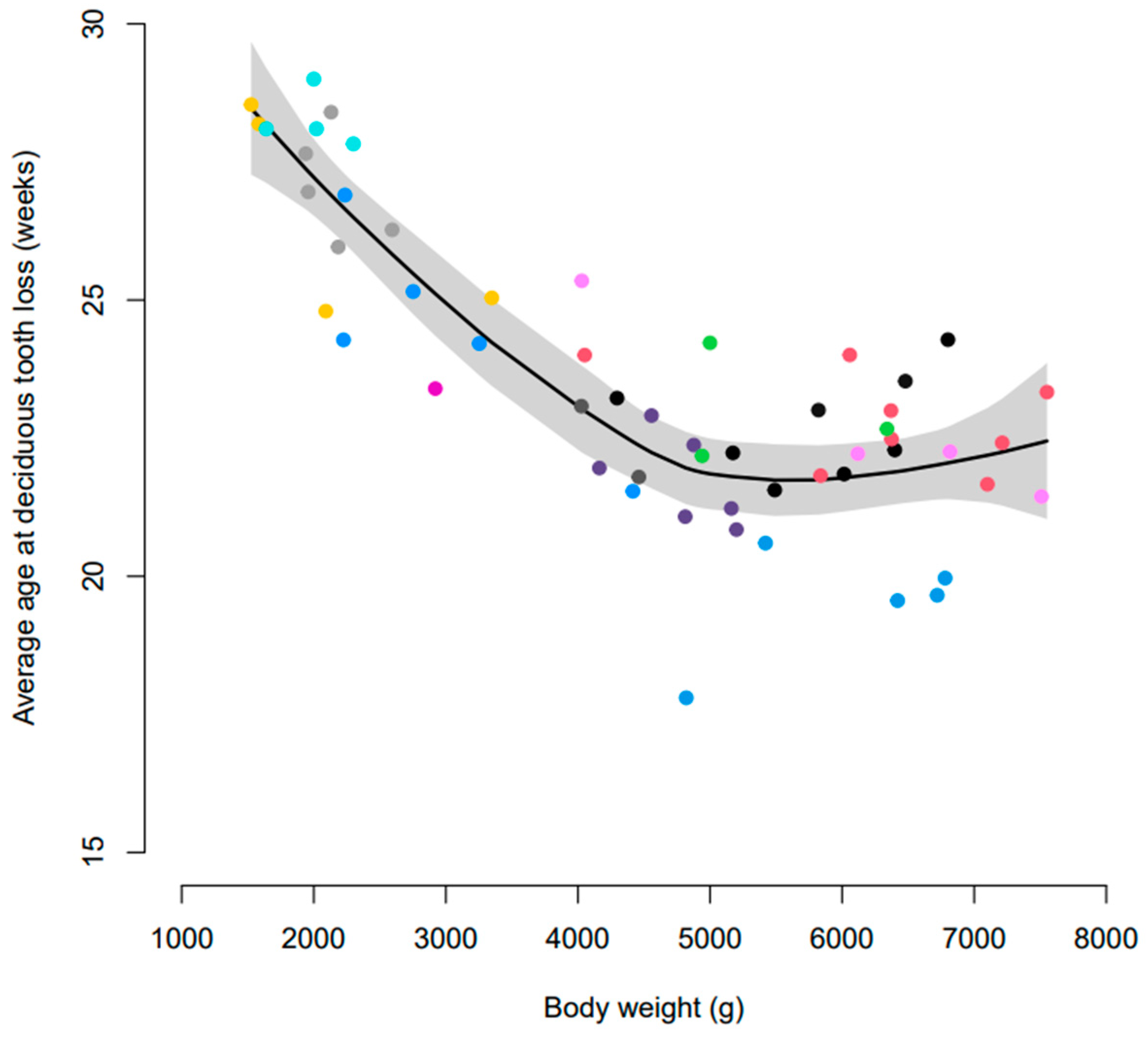
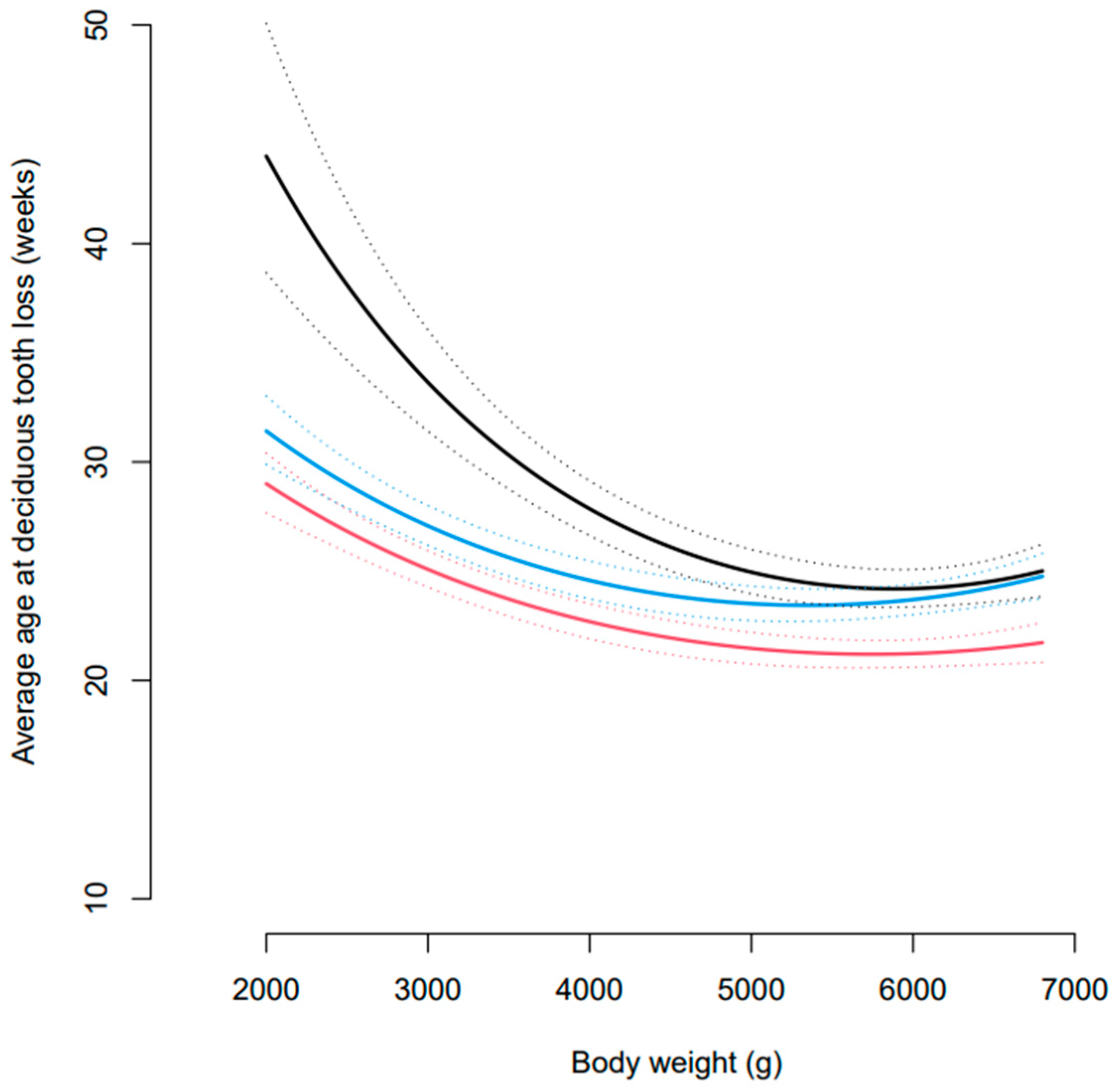
3.1. Age at Exfoliation of Deciduous Dentition
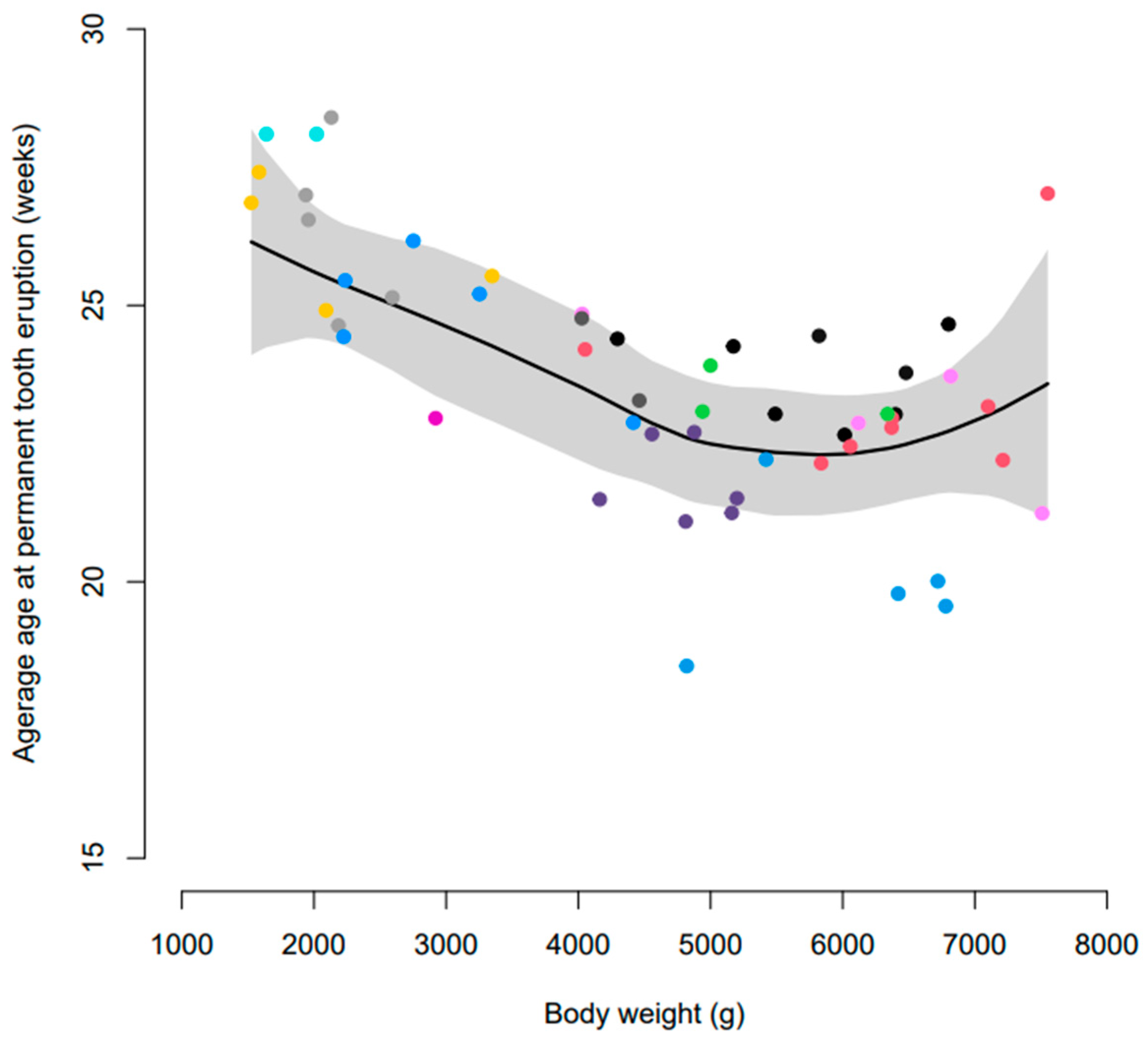
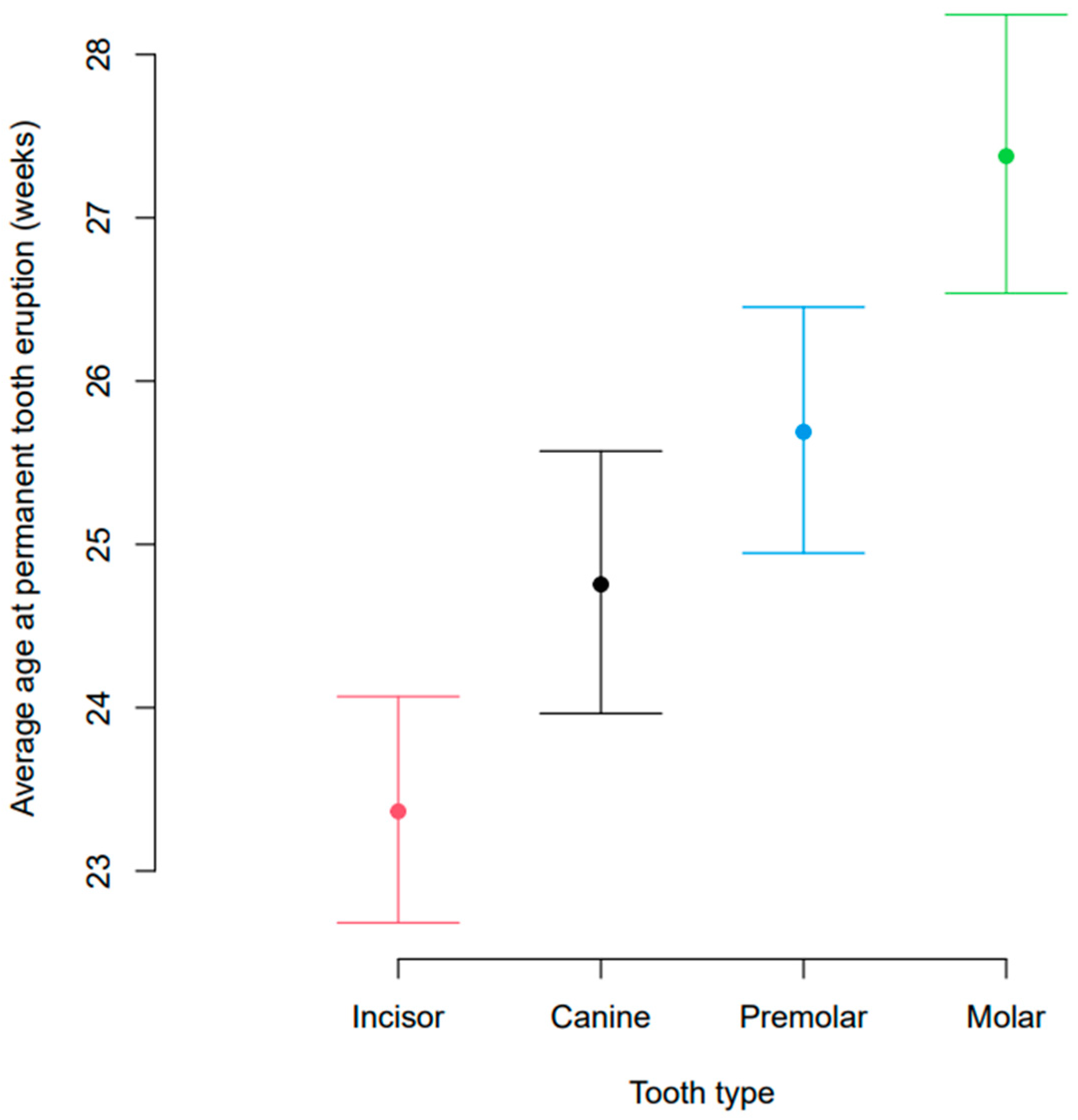
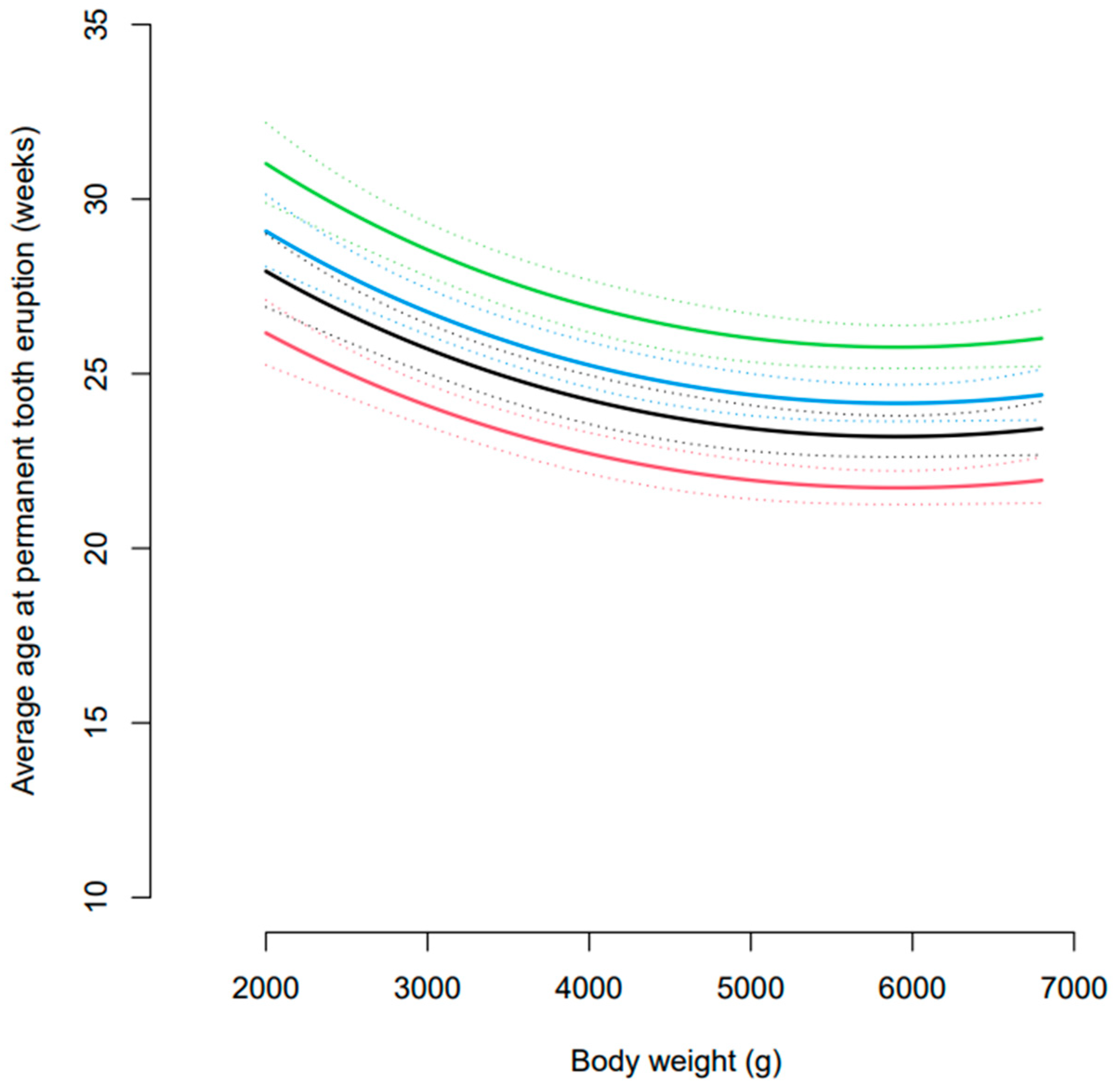
3.2. Age at Eruption of Permanent Dentition
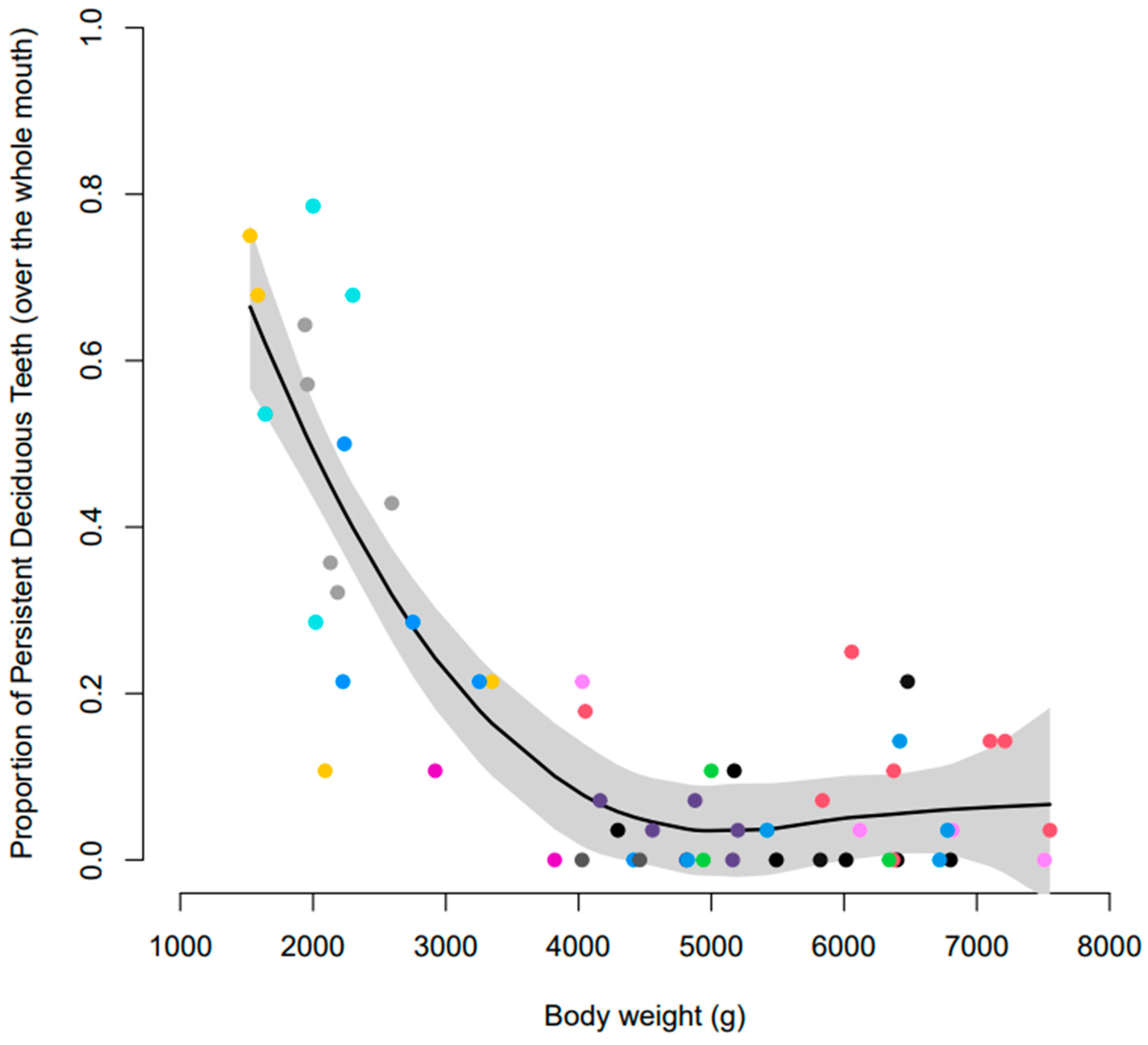
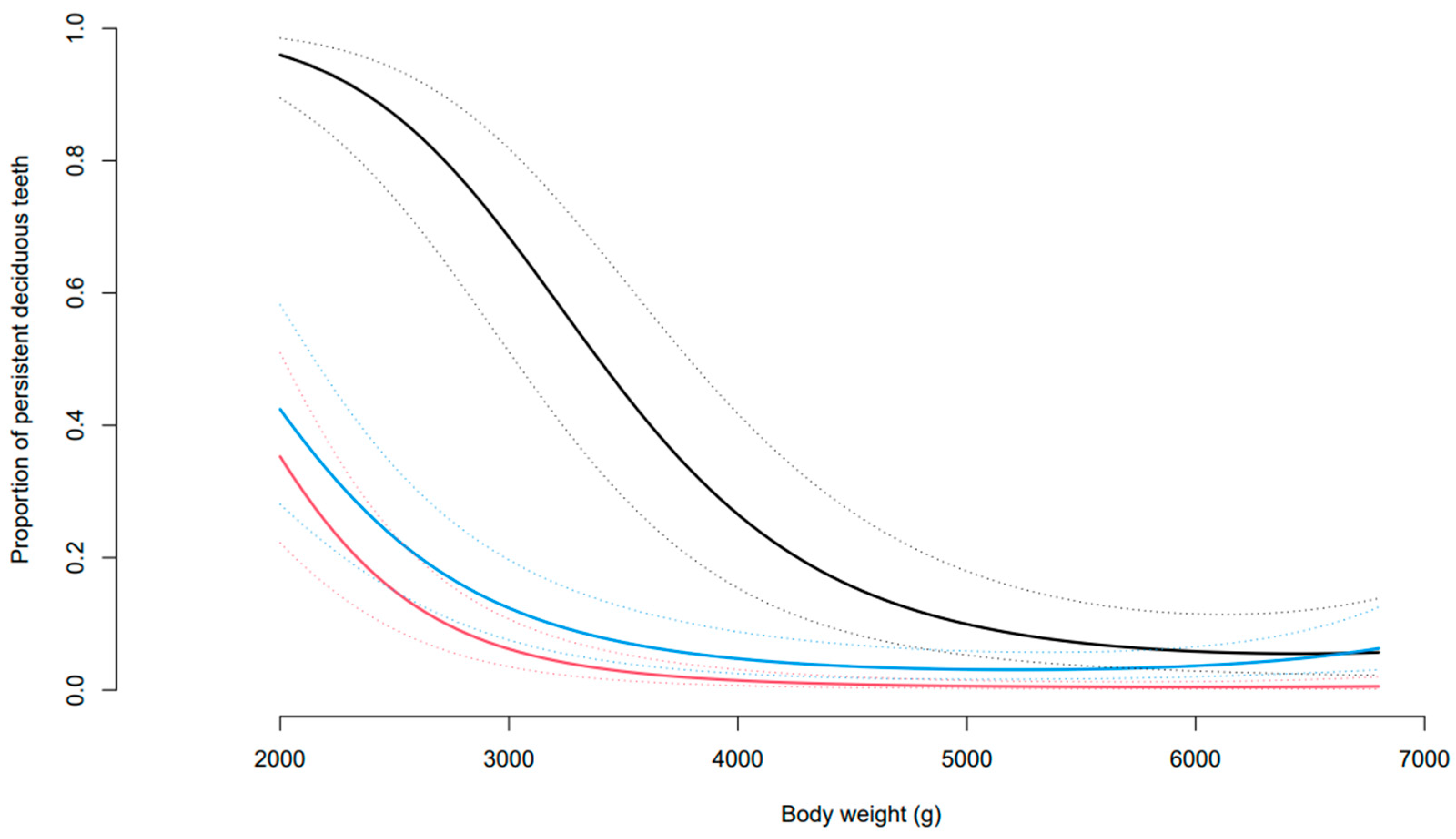
