Simple Summary
Claw health condition is of major importance for modern hyperprolific sow productivity and longevity and pig operations profitability. Infrared technologies (IRTs) have been used in human medicine and recently in the claw health and foot temperature distribution evaluation of purebred sows. The current study presents the results of monitoring claw length, claw lesion, anisodactylia, backfat thickness, mobility and reproductive performance evolution from weaning age to second parturition in replacement gilts of different genetic lines (PIC, DANBRED and TOPIGS) in three farrow-to-finish herds in Greece, concurrently with temperature distribution evaluation using infrared technologies. The majority of the assessed characteristics showed statistical differences across herds at the first and second parturition, as well as at the weaning age. The fact that the three herds’ housing, flooring, management and feeding procedures were essentially the same suggests that there may be genetic variation in the analyzed parameters’ conformation. However, more research is necessary to clarify the relationships between the temperature distribution of the feet and the claw health indicators, mobility, backfat thickness and reproductive efficiency. The development of infrared technology as a practical, accurate and dependable technique for the early diagnosis of claw health issues in replacement gilts may be crucial for practicing veterinarians.
Abstract
Feet infrared temperature is associated with feet health and may affect the reproductive performance of sows. In total, 137, 98 and 114 replacement gilts were selected at the age of weaning from 3 herds—A, B and C—with different genetic lines. Dorsal claw length was measured, and anisodactylia was measured in all four feet, at weaning age, and at those gilts that completed their first and second farrowing. At the first and second farrowing stage, the infrared temperature distribution, dew/claw length and backfat thickness were measured concurrently with claw lesion and mobility score evaluation. The maximum temperature significantly differed (p < 0.01) among herds, in the rear feet and in all four feet at the first and second farrowing respectively. Claw lengths statistically differed among herds at all stages (p < 0.05). Anisodactylia in rear feet was lower in herd A (p < 0.05) at weaning, and in herd C at the first and second farrowing (p < 0.05). In addition, the claw lesion score, mobility, backfat thickness and reproductive performance statistically differed among herds (p < 0.05). It is shown that even at an early stage of their reproductive life, claw length differences exist in replacement gilts of different genetic lines.
1. Introduction
Claw health in modern hyperprolific sows is important for their longevity, productivity and welfare. It is well recognized that sows with lameness experience pain and that their well-being is compromised. One of the primary reasons of sow lameness is the presence and severity of claw lesions [1]. Inconsistencies in the hoof formation, such as overgrowth and differences in the length of the medial and lateral claws, have been linked to lameness and claw abnormalities [2,3]. Claw length disparities have been associated with an increase in claw lesions frequency [4]. Claw lesion estimation is a labor- and time-intensive process, and clinical lameness can only be visually observed when a major claw lesion has occurred. The pig business might benefit from a method for the early diagnosis of affected damaged tissue in feet [5]. As a helpful non-invasive technology, infrared thermography (IRT) has been widely employed in human and veterinary medicine for diagnostic purposes [6,7,8,9]. Changes brought on by the progression of inflammation in the underlying tissues can be quickly identified using IR technology [10]. Other studies have connected changes in surface temperature to increased blood flow to tissues during inflammatory conditions [10,11,12]. The use of IRT to recognize lameness in livestock has increased largely in the last years since it represents a non-invasive method, ease of automation and low cost [13,14].
In sows, claw lesions are common, and their evaluation has been under debate in recent years. A proposed categorization system for claw lesions has been employed in the past [15]. It is based on a rating scale and includes fractures in the wall and toe, white line and heel–sole junction disruptions, heel curtailments and overgrowth. Others have used a scale with three or four [16] criteria to evaluate the length of the claw and dew claw, as well as lesions. More recently, a scoring system applied was “Zeugenklauwencheck” [16] and the Zinpro Foot First method [17].
