Antioxidants and Oxidants in Boar Spermatozoa and Their Surrounding Environment Are Associated with AMPK Activation during Liquid Storage
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Semen Handling and Experiment Design
2.2. Assessment of Sperm Quality and Functionality
2.3. Measurement of Antioxidants and Oxidants in Boar Spermatozoa and SF
2.4. Determination of Intracellular ATP, ADP, and AMP Content
2.5. Western Blotting
2.6. Statistical Analysis
3. Results
3.1. Boar Sperm Quality and Functionality Deteriorated with Liquid Storage Time at 17 °C
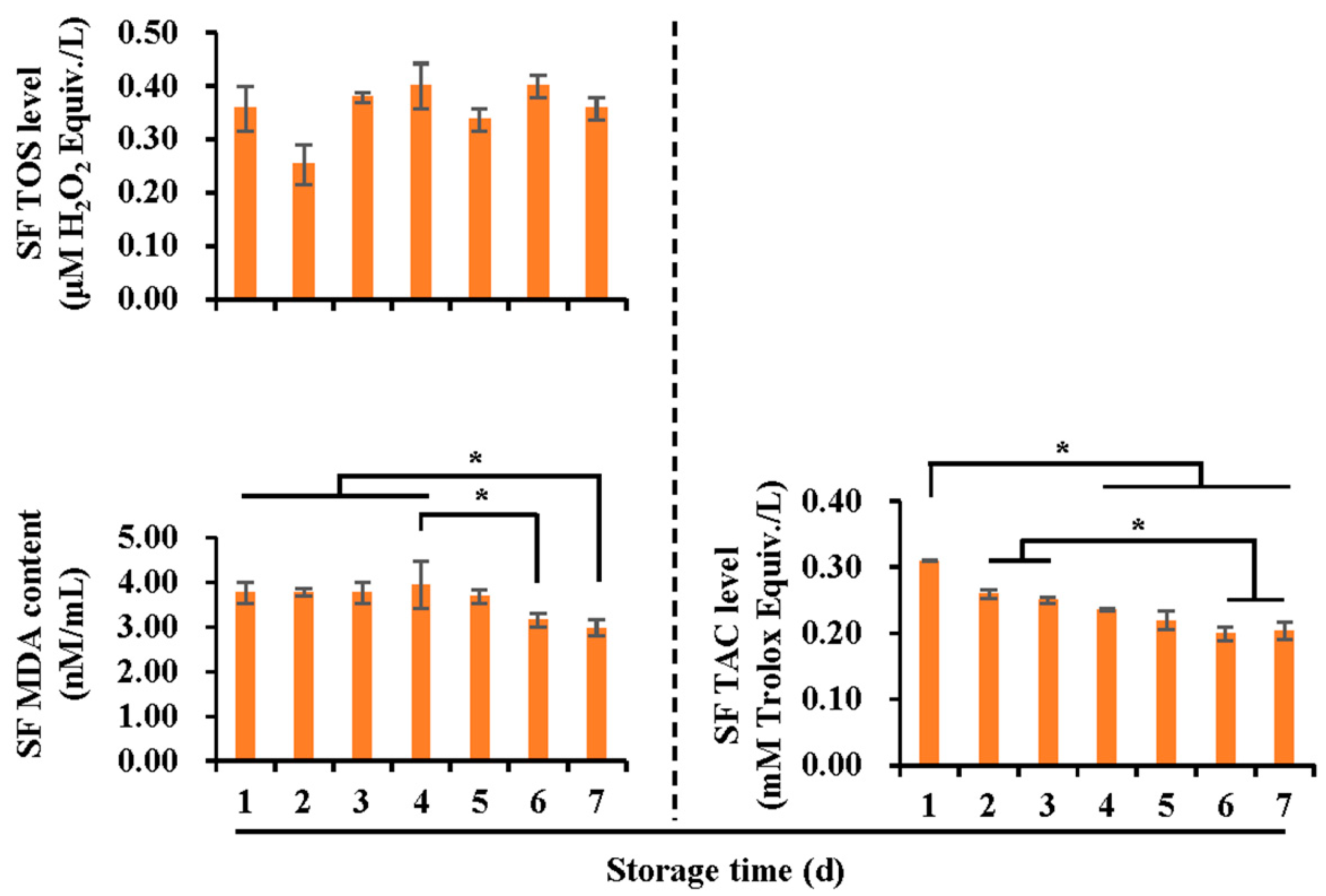
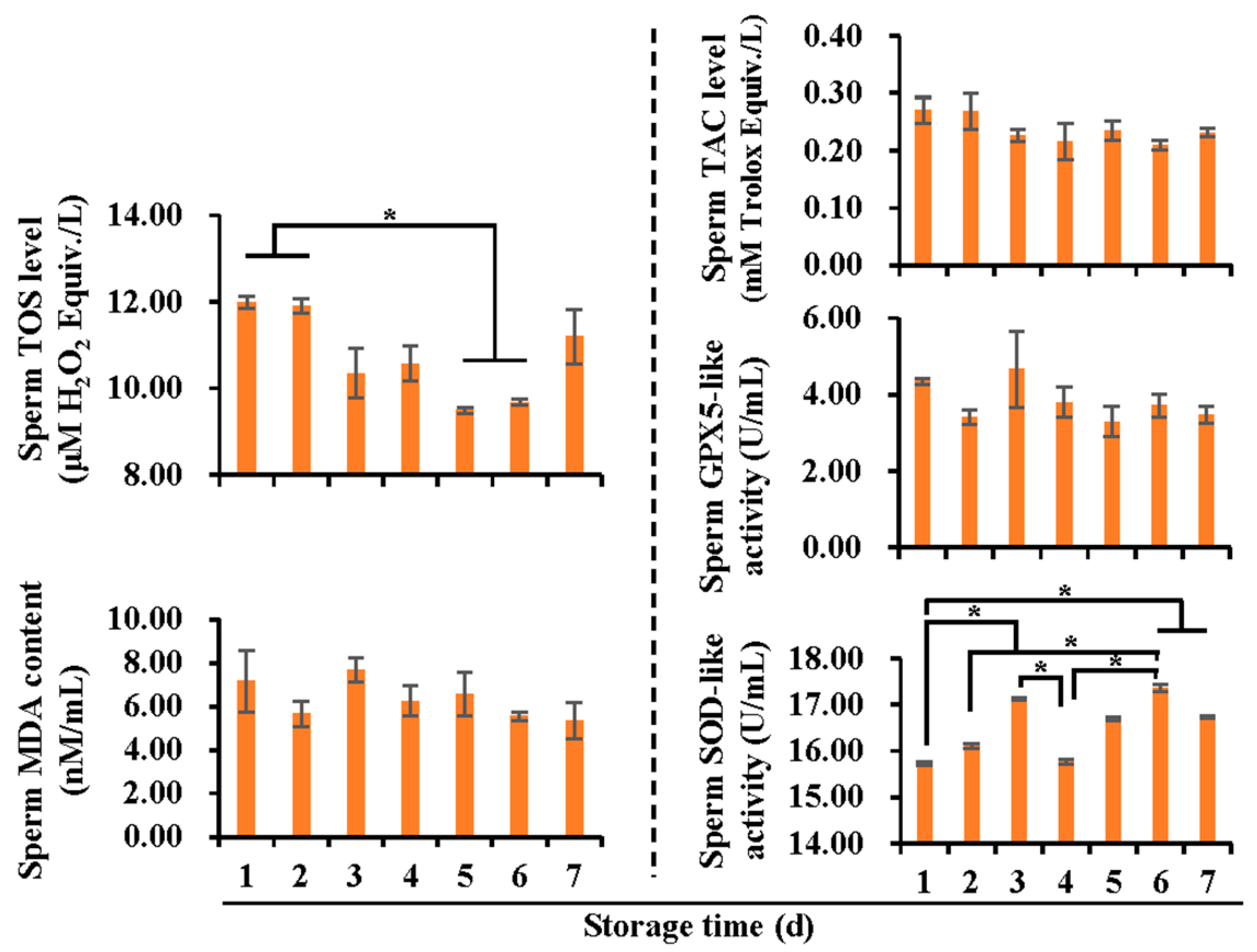
3.2. Effects of Liquid Storage Time at 17 °C on Antioxidant and Oxidant Levels in Boar Spermatozoa and SF
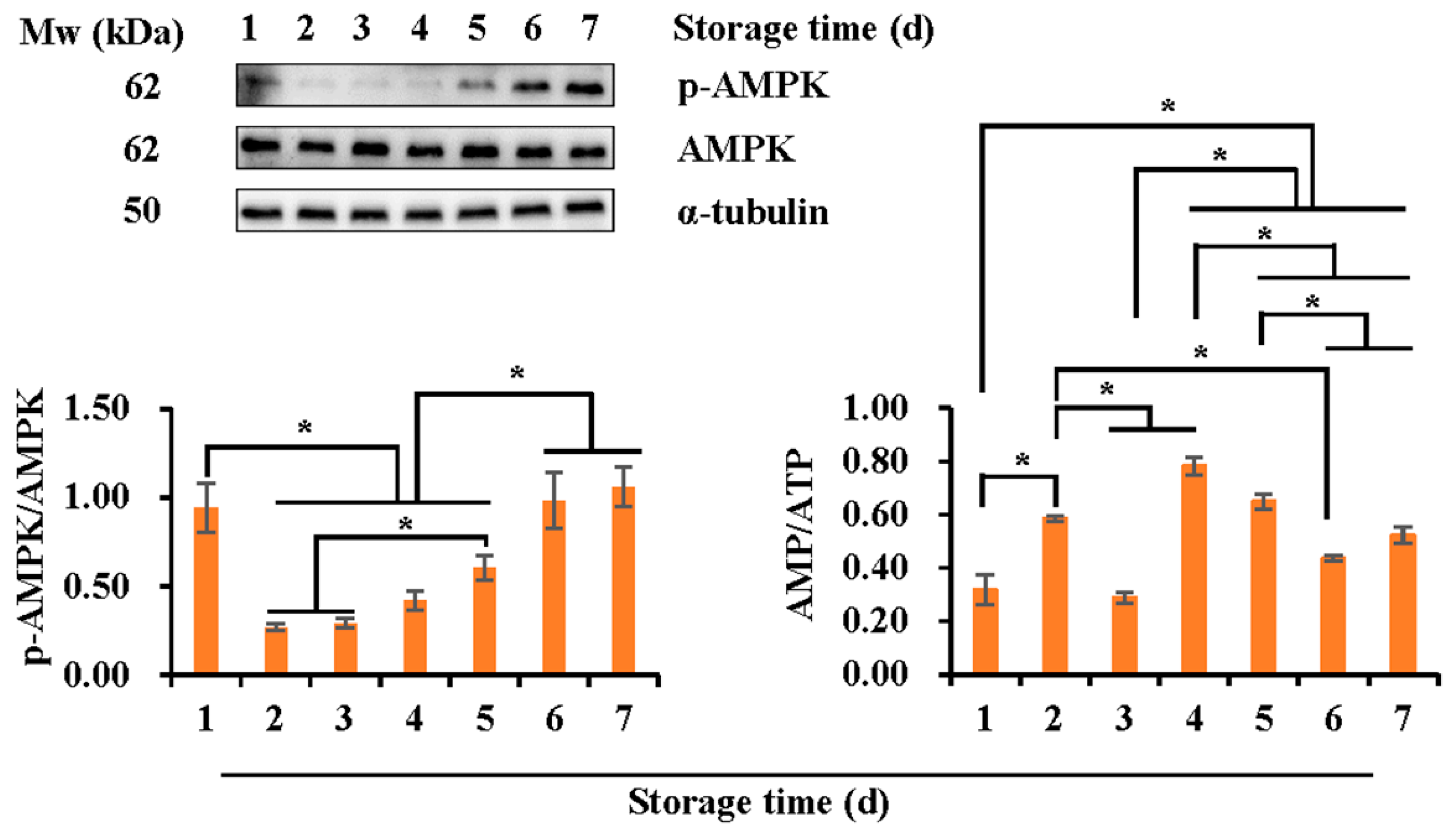
3.3. Effects of Liquid Storage Time at 17 °C on Expression of the Phosphorylated AMPK and the Intracellular AMP/ATP Ratio
3.4. Correlation Analyses
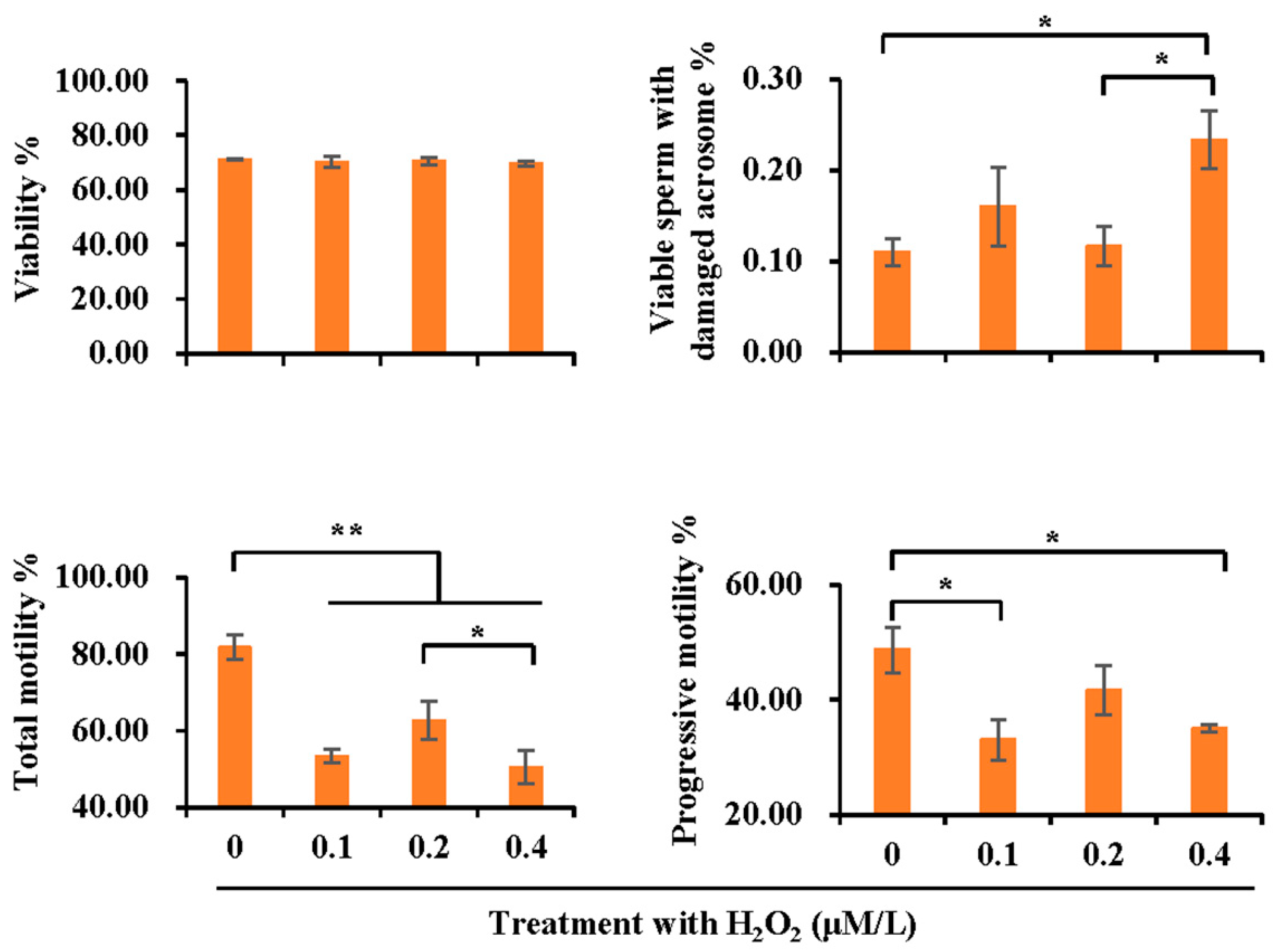
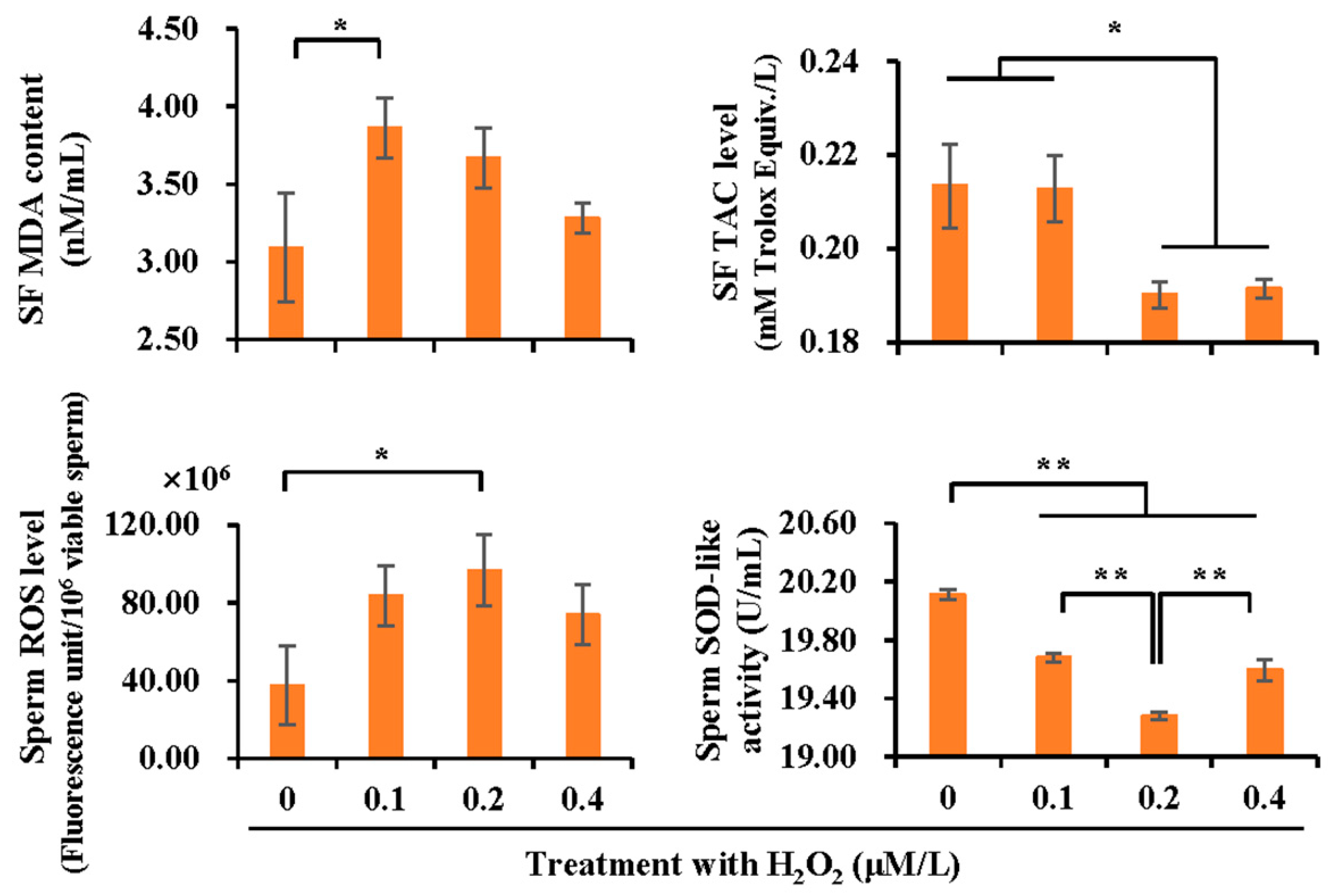
3.5. Effect of H2O2 Treatment on Sperm Quality
3.6. Effect of H2O2 Treatment on Antioxidant and Oxidant Levels in Boar Spermatozoa and SF
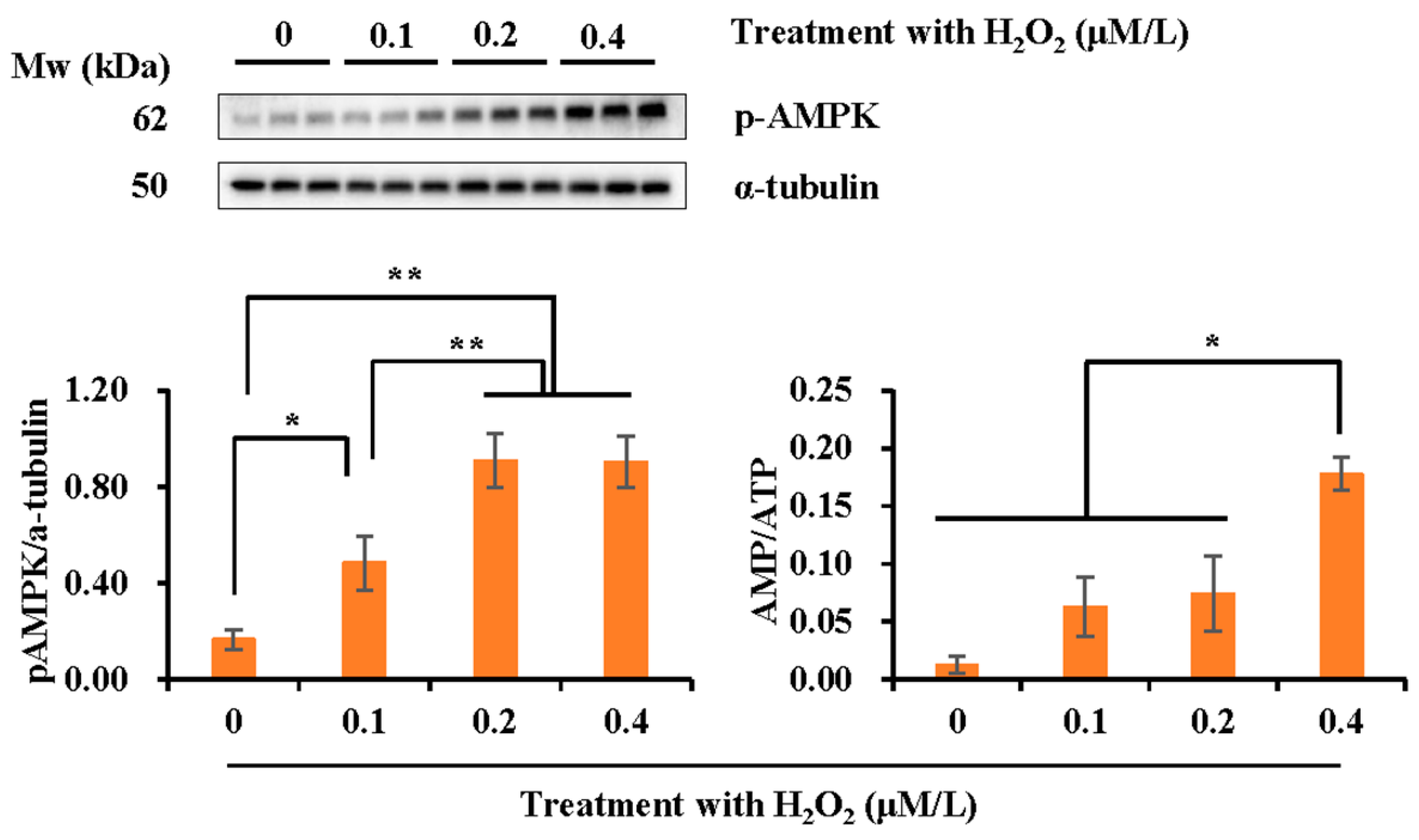
3.7. Effect of H2O2 Treatment on AMPK Phosphorylation and AMP/ATP Ratio of Boar Spermatozoa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knox, R.V. Artificial insemination in pigs today. Theriogenology 2016, 85, 83–93. [Google Scholar] [CrossRef]
- Waberski, D.; Henning, H.; Petrunkina, A.M. Assessment of storage effects in liquid preserved boar semen. Reprod. Domest. Anim. 2011, 46 (Suppl. S2), 45–48. [Google Scholar] [CrossRef]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Barranco, I.; Tvarijonaviciute, A.; Molina, M.F.; Martinez, E.A.; Rodriguez-Martinez, H.; Parrilla, I.; Roca, J. Seminal plasma antioxidants are directly involved in boar sperm cryotolerance. Theriogenology 2018, 107, 27–35. [Google Scholar] [CrossRef]