3.3. Incidence of PDT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiggs, R.; Lobprise, H. Pedodontics. In Veterinary Dentistry, Principles and Practice; Raven-Lippincott: Philadelphia, PA, USA, 1997; pp. 167–185. [Google Scholar]
- Harvey, C.; Emily, P. Function, formation, and anatomy of oral structures in carnivores. In Small Animal Dentistry; Mosby-Year Book: St. Louis, MO, USA, 1993. [Google Scholar]
- Hale, F. Juvenille Veterinary Dentistry. Vet. Clin. N. Am. Small Anim. Pract. 2005, 35, 789–817. [Google Scholar] [CrossRef]
- Hobson, P. Extraction of retained primary canine teeth in the dog. J. Vet. Dent. 2005, 22, 132–137. [Google Scholar] [CrossRef]
- Niemiec, B.A. Oral pathology. Top. Companion Anim. Med. 2008, 23, 59–71. [Google Scholar] [CrossRef]
- Niemiec, B.A. Conditions common in small and toy breed dogs. In Breed Predispositions to Dental and Oral Disease in Dogs; Wiley Online Library: New York, NY, USA, 2021; pp. 1–38. [Google Scholar]
- O’Neill, D.G.; Packer, R.M.A.; Lobb, M.; Church, D.B.; Brodbelt, D.C.; Pegram, C. Demography and commonly recorded clinical conditions of Chihuahuas under primary veterinary care in the UK in 2016. BMC Vet. Res. 2020, 16, 42. [Google Scholar] [CrossRef]
- Harvey, C.; Emily, P. Occlusion, occlusive abnormalities and orthodontic treatment. In Small Animal Dentistry; Mosby-Year Book: St. Louis, MO, USA, 1993. [Google Scholar]
- Baxter, C. Should that tooth be extracted? Part 1: Problems associated with deciduous dentition in puppies. UK Vet. Companion Anim. 2009, 14, 58–61. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Therneau, T.M. A package for Survival Analysis in S Version 2.38, ed. 2015. Available online: https://CRAN.R-project.org/package=survival (accessed on 14 June 2023).
