16S rRNA Gene Amplicon Sequencing of Gut Microbiota Affected by Four Probiotic Strains in Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Bacterial Concentration Results
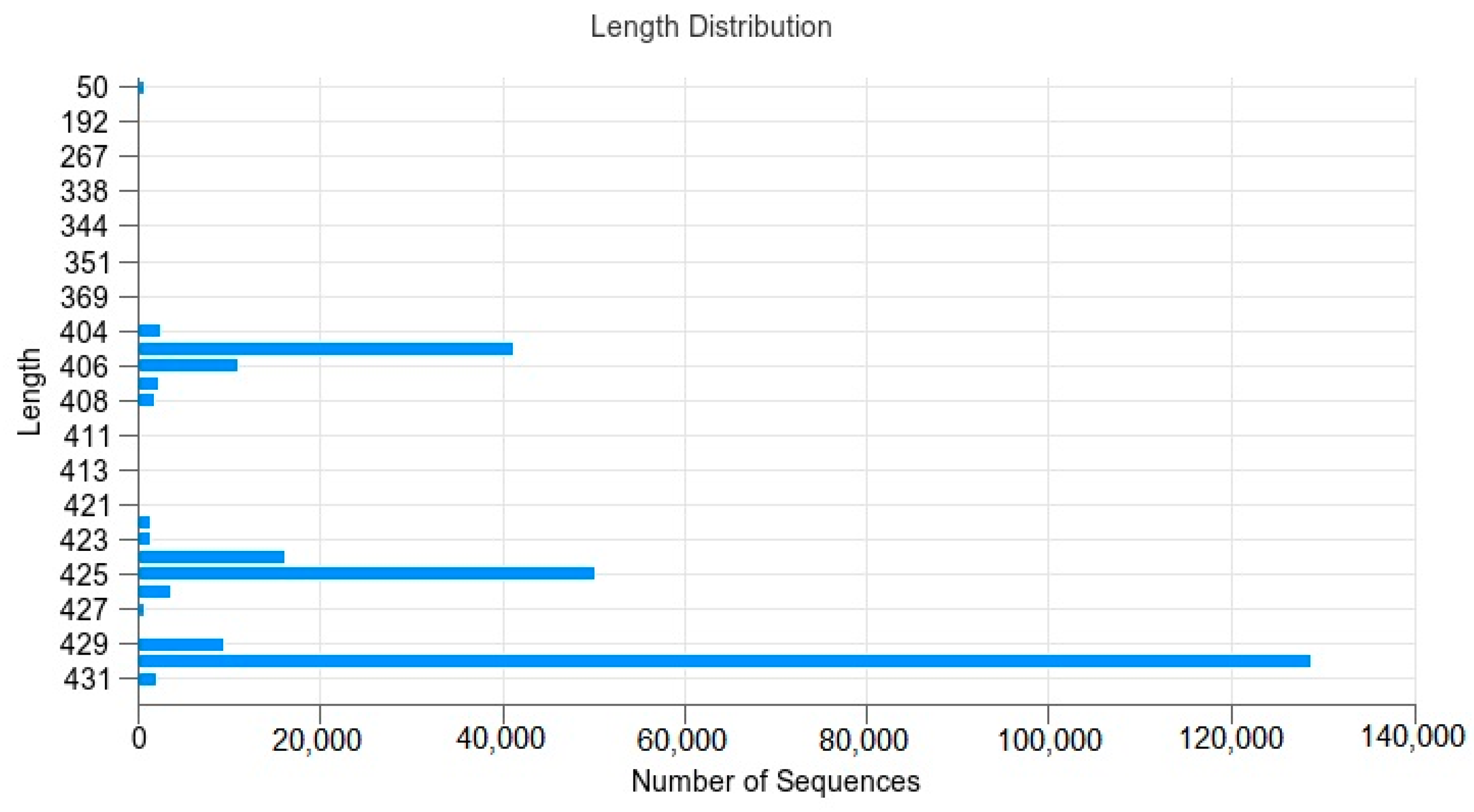
3.2. Sequence Processing
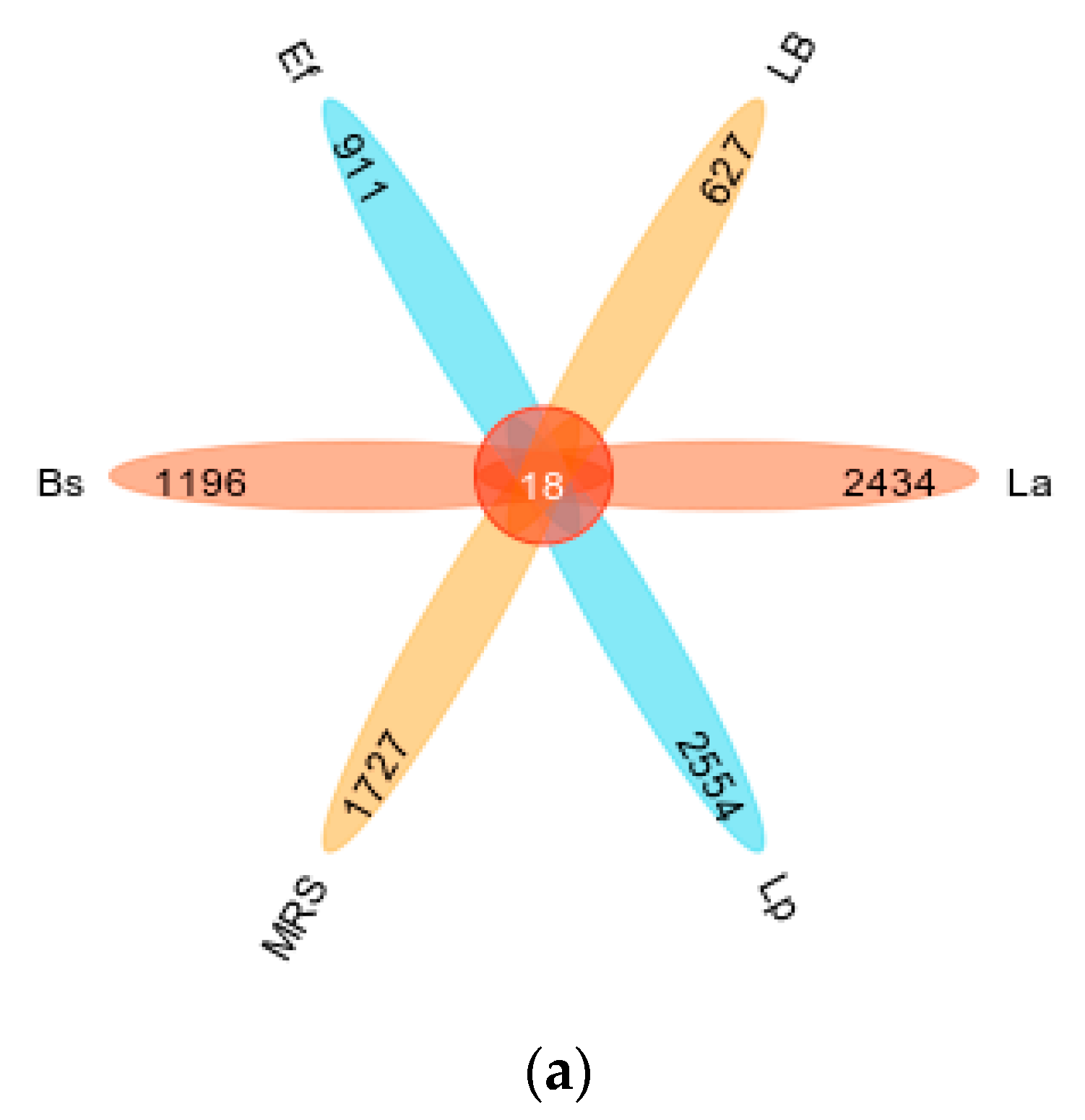
3.3. Four Probiotics Affect Gut Microbiota Diversity in Mice
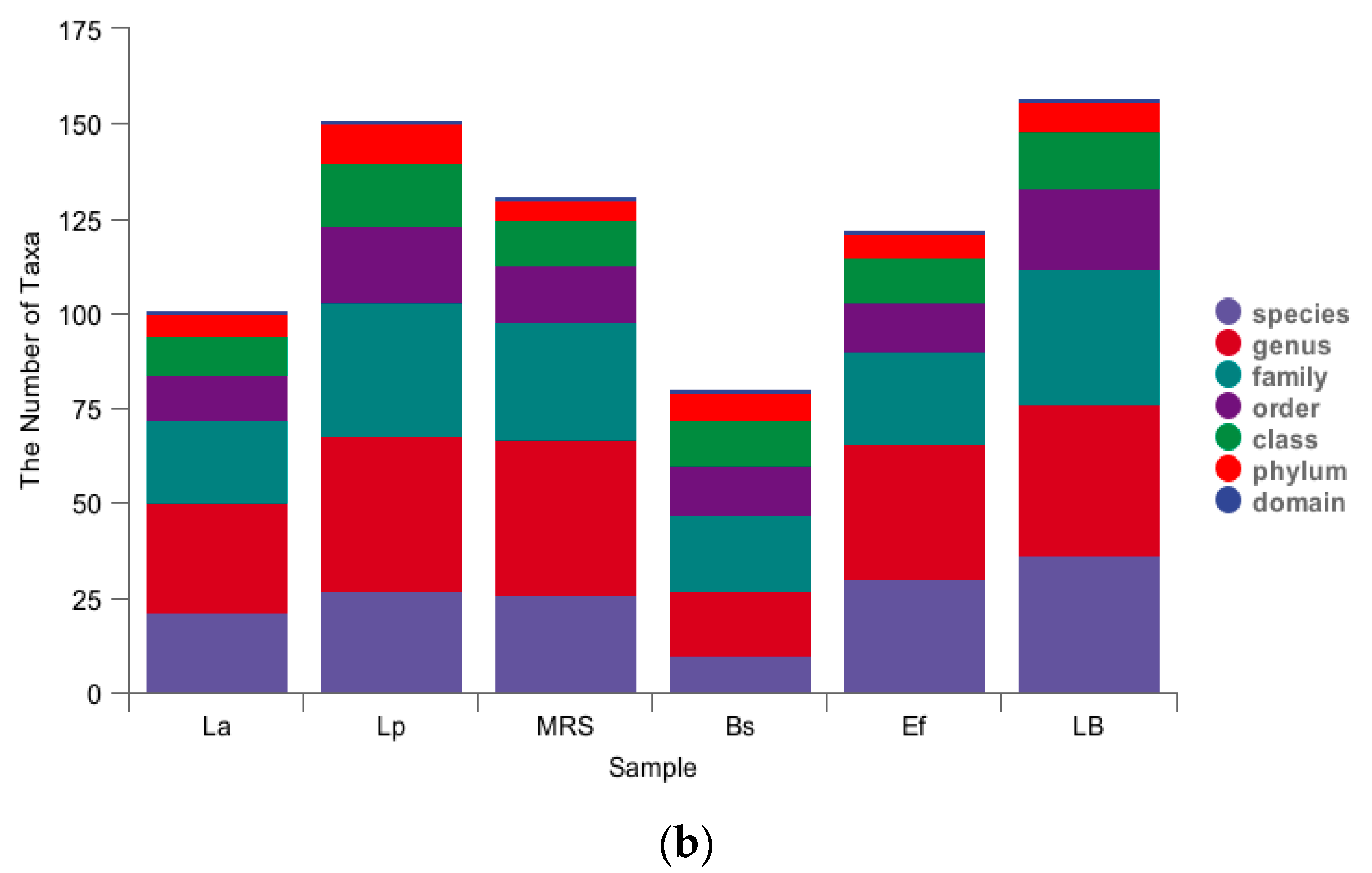
3.4. Four Probiotics Alter the Distribution of Gut Microbiota in Mice
3.5. α-Diversity Analysis
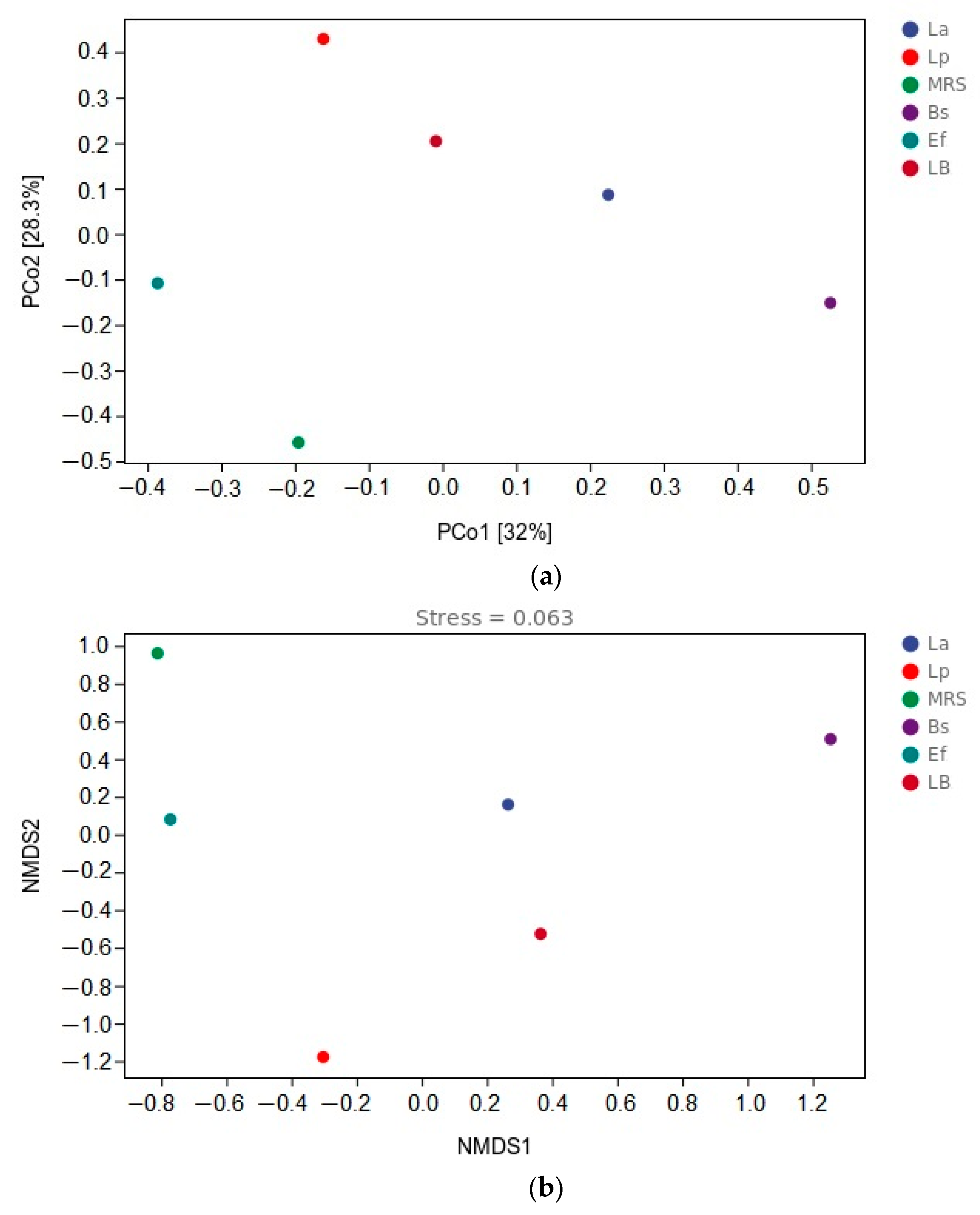
3.6. β-Diversity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Kc, D.; Sumner, R.; Lippmann, S. Gut microbiota and health. Postgrad. Med. 2020, 132, 274. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Bibbo, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar] [PubMed]
- Simren, M.; Barbara, G.; Flint, H.J.; Spiegel, B.M.; Spiller, R.C.; Vanner, S.; Verdu, E.F.; Whorwell, P.J.; Zoetendal, E.G.; Rome Foundation, C. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut 2013, 62, 159–176. [Google Scholar] [CrossRef]
- Bibbo, S.; Lopetuso, L.R.; Ianiro, G.; Di Rienzo, T.; Gasbarrini, A.; Cammarota, G. Role of microbiota and innate immunity in recurrent Clostridium difficile infection. J. Immunol. Res. 2014, 2014, 462740. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Gaborit, B.; Dutour, A.; Clement, K. Gut microbiota and non-alcoholic fatty liver disease: New insights. Clin. Microbiol. Infect. 2013, 19, 338–348. [Google Scholar] [CrossRef]
- D’Aversa, F.; Tortora, A.; Ianiro, G.; Ponziani, F.R.; Annicchiarico, B.E.; Gasbarrini, A. Gut microbiota and metabolic syndrome. Intern. Emerg. Med. 2013, 8 (Suppl. S1), S11–S15. [Google Scholar] [CrossRef]