- Khoi, H.X.; Shimizu, K.; Yoneda, Y.; Minagawa, I.; Abe, Y.; Kuwabara, Y.; Sasanami, T.; Kohsaka, T. Monitoring the reactive oxygen species in spermatozoa during liquid storage of boar semen and its correlation with sperm motility, free thiol content and seasonality. Andrologia 2021, 53, e14237. [Google Scholar] [CrossRef]
- Waheed, M.M.; Gouda, E.M.; Khalifa, T.A. Impact of seminal plasma superoxide dismutase and glutathione peroxidase on cryopreserved buffalo spermatozoa. Anim. Reprod. Sci. 2013, 142, 126–130. [Google Scholar] [CrossRef]
- Gürler, H.; Calisici, O.; Bollwein, H. Inter- and intra-individual variability of total antioxidant capacity of bovine seminal plasma and relationships with sperm quality before and after cryopreservation. Anim. Reprod. Sci. 2015, 155, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Partyka, A.; Lukaszewicz, E.; Niżański, W. Lipid peroxidation and antioxidant enzymes activity in avian semen. Anim. Reprod. Sci. 2012, 134, 184–190. [Google Scholar] [CrossRef]
- Neagu, V.R.; García, B.M.; Rodríguez, A.M.; Ferrusola, C.O.; Bolaños, J.M.; Fernández, L.G.; Tapia, J.A.; Peña, F.J. Determination of glutation peroxidase and superoxide dismutase activities in canine seminal plasma and its relation with sperm quality and lipid peroxidation post thaw. Theriogenology 2011, 75, 10–16. [Google Scholar] [CrossRef]
- Catalán, J.; Yánez-Ortiz, I.; Tvarijonaviciute, A.; González-Aróstegui, L.G.; Rubio, C.P.; Barranco, I.; Yeste, M.; Miró, J. Seminal plasma antioxidants are related to sperm cryotolerance in the horse. Antioxidants 2022, 11, 1279. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; Mateo-Otero, Y.; Delgado-Bermúdez, A.; Bucci, D.; Tamanini, C.; Yeste, M.; Barranco, I. Role of exogenous antioxidants on the performance and function of pig sperm after preservation in liquid and frozen states: A systematic review. Theriogenology 2021, 173, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Jones, K.T.; Robertson, S.A. Reactive oxygen species and sperm function--in sickness and in health. J. Androl. 2012, 33, 1096–1106. [Google Scholar] [CrossRef]