- Therneau, T.M.; Grambsch, P.M. The cox model. In Modeling Survival Data: Extending the Cox Model; Springer: Berlin/Heidelberg, Germany, 2000; pp. 39–77. [Google Scholar]
- Garn, S.M.; Lewis, A.B.; Kerewsky, R.S. Genetic, Nutritional, and Maturational Correlates of Dental Development. J. Dent. Res. 1965, 44, 228–242. [Google Scholar] [CrossRef]
- Townsend, G.; Richards, L.; Messer, L.B.; Hughes, T.; Pinkerton, S.; Seow, K.; Gotjamanos, T.; Gully, N.; Bockmann, M. Genetic and environmental influences on dentofacial structures and oral health: Studies of Australian twins and their families. Twin Res. Hum. Genet. 2006, 9, 727–732. [Google Scholar] [CrossRef]
- Pelsmaekers, B.; Loos, R.; Carels, C.; Derom, C.; Vlietinck, R. The genetic contribution to dental maturation. J. Dent. Res. 1997, 76, 1337–1340. [Google Scholar] [CrossRef]
- Hughes, T.; Bockmann, M.; Seow, K.; Gotjamanos, T.; Gully, N. Strong genetic control of emergence of human primary incisor. J. Dent. Res. 2007, 86, 1160–1165. [Google Scholar] [CrossRef]
- Geller, F.; Feenstra, B.; Zhang, H.; Shaffer, J.R.; Hansen, T.; Esserlind, A.-L.; Boyd, H.A.; Nohr, E.A.; Timpson, N.J.; Fatemifar, G.; et al. Genome-wide association study identifies four loci associated with eruption of permanent teeth. PLoS Genet. 2011, 7, e1002275. [Google Scholar] [CrossRef]
- Pillas, D.; Hoggart, C.J.; Evans, D.M.; O’Reilly, P.F.; Sipilä, K.; Lähdesmäki, R.; Millwood, I.Y.; Kaakinen, M.; Netuveli, G.; Blane, D.; et al. Genome-wide association study reveals multiple loci associated with primary tooth development during infancy. PLoS Genet. 2010, 6, e1000856. [Google Scholar] [CrossRef]
- Fatemifar, G.; Hoggart, C.J.; Paternoster, L.; Kemp, J.P.; Prokopenko, I.; Horikoshi, M.; Wright, V.J.; Tobias, J.H.; Richmond, S.; Zhurov, A.I.; et al. Genome-wide association study of primary tooth eruption identifies pleiotropic loci associated with height and craniofacial distances. Hum. Mol. Genet. 2013, 22, 3807–3817. [Google Scholar] [CrossRef]
- Liu, H.; Deng, H.; Cao, C.F.; Ono, H. Genetic analysis of dental traits in 82 pairs of female-female twins. Chin. J. Dent. Res. Off. J. Sci. Sect. Chin. Stomatol. Assoc. 1998, 1, 12–16. [Google Scholar]
- Liu, H.; Deng, H.; Cao, C. Genetic analysis of tooth development and eruption in 82 pairs of female-female twins. Zhonghua Kou Qiang Yi Xue Za Zhi = Zhonghua Kouqiang Yixue Zazhi = Chin. J. Stomatol. 1999, 34, 159–161. [Google Scholar]
- Hagg, U.; Taranger, J. Timing of tooth emergence. A prospective longitudinal study of Swedish urban children from birth to 18 years. Swed. Dent. J. 1986, 10, 195–206. [Google Scholar]
- Mugonzibwa, E.A.; Kuijpers-Jagtman, A.M.; Laine-Alava, M.T.; Hof, M.A.V. Emergence of permanent teeth in Tanzanian children. Community Dent. Oral Epidemiol. 2002, 30, 455–462. [Google Scholar] [CrossRef]
- Nystrom, M.; Kleemola-Kujala, E.; Evalahti, M.; Peck, L.; Kataja, M. Emergence of permanent teeth and dental age in a series of Finns. Acta Odontol. Scand. 2001, 59, 49–56. [Google Scholar] [CrossRef]
- Sharma, K.; Mittal, S. Permanent tooth emergence in Gujjars of Punjab, India. Anthropol. Anz. Bericht. Uber Die Biol.-Anthropol. Lit. 2001, 59, 165–178. [Google Scholar] [CrossRef]
- Nonaka, K.; Ichiki, A.; Miura, T. Changes in the eruption order of the first permanent tooth and their relation to season of birth in Japan. Am. J. Phys. Anthropol. 1990, 82, 191–198. [Google Scholar] [CrossRef]
- Brown, T. Tooth emergence in Australian Aboriginals. Ann. Hum. Biol. 1978, 5, 41–54. [Google Scholar] [CrossRef]
- Gaur, R.; Singh, N.Y. Emergence of permanent teeth among the meiteis of Manipur, India. Am. J. Hum. Biol. Off. J. Human. Biol. Counc. 1994, 6, 321–328. [Google Scholar] [CrossRef]
- Garcia-Godoy, F.; D’Iaz, A.N.; del Valle, J.M.; Arana, E.J. Timing of permanent tooth emergence in a Southeastern Dominican schoolchildren population sample. Community Dent. Oral Epidemiol. 1982, 10, 43–46. [Google Scholar] [CrossRef]
- Chaillet, N.; Nystrom, M.; Demirjian, A. Comparison of dental maturity in children of different ethnic origins: International maturity curves for clinicians. J. Forensic Sci. 2005, 50, 1164–1174. [Google Scholar] [CrossRef]
- Höuffding, J.; Maeda, M.; Yamaguchi, K.; Tsuji, H.; Kuwabara, S.; Nohara, Y.; Yoshida, S. Emergence of permanent teeth and onset of dental stages in Japanese children. Community Dent. Oral Epidemiol. 1984, 12, 55–58. [Google Scholar] [CrossRef]
- Kanagaratnam, S.; Schluter, P.J. The age of permanent tooth emergence in children of different ethnic origin in the Auckland region: A cross-sectional study. N. Z. Dent. J. 2012, 108, 55–61. [Google Scholar]