- Bibbo, S.; Dore, M.P.; Pes, G.M.; Delitala, G.; Delitala, A.P. Is there a role for gut microbiota in type 1 diabetes pathogenesis? Ann. Med. 2017, 49, 11–22. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, J.M. The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience 2016, 324, 131–139. [Google Scholar] [CrossRef]
- Dudek-Wicher, R.K.; Junka, A.; Bartoszewicz, M. The influence of antibiotics and dietary components on gut microbiota. Prz. Gastroenterol. 2018, 13, 85–92. [Google Scholar] [CrossRef]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The Gut Microbiota of Laying Hens and Its Manipulation with Prebiotics and Probiotics To Enhance Gut Health and Food Safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Ragan, M.V.; Wala, S.J.; Goodman, S.D.; Bailey, M.T.; Besner, G.E. Next-Generation Probiotic Therapy to Protect the Intestines From Injury. Front. Cell. Infect. Microbiol. 2022, 12, 863949. [Google Scholar] [CrossRef]
- Ruiz, L.; Margolles, A.; Sanchez, B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front. Microbiol. 2013, 4, 396. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, A.; Nordone, S.K.; Zhang, L.; Stoeker, L.L.; LaVoy, A.S.; Klaenhammer, T.R.; Dean, G.A. Dissimilar properties of two recombinant Lactobacillus acidophilus strains displaying Salmonella FliC with different anchoring motifs. Appl. Environ.Microbiol. 2011, 77, 6587–6596. [Google Scholar] [CrossRef]
- Wells, J.M.; Mercenier, A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 2008, 6, 349–362. [Google Scholar] [CrossRef]