- Martin-Hidalgo, D.; Hurtado de Llera, A.; Calle-Guisado, V.; Gonzalez-Fernandez, L.; Garcia-Marin, L.; Bragado, M.J. AMPK function in mammalian spermatozoa. Int. J. Mol. Sci. 2018, 19, 3293. [Google Scholar] [CrossRef] [PubMed]
- Hurtado de Llera, A.; Martin-Hidalgo, D.; Gil, M.C.; Garcia-Marin, L.J.; Bragado, M.J. AMP-activated kinase AMPK is expressed in boar spermatozoa and regulates motility. PLoS ONE 2012, 7, e38840. [Google Scholar] [CrossRef]
- Tartarin, P.; Guibert, E.; Touré, A.; Ouiste, C.; Leclerc, J.; Sanz, N.; Brière, S.; Dacheux, J.L.; Delaleu, B.; McNeilly, J.R.; et al. Inactivation of AMPKα1 induces asthenozoospermia and alters spermatozoa morphology. Endocrinology 2012, 153, 3468–3481. [Google Scholar] [CrossRef]
- Hurtado de Llera, A.; Martin-Hidalgo, D.; Rodriguez-Gil, J.E.; Gil, M.C.; Garcia-Marin, L.J.; Bragado, M.J. AMP-activated kinase, AMPK, is involved in the maintenance of plasma membrane organization in boar spermatozoa. Biochim. Biophys. Acta 2013, 1828, 2143–2151. [Google Scholar] [CrossRef]
- Shabani Nashtaei, M.; Amidi, F.; Sedighi Gilani, M.A.; Aleyasin, A.; Bakhshalizadeh, S.; Naji, M.; Nekoonam, S. Protective features of resveratrol on human spermatozoa cryopreservation may be mediated through 5′ AMP-activated protein kinase activation. Andrology 2017, 5, 313–326. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Ma, G.; Bai, W.; Fan, X.; Lv, Y.; Luo, J.; Zeng, W. 5′-AMP-activated protein kinase regulates goat sperm functions via energy metabolism in vitro. Cell Physiol. Biochem. 2018, 47, 2420–2431. [Google Scholar] [CrossRef] [PubMed]
- Trefts, E.; Shaw, R.J. AMPK: Restoring metabolic homeostasis over space and time. Mol. Cell 2021, 81, 3677–3690. [Google Scholar] [CrossRef]
- Hinchy, E.C.; Gruszczyk, A.V.; Willows, R.; Navaratnam, N.; Hall, A.R.; Bates, G.; Bright, T.P.; Krieg, T.; Carling, D.; Murphy, M.P. Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. J. Biol. Chem. 2018, 293, 17208–17217. [Google Scholar] [CrossRef]