- Kochhar, R.; Richardson, A. The chronology and sequence of eruption of human permanent teeth in Northern Ireland. Int. J. Paediatr. Dent. 1998, 8, 243–252. [Google Scholar] [CrossRef]
- Pahel, B.T.; Vann, W.F., Jr.; Divaris, K.; Rozier, R.G. A Contemporary Examination of First and Second Permanent Molar Emergence. J. Dent. Res. 2017, 96, 1115–1121. [Google Scholar] [CrossRef]
- Sajjadian, N.; Shajari, H.; Jahadi, R.; Barakat, M.G.; Sajjadian, A. Relationship between birth weight and time of first deciduous tooth eruption in 143 consecutively born infants. Pediatr. Neonatol. 2010, 51, 235–237. [Google Scholar] [CrossRef]
- Billewicz, W.Z.; McGregor, I.A. Eruption of permanent teeth in West African (Gambian) children in relation to age, sex and physique. Ann. Hum. Biol. 1975, 2, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Viscardi, R.; Rombery, B.; Abrams, R. Delayed primary tooth eruption in premature infant: Relationship to neonatal factors. Pediatr. Dent. 1994, 16, 23–28. [Google Scholar]
- Fadavi, S.; Punwani, I.C.; Adeni, S.; Vidyasagar, D. Eruption pattern in the primary dentition of premature low-birth-weight children. ASDC J. Dent. Child. 1992, 59, 120–122. [Google Scholar]
- Lawoyin, T.; Lawoyin, D.; Lawoyin, J. Epidemiological study of some factors related to deciduous tooth eruption. Afr. Dent. J. 1996, 10, 19–23. [Google Scholar] [PubMed]
- Correa-Faria, P.; Leite-Faria, L.; Viana, A.N.; Marques, L.S.; Ferreira, F.O.; Ramos-Jorge, M.L. Factors associated with number of erupted primary teeth in Brazilian children: A cross-sectional study. J. Dent. Child. 2013, 80, 111–114. [Google Scholar]
- Soliman, N.L.; El-Zainy, M.A.; Hassan, R.M.; Aly, R.M. Relationship of deciduous teeth emergence with physical growth. Indian J. Dent. Res. 2012, 23, 236–240. [Google Scholar]
- Shuper, A.; Sarnat, H.; Mimouni, F.; Mimouni, M.; Varsano, I. Deciduous tooth eruption in Israeli children. A cross-sectional study. Clin. Pediatr. 1985, 24, 342–344. [Google Scholar] [CrossRef]
- Haddad, A.; Correa, M. The relationship between the number of erupted teeth and the child’s height and weight: A cross-sectioanl study. J. Pediatr. Dent. 2005, 29, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Kutesa, A.; Nkamba, E.M.; Muwazi, L.; Buwembo, W.; Rwenyonyi, C.M. Weight, height and eruption times of permanent teeth of children aged 4-15 years in Kampala, Uganda. BMC Oral Health 2013, 13, 15. [Google Scholar] [CrossRef]
- Lobprise, H. Canine periodontal disease overview. Vet. Tech. 2006, 27, 168–174. [Google Scholar]
- Tutt, C.; Deeprose, J.; Crossley, D. BSAVA Manual of Canine and Feline Dentistry; British Small Animal Veterinary Association: Gloucestershire, UK, 2007. [Google Scholar]
- Albuquerque, C.; Morinha, F.; Requicha, J.; Martins, T.; Dias, I.; Guedes-Pinto, H.; Bastos, E.; Viegas, C. Canine periodontitis: The dog as an important model for periodontal studies. Vet. J. 2012, 191, 299–305. [Google Scholar] [CrossRef]
- Gioso, M.A.; Shofer, F.; Barros, P.S.; Harvey, C.E. Mandible and mandibular first molar tooth measurements in dogs: Relationship of radiographic height to body weight. J. Vet. Dent. 2001, 18, 65–68. [Google Scholar] [CrossRef] [PubMed]
- McKeown, M. Craniofacial variability and its relationship to disharmony of the jaws and teeth. J. Anat. 1975, 119 Pt 3, 579–588. [Google Scholar]
- Potter, R.H.; Nance, W.E.; Yu, P.L.; Davis, W.B. A twin study of dental dimension. II. Independent genetic determinants. Am. J. Phys. Anthropol. 1976, 44, 397–412. [Google Scholar] [CrossRef]
- Borissov, I.; Sivrev, D.; Milev, N. Incidence of some teeth and occlusion abnormlities in dogs: A retrospective study. Bulg. J. Vet. Med. 2004, 7, 245–250. [Google Scholar]
- Butkovic, V.; Šehič, M.; Stanin, D.; Šimpraga, M.; Capak, D.; Kos, J. Dental diseases of dogs: A retrospective study of radiological data. Acta Vet. Brno 2001, 70, 203–208. [Google Scholar] [CrossRef]
- Isogai, H.; Isogai, E.; Okamoto, H.; Shirakawa, H.; Nakamura, F.; Matsumoto, T.; Watanabe, T.; Miura, H.; Aoi, Y.; Kagota, W.; et al. Epidemiological Study on Periodontal Diseases and some Other Dental Disorders in Dogs. Jpn. J. Vet. Sci. 1989, 51, 1151–1162. [Google Scholar] [CrossRef]
- Aktan, A.M.; Kara, I.; Şener, I.; Bereket, C.; Çelik, S.; Kırtay, M.; Çiftçi, M.E.; Arıcı, N. An evaluation of factors associated with persistent primary teeth. Eur. J. Orthod. 2012, 34, 208–212. [Google Scholar] [CrossRef]
- Nordquist, I.; Lennartsson, B.; Paulander, J. Primary teeth in adults—A pilot study. Swed. Dent. J. 2005, 29, 27–34. [Google Scholar] [PubMed]