- Bull, M.J.; Jolley, K.A.; Bray, J.E.; Aerts, M.; Vandamme, P.; Maiden, M.C.; Marchesi, J.R.; Mahenthiralingam, E. The domestication of the probiotic bacterium Lactobacillus acidophilus. Sci. Rep. 2014, 4, 7202. [Google Scholar] [CrossRef]
- Russo, P.; Arena, M.P.; Fiocco, D.; Capozzi, V.; Drider, D.; Spano, G. Lactobacillus plantarum with broad antifungal activity: A promising approach to increase safety and shelf-life of cereal-based products. Int. J. Food Microbiol. 2017, 247, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Maughan, H.; Van der Auwera, G. Bacillus taxonomy in the genomic era finds phenotypes to be essential though often misleading. Infect. Genet. Evol. 2011, 11, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Stathopoulos, C.; Abu-Jdayil, B.; Esposito, G.; Baig, M.; Turner, M.S.; Baba, A.S.; Apostolopoulos, V.; Al-Nabulsi, A.; Osaili, T. Exopolysaccharide produced by potential probiotic Enterococcus faecalis MS79: Characterization, bioactivities and rheological properties influenced by salt and pH. Lwt-Food Sci. Technol. 2020, 131, 109741. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus As Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed]
- Cutting, S.M.; Ricca, E. Bacterial spore-formers: Friends and foes. FEMS Microbiol. Lett. 2014, 358, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.A.; Duc, L.H.; Cutting, S.M. The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 2005, 29, 813–835. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Yegani, M.; Korver, D.R. Factors affecting intestinal health in poultry. Poult. Sci. 2008, 87, 2052–2063. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Han, J.; Sun, Y.; Lei, L.; Yuan, J.; Qiao, Z.; Men, J.; Wang, X.; Guo, Y.; Wang, Q.; et al. Probiotics ameliorate growth retardation of glyphosate by regulating intestinal microbiota and metabolites in crucian carp (Carassius auratus). Sci. Total. Environ. 2022, 851, 158260. [Google Scholar] [CrossRef]
- Vemuri, R.; Shinde, T.; Gundamaraju, R.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Martoni, C.J.; Eri, R. Lactobacillus acidophilus DDS-1 Modulates the Gut Microbiota and Improves Metabolic Profiles in Aging Mice. Nutrients 2018, 10, 1255. [Google Scholar] [CrossRef]
- Tong, T.; Niu, X.; Li, Q.; Ling, Y.; Li, Z.; Liu, J.; Zhang, M.; Bai, Z.; Xia, R.; Wu, Z.; et al. The Effect of Lactobacillus plantarum BW2013 on The Gut Microbiota in Mice Analyzed by 16S rRNA Amplicon Sequencing. Pol. J. Microbiol. 2021, 70, 235–243. [Google Scholar] [CrossRef]
- Wang, T.; Teng, K.; Liu, Y.; Shi, W.; Zhang, J.; Dong, E.; Zhang, X.; Tao, Y.; Zhong, J. Lactobacillus plantarum PFM 105 Promotes Intestinal Development Through Modulation of Gut Microbiota in Weaning Piglets. Front. Microbiol. 2019, 10, 90. [Google Scholar] [CrossRef]
- Li, A.; Jiang, X.; Wang, Y.; Zhang, L.; Zhang, H.; Mehmood, K.; Li, Z.; Waqas, M.; Li, J. The impact of Bacillus subtilis 18 isolated from Tibetan yaks on growth performance and gut microbial community in mice. Microb. Pathog. 2019, 128, 153–161. [Google Scholar] [CrossRef]