- Hurtado de Llera, A.; Martin-Hidalgo, D.; Gil, M.C.; Garcia-Marin, L.J.; Bragado, M.J. The calcium/CaMKKalpha/beta and the cAMP/PKA pathways are essential upstream regulators of AMPK activity in boar spermatozoa. Biol. Reprod. 2014, 90, 29. [Google Scholar] [CrossRef]
- Omar, B.; Zmuda-Trzebiatowska, E.; Manganiello, V.; Göransson, O.; Degerman, E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: Roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell Signal. 2009, 21, 760–766. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Combarnous, Y.; Praud, C.; Duittoz, A.; Blesbois, E. Ca2+/calmodulin-dependent protein kinase kinases (CaMKKs) effects on AMP-activated protein kinase (AMPK) regulation of chicken sperm functions. PLoS ONE 2016, 11, e0147559. [Google Scholar] [CrossRef]
- Martinez-Alborcia, M.J.; Valverde, A.; Parrilla, I.; Vazquez, J.M.; Martinez, E.A.; Roca, J. Detrimental effects of non-functional spermatozoa on the freezability of functional spermatozoa from boar ejaculate. PLoS ONE 2012, 7, e36550. [Google Scholar] [CrossRef]
- Alkmin, D.V.; Perez-Patiño, C.; Barranco, I.; Parrilla, I.; Vazquez, J.M.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Boar sperm cryosurvival is better after exposure to seminal plasma from selected fractions than to those from entire ejaculate. Cryobiology 2014, 69, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, H.D.; Welch, G.R. Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in Percoll-treated viable boar sperm using fluorescence-activated flow cytometry. J. Anim. Sci. 2006, 84, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Wallner, U.; Schmicke, M.; Waberski, D.; Henning, H. Energy metabolic state in hypothermically stored boar spermatozoa using a revised protocol for efficient ATP extraction. Biol. Open 2016, 5, 1743–1751. [Google Scholar] [CrossRef]