- Dashash, M.; Al-Jazar, N. Timing and sequence of emergence of permanent teeth in Syrian schoolchildren. J. Investig. Clin. Dent. 2017, 9, e12311. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.E.; Kamalwand, N.; Jurgen, S.W.; Scheuer, H.A. Die Durchbruchszeiten der bleibenden Dentition bei Jungen in Teheran (Iran) [Eruption times of permanent teeth in male children and adolescents of Tehran (Iran)]. Arch. Fur Kriminol. 2007, 219, 145–168. (In German) [Google Scholar]
- Khatskevich, G.A.; Bogomolova, I.A. Sroki prorezyvaniia postoiannykh zubov u shkol’nikov Sankt-Peterburga [Time of permanent teeth eruption in schoolchildren of Saint-Petersburg]. Stomatologiia 2004, 83, 53–57. (In Russian) [Google Scholar] [PubMed]
- Friedrich, R.E.; Habib, S.; Scheuer, H.A. Die Durchbruchszeiten der permanenten Dentition bei Kindern und Jugendlichen in Latakia (Syrien) [Eruption times of permanent teeth in children and adolescents in Latakia (Syria)]. Arch. Fur Kriminol. 2009, 223, 84–97. (In German) [Google Scholar]
- Friedrich, R.E.; Katerji, H.; Wedl, J.S.; Scheuer, H.A. Die Zahndurchbruchszeiten der bleibenden Dentition bei Jungen und Madchen in Paderborn, Westfalen [Eruption times of permanent teeth in children and adolescents of Paderborn, Westphalia, Germany]. Arch. Fur Kriminol. 2006, 217, 20–35. (In German) [Google Scholar]
- Friedrich, R.E.; Leist, A.; Scheuer, H.A. Die Durchbruchszeiten der permanenten Dentition bei Kindern und Jugendlichen im Saarland [Eruption times of permanent teeth in children and adolescents in the German state of Saarland]. Arch. Fur Kriminol. 2008, 222, 73–104. (In German) [Google Scholar]
- Kaur, B.; Singh, R. One year follow-up study of stature, weight, emergence of dentition, and sexual maturation of well-nourished indian girls from birth to 20 years. Am. J. Hum. Biol. Off. J. Human. Biol. Counc. 1994, 6, 425–436. [Google Scholar] [CrossRef]
- Agarwal, K.N.; Gupta, R.; Faridi, M.M.; Kalra, N. Permanent dentition in Delhi boys of age 5–14 years. Indian Pediatr. 2004, 41, 1031–1035. [Google Scholar]
| Variable | Description |
|---|---|
| Litter | Dogs’ litter group |
| Age | Dogs’ age (weeks) at time of measurement |
| Body weight | Dogs’ weight (kg) at end of study (28 weeks of age) |
| Tooth type | Incisor, canine, premolar, molar |
| Persistent deciduous tooth indicator | Defined as whether deciduous teeth were present in the mouth at the same time as their permanent counterparts. Not persistent—0; persistent—1 |
| Surgery indicator | Deciduous dentition, which was not naturally exfoliated by 32 weeks of age and required surgical intervention. No surgery—0; surgery—1 |
| Tooth Type | Body Weight | ||
|---|---|---|---|
| ≤3 kg | >3 kg and ≤5 kg | >5 kg | |
| Incisor | 25.8 (2.8) | 20.8 (2.5) | 20.6 (2.5) |
| Canine | 28.3 (0.6) | 25.5 (2.8) | 23.8 (2.7) |
| Premolar | 26.9 (2.2) | 23.6 (2.7) | 23.0 (2.8) |
| Tooth Type | Body Weight | ||
|---|---|---|---|
| ≤3 kg | >3 kg and ≤5 kg | >5 kg | |
| Incisor | 24.4 (2.3) | 21.2(2.4) | 21.1 (2.5) |
| Canine | 26.8 (1.7) | 22.7 (1.6) | 22.7 (1.6) |
| Premolar | 26.1 (2.6) | 24.1 (3.4) | 22.9 (3.0) |
| Molar | 26.4 (2.9) | 25.7 (2.9) | 24.4 (3.4) |
| Tooth Type | Body Weight | ||
|---|---|---|---|
| ≤3 kg | >3 kg and ≤5 kg | >5 kg | |
| Incisor | 0.349 (0.477) | 0.017 (0.128) | 0.013 (0.115) |
| Canine | 0.891 (0.312) | 0.250 (0.433) | 0.120 (0.325) |
| Premolar | 0.411 (0.492) | 0.061 (0.240) | 0.087 (0.281) |
| Tooth Type | Body Weight | ||
|---|---|---|---|
| ≤3 kg | >3 kg and ≤5 kg | >5 kg | |
| Incisor | 0.188 (0.390) | 0.011 (0.105) | 0.003 (0.058) |
| Canine | 0.641 (0.480) | 0.167 (0.373) | 0.080 (0.271) |
| Premolar | 0.234 (0.424) | 0.044 (0.206) | 0.080 (0.271) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallis, C.; Solmi, F.; Pesci, I.; Desforges, N.; Holcombe, L.J. Development of Yorkshire Terrier Dentition. Vet. Sci. 2023, 10, 406. https://doi.org/10.3390/vetsci10070406
Wallis C, Solmi F, Pesci I, Desforges N, Holcombe LJ. Development of Yorkshire Terrier Dentition. Veterinary Sciences. 2023; 10(7):406. https://doi.org/10.3390/vetsci10070406
Chicago/Turabian StyleWallis, Corrin, Francesca Solmi, Ilaria Pesci, Neil Desforges, and Lucy J. Holcombe. 2023. "Development of Yorkshire Terrier Dentition" Veterinary Sciences 10, no. 7: 406. https://doi.org/10.3390/vetsci10070406
APA StyleWallis, C., Solmi, F., Pesci, I., Desforges, N., & Holcombe, L. J. (2023). Development of Yorkshire Terrier Dentition. Veterinary Sciences, 10(7), 406. https://doi.org/10.3390/vetsci10070406