- Wang, W.; Cai, H.; Zhang, A.; Chen, Z.; Chang, W.; Liu, G.; Deng, X.; Bryden, W.L.; Zheng, A. Enterococcus faecalis Modulates the Gut Microbiota of Broilers and Enhances Phosphorus Absorption and Utilization. Animals 2020, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhao, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Narbad, A.; Chen, W. A mixture of Lactobacillus species isolated from traditional fermented foods promote recovery from antibiotic-induced intestinal disruption in mice. J. Appl. Microbiol. 2018, 124, 842–854. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Sansonetti, P.J.; Marteyn, B.S. Shigella Diversity and Changing Landscape: Insights for the Twenty-First Century. Front. Cell. Infect. Microbiol. 2016, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gong, L.; Zhou, Y.; Tang, L.; Zeng, Z.; Wang, Q.; Zou, P.; Yu, D.; Li, W. Probiotic Paenibacillus polymyxa 10 and Lactobacillus plantarum 16 enhance growth performance of broilers by improving the intestinal health. Anim. Nutr. 2021, 7, 829–840. [Google Scholar] [CrossRef]
- Karimaei, S.; Sadeghi, J.; Asadian, M.; Esghaei, M.; Pourshafie, M.R.; Talebi, M. Antibacterial potential and genetic profile of Enterococcus faecalis strains isolated from human normal flora. Microb. Pathog. 2016, 96, 67–71. [Google Scholar] [CrossRef]
- Zare Mirzaei, E.; Lashani, E.; Davoodabadi, A. Antimicrobial properties of lactic acid bacteria isolated from traditional yogurt and milk against Shigella strains. GMS Hyg. Infect. Control 2018, 13, Doc01. [Google Scholar] [CrossRef]
- Apas, A.L.; Dupraz, J.; Ross, R.; Gonzalez, S.N.; Arena, M.E. Probiotic administration effect on fecal mutagenicity and microflora in the goat’s gut. J. Biosci. Bioeng. 2010, 110, 537–540. [Google Scholar] [CrossRef]