- Tremoen, N.H.; Gaustad, A.H.; Andersen-Ranberg, I.; van Son, M.; Zeremichael, T.T.; Frydenlund, K.; Grindflek, E.; Våge, D.I.; Myromslien, F.D. Relationship between sperm motility characteristics and ATP concentrations, and association with fertility in two different pig breeds. Anim. Reprod. Sci. 2018, 193, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Dziekońska, A.; Świąder, K.; Koziorowska-Gilun, M.; Mietelska, K.; Zasiadczyk, Ł.; Kordan, W. Effect of boar ejaculate fraction, extender type and time of storage on quality of spermatozoa. Pol. J. Vet. Sci. 2017, 20, 77–84. [Google Scholar] [CrossRef]
- Dziekonska, A.; Fraser, L.; Majewska, A.; Lecewicz, M.; Zasiadczyk, L.; Kordan, W. Effect of commercial long-term extenders on metabolic activity and membrane integrity of boar spermatozoa stored at 17 degrees C. Pol. J. Vet. Sci. 2013, 16, 517–525. [Google Scholar] [CrossRef]
- Cerolini, S.; Maldjian, A.; Surai, P.; Noble, R. Viability, susceptibility to peroxidation and fatty acid composition of boar semen during liquid storage. Anim. Reprod. Sci. 2000, 58, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Xue, L.; Cao, J.; Xie, Y.; Xiao, T.; Fang, S. Caffeic acid phenethyl ester (CAPE) improves boar sperm quality and antioxidant capacity in liquid preservation (17 °C) linked to AMPK activity maintenance. Front. Vet. Sci. 2022, 9, 904886. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Xie, Y.; Pan, J.; Chen, Q.; Xiao, T.; Fang, S. The antibacterial and antioxidant roles of buckwheat honey (BH) in liquid preservation of boar semen. Biomed. Res. Int. 2021, 2021, 5573237. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kou, Z.; Hu, B.; Li, Y.; Cai, R.; Gao, L.; Chu, G.; Yang, G.; Pang, W. Boar seminal plasma improves sperm quality by enhancing its antioxidant capacity during liquid storage at 17 °C. Zygote 2022, 30, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Baker, M.A.; De Iuliis, G.N.; Nixon, B. New insights into sperm physiology and pathology. Handb. Exp. Pharmacol. 2010, 198, 99–115. [Google Scholar]
- Das, S.; Nandi, P.R.; Sarkar, P.; Tudu, K.C.; Rai, S.; Behera, R.; Mandal, A.; Mondal, M.; Karunakaran, M. Effect of superoxide dismutase, catalase, and glutathione reductase supplementation on cryopreservation of Black Bengal buck semen. Trop. Anim. Health Prod. 2021, 53, 552. [Google Scholar] [CrossRef]