- Yang, J.; Bindels, L.B.; Segura Munoz, R.R.; Martinez, I.; Walter, J.; Ramer-Tait, A.E.; Rose, D.J. Disparate Metabolic Responses in Mice Fed a High-Fat Diet Supplemented with Maize-Derived Non-Digestible Feruloylated Oligo- and Polysaccharides Are Linked to Changes in the Gut Microbiota. PLoS ONE 2016, 11, e0146144. [Google Scholar] [CrossRef]
- Tett, A.; Pasolli, E.; Masetti, G.; Ercolini, D.; Segata, N. Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 2021, 19, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, E.S.; Mariyatun, M.; Putri Manurung, N.E.; Hasan, P.N.; Therdtatha, P.; Mishima, R.; Komalasari, H.; Mahfuzah, N.A.; Pamungkaningtyas, F.H.; Yoga, W.K.; et al. Effect of probiotic Lactobacillus plantarum Dad-13 powder consumption on the gut microbiota and intestinal health of overweight adults. World J. Gastroenterol. 2021, 27, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, X.; Long, Y.; Zhong, H.; Wang, P.; Yuan, P.; Zhang, X.; Che, L.; Feng, B.; Li, J.; et al. Dietary supplementation with Lactobacillus plantarum modified gut microbiota, bile acid profile and glucose homoeostasis in weaning piglets. Br. J. Nutr. 2020, 124, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tsai, T.; Wei, X.; Zuo, B.; Davis, E.; Rehberger, T.; Hernandez, S.; Jochems, E.J.M.; Maxwell, C.V.; Zhao, J. Effect of Lactylate and Bacillus subtilis on Growth Performance, Peripheral Blood Cell Profile, and Gut Microbiota of Nursery Pigs. Microorganisms 2021, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- Mamuad, L.L.; Kim, S.H.; Biswas, A.A.; Yu, Z.; Cho, K.K.; Kim, S.B.; Lee, K.; Lee, S.S. Rumen fermentation and microbial community composition influenced by live Enterococcus faecalis supplementation. AMB Express 2019, 9, 123. [Google Scholar] [CrossRef]