- Martin-Hidalgo, D.; Hurtado de Llera, A.; Yeste, M.; Cruz Gil, M.; Bragado, M.J.; Garcia-Marin, L.J. Adenosine monophosphate-activated kinase, AMPK, is involved in the maintenance of the quality of extended boar semen during long-term storage. Theriogenology 2013, 80, 285–294. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.E.; Xu, H.E.; Melcher, K. Structure and Physiological Regulation of AMPK. Int. J. Mol. Sci. 2018, 19, 3534. [Google Scholar] [CrossRef]
- Niki, E. Assessment of antioxidant capacity of natural products. Curr. Pharm. Biotechnol. 2010, 11, 801–809. [Google Scholar] [CrossRef]
- Barranco, I.; Tvarijonaviciute, A.; Perez-Patiño, C.; Parrilla, I.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. High total antioxidant capacity of the porcine seminal plasma (SP-TAC) relates to sperm survival and fertility. Sci. Rep. 2015, 5, 18538. [Google Scholar] [CrossRef]
- He, Y.; Li, D.; Zhang, W.; Tian, X.; Pang, W.; Du, R.; Yang, G.; Yu, T. Boar sperm quality and oxidative status as affected by rosmarinic acid at 17 °C. Trop. Anim. Health Prod. 2020, 52, 2169–2177. [Google Scholar] [CrossRef]
- Sun, L.; Fan, X.; Zeng, Y.; Wang, L.; Zhu, Z.; Li, R.; Tian, X.; Wang, Y.; Lin, Y.; Wu, D.; et al. Resveratrol protects boar sperm in vitro via its antioxidant capacity. Zygote 2020, 28, 417–424. [Google Scholar] [CrossRef]
- Wysocki, P.; Orzołek, A.; Strzeżek, J.; Koziorowska-Gilun, M.; Zasiadczyk, Ł.; Kordan, W. The activity of N-acetyl-β-hexosaminidase in boar seminal plasma is linked with semen quality and its suitability for cryopreservation. Theriogenology 2015, 83, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zhu, Z.; Bai, W.; Li, R.; Zheng, Y.; Tian, X.; Wu, D.; Lu, H.; Wang, Y.; Zeng, W. Proline Protects Boar Sperm against Oxidative Stress through Proline Dehydrogenase-Mediated Metabolism and the Amine Structure of Pyrrolidine. Animals 2020, 10, 1549. [Google Scholar] [CrossRef]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.K.; Talevi, R. Mitochondrial