| Number. | Strain | Source | Preservation Location |
|---|---|---|---|
| 1 | L. plantarum | Separated from mink manure in August 2017 | Laboratory of Veterinary Microbiology and Immunology, College of Veterinary Medicine, Qingdao Agricultural University |
| 2 | L. acidophilus | Separated from mink manure in August 2017 | Laboratory of Veterinary Microbiology and Immunology, College of Veterinary Medicine, Qingdao Agricultural University |
| 3 | B. subtilis | Separated from mink manure in August 2017 | Laboratory of Veterinary Microbiology and Immunology, College of Veterinary Medicine, Qingdao Agricultural University |
| 4 | E.faecalis | Separated from mink manure in August 2017 | Laboratory of Veterinary Microbiology and Immunology, College of Veterinary Medicine, Qingdao Agricultural University |
| Time | 6 h | 8 h | 10 h | 12 h | 14 h | |
|---|---|---|---|---|---|---|
| Strains | ||||||
| L. plantarum | 6.01 × 108 | 1.72 × 109 | 2.34 × 109 | 2.58 × 109 | 2.62 × 109 | |
| L. acidophilus | 2.98 × 108 | 1.35 × 109 | 2.35 × 109 | 3.55 × 109 | 3.59 × 109 | |
| B. subtilis | 1.78 × 109 | 2.28 × 109 | 2.62 × 109 | 2.65 × 109 | 2.66 × 109 | |
| E.faecalis | 2.20 × 109 | 2.61 × 109 | 2.82 × 109 | 2.90 × 109 | 2.91 × 109 | |
| Sample ID | Input | Filtered | Denoised | Merged | Non-Chimeric | Non-Singleton |
|---|---|---|---|---|---|---|
| La | 77,695 | 71,285 | 69,469 | 63,712 | 44,755 | 42,656 |