dysfunction and oxidative stress caused by cryopreservation in reproductive cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Shen, H.M. Critical role of AMPK in redox regulation under glucose starvation. Redox Biol. 2019, 25, 101154. [Google Scholar] [CrossRef]
- Zmijewski, J.W.; Banerjee, S.; Bae, H.; Friggeri, A.; Lazarowski, E.R.; Abraham, E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J. Biol. Chem. 2010, 285, 33154–33164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Y.; Wu, R.Y.; Zhao, Y.; Xu, C.S.; Zhang, W.D.; Ge, W.; Liu, J.; Sun, Z.Y.; Zou, S.H.; Shen, W. Ochratoxin A exposure decreased sperm motility via the AMPK and PTEN signaling pathways. Toxicol. Appl. Pharmacol. 2018, 340, 49–57. [Google Scholar] [CrossRef]
- Li, R.N.; Zhu, Z.D.; Zheng, Y.; Lv, Y.H.; Tian, X.E.; Wu, D.; Wang, Y.J.; Zeng, W.X. Metformin improves boar sperm quality via 5′-AMP-activated protein kinase-mediated energy metabolism. Zool. Res. 2020, 41, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.Y.; Lv, D.L.; Zhang, X.; Du, Y.Q.; Yuan, Y.T.; Chen, M.J.; Xi, H.M.; Li, Y.; Han, N.; Hu, J.H. Rosmarinic acid improves boar sperm quality, antioxidant capacity and energy metabolism at 17 °C via AMPK activation. Reprod. Domest. Anim. 2020, 55, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, R.C.; Samborska, B.; Faubert, B.; Ma, E.H.; Gravel, S.P.; Andrzejewski, S.; Raissi, T.C.; Pause, A.; St-Pierre, J.; Jones, R.G. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 2017, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jager, S.; Handschin, C.; Pierre, J.; Spiegelman, B. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1 alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
| Liquid Storage Time at 17 °C (d) | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Total Motility (%) | 71.92 ± 3.87 | 58.36 ± 4.85 | 51.96 ± 5.82 | 51.80 ± 5.85 | 57.92 ± 5.65 | 57.96 ± 5.48 | 54.60 ± 5.14 |
| Progressive Motility (%) | 45.44 ± 3.06 | 40.20 ± 3.60 | 35.96 ± 4.12 | 34.32 ± 4.05 | 37.60 ± 3.62 | 39.12 ± 3.92 | 35.60 ± 3.37 |
| Viability (%) | 74.20 ± 1.24 ac | 74.78 ± 1.25 a | 74.51 ± 1.22 a | 70.28 ± 1.57 b | 72.28 ± 1.55 ab | 70.55 ± 1.24 bc | 69.80 ± 1.52 b |
| Damaged acrosomal membrane in viable sperm (%) | 1.54 ± 0.29 | 1.35 ± 0.32 | 1.12 ± 0.18 | 1.84 ± 0.38 | 1.76 ± 0.31 | 1.31 ± 0.22 | 1.38 ± 0.24 |
| Intracellular ROS production (106 Fluorescence unit/106 viable sperm) | 20.22 ± 1.72 a | 7.33 ± 0.74 bc | 11.92 ± 1.38 cd | 10.88 ± 1.26 bcd | 11.92 ± 0.94 ad | 7.32 ± 0.53 b | 6.81 ± 0.56 b |
| Mitochondrial membrane potential (%) | 55.90 ± 6.84 ab | 63.26 ± 5.05 a | 48.36 ± 5.21 ab | 38.93 ± 5.09 ab | 60.00 ± 5.49 a | 35.60 ± 5.59 b | 40.87 ± 4.48 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhao, W.; Zhu, J.; Ju, H.; Liang, M.; Wang, S.; Chen, S.; Ferreira-Dias, G.; Liu, Z. Antioxidants and Oxidants in Boar Spermatozoa and Their Surrounding Environment Are Associated with AMPK Activation during Liquid Storage. Vet. Sci. 2023, 10, 214. https://doi.org/10.3390/vetsci10030214
Li J, Zhao W, Zhu J, Ju H, Liang M, Wang S, Chen S, Ferreira-Dias G, Liu Z. Antioxidants and Oxidants in Boar Spermatozoa and Their Surrounding Environment Are Associated with AMPK Activation during Liquid Storage. Veterinary Sciences. 2023; 10(3):214. https://doi.org/10.3390/vetsci10030214
Chicago/Turabian StyleLi, Junwei, Wenming Zhao, Jiaqiao Zhu, Huiming Ju, Ming Liang, Shuaibiao Wang, Shufang Chen, Graça Ferreira-Dias, and Zongping Liu. 2023. "Antioxidants and Oxidants in Boar Spermatozoa and Their Surrounding Environment Are Associated with AMPK Activation during Liquid Storage" Veterinary Sciences 10, no. 3: 214. https://doi.org/10.3390/vetsci10030214
APA StyleLi, J., Zhao, W., Zhu, J., Ju, H., Liang, M., Wang, S., Chen, S., Ferreira-Dias, G., & Liu, Z. (2023). Antioxidants and Oxidants in Boar Spermatozoa and Their Surrounding Environment Are Associated with AMPK Activation during Liquid Storage. Veterinary Sciences, 10(3), 214. https://doi.org/10.3390/vetsci10030214