| Lp | 132,321 | 122,785 | 119,571 | 109,512 | 72,120 | 70,366 |
| MRS | 57,511 | 53,107 | 51,734 | 47,739 | 34,229 | 32,166 |
| Bs | 57,891 | 53,649 | 52,800 | 50,650 | 36,812 | 35,819 |
| Ef | 56,746 | 52,825 | 51,550 | 47,408 | 36,800 | 36,000 |
| LB | 77,804 | 73,483 | 72,382 | 69,136 | 49,334 | 48,793 |
| Sample | Chao1 | Shannon | Simpson |
|---|---|---|---|
| La | 3555.3 | 8.06856 | 0.958022 |
| Lp | 3532.69 | 7.7823 | 0.961972 |
| MRS | 2582.61 | 8.39776 | 0.964692 |
| Bs | 1839.56 | 6.62015 | 0.88648 |
| Ef | 1512.04 | 6.2814 | 0.904921 |
| LB | 1214.77 | 6.23755 | 0.901051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; He, F.; Yu, D.; Xu, H.; Li, N.; Cao, Z.; Wen, J. 16S rRNA Gene Amplicon Sequencing of Gut Microbiota Affected by Four Probiotic Strains in Mice. Vet. Sci. 2023, 10, 288. https://doi.org/10.3390/vetsci10040288
Ren J, He F, Yu D, Xu H, Li N, Cao Z, Wen J. 16S rRNA Gene Amplicon Sequencing of Gut Microbiota Affected by Four Probiotic Strains in Mice. Veterinary Sciences. 2023; 10(4):288. https://doi.org/10.3390/vetsci10040288
Chicago/Turabian StyleRen, Jianwei, Fang He, Detao Yu, Hang Xu, Nianfeng Li, Zhi Cao, and Jianxin Wen. 2023. "16S rRNA Gene Amplicon Sequencing of Gut Microbiota Affected by Four Probiotic Strains in Mice" Veterinary Sciences 10, no. 4: 288. https://doi.org/10.3390/vetsci10040288
APA StyleRen, J., He, F., Yu, D., Xu, H., Li, N., Cao, Z., & Wen, J. (2023). 16S rRNA Gene Amplicon Sequencing of Gut Microbiota Affected by Four Probiotic Strains in Mice. Veterinary Sciences, 10(4), 288. https://doi.org/10.3390/vetsci10040288