It can be hypothesized that sows with abnormalities in claw length concurrently with increased incidence of claw lesions, would affect their ability to move as a result. Claw overgrowth and lesions have been linked to mobility difficulties in sows [2,18], as have weight distribution changes to the heel in cases of enlarged nails, which makes walking difficult [19]. Overgrowth of weight-bearing claws can affect sows’ lying down, postural and feeding behavior, with a negative impact on sows’ welfare and productivity [20]. Thus far, comparison of mobility issues together with claw length abnormalities and claw lesions has not been extensively evaluated in gilts of different genetic backgrounds. As mobility and claw health issues are a common reason for sow cull from a herd, it would be worthwhile to investigate whether such issues exist and persist from the beginning of the reproductive life of replacement gilts and whether differences exist among genetic lines. Furthermore, it could be hypothesized that gilts with claw abnormalities would have impaired mobility, either at the gestation group pens or at the lactation crates, which might subsequently affect their reproductive performance. Under such conditions, it is plausible that a gilt or a sow might be reluctant to frequently stand up to consume feed. Thus, during the high-metabolic-demand period of lactation, increased backfat tissue mobilization might occur.
Therefore, the current study’s aim was to further examine, using IRT devices, the temperature gradient in the lower front and rear feet of replacement gilts, while also monitoring the hypertrophy of their claws, the evolution of anisodactylia, the occurrence of claw lesions, the depth of their backfat, their mobility and their reproductive efficiency.
2. Materials and Methods
2.1. Herds and Gilts Included in the Study
The recent investigation with purebred sows that took part in the same research effort was done in the same herds as the current study [21]. In short, herds were situated in the Prefecture of Larisa (Herd A and C) and Kozani (Herd B), and the purebred breeding sows belonged to the PIC, TOPIGS and DANBRED genetic lines, respectively. Further, the housing, flooring and managerial practices at the insemination, lactation and gestation facilities were almost similar among herds, as described in detail in the aforementioned study. At the age of weaning, 349 crossbred replacement gilts were randomly selected, including 137, 98 and 114 (in herds A, B and C, respectively). Among the replacement gilts, 132, 84 and 110 finished their first farrowing (in herds A, B and C, respectively). In herds A, B, and C, there were 55, 68 and 47 individuals that finished the second farrowing.
2.2. Housing and Management
Housing and management at the insemination, gestation and lactation facilities are described in detail in the aforementioned study [21].
2.3. IRT Measurements
We followed the measurement procedure as previously described in our recent publication on purebred GP sows [21]. The areas of interest in each foot are shown in Figure 1.
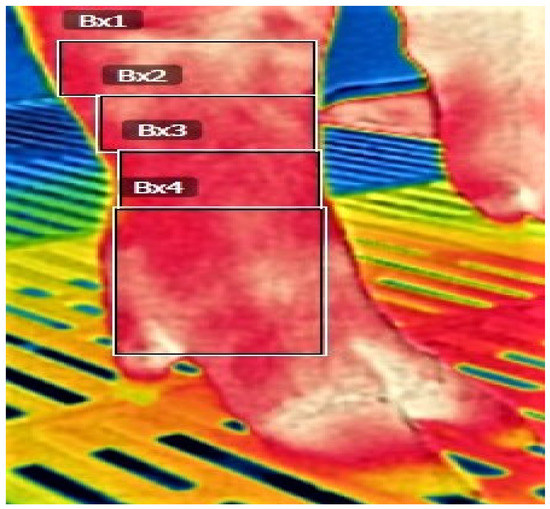
Figure 1.
Areas of temperature measurement by IR camera in sows of different genetic lines in three farrow-to-finish herds in Greece: IRT1(Bx1) Carpus-Tarsus, IRT2(Bx2) Upper metacarpi/metatarsi, IRT3(Bx3) Lower metacarpi/metatarsi, IRT4(Bx4) Phalanges.
2.4. Claw Length Measurements and Anisodactylia
At weaning day, the claw lengths of all female piglets selected as replacement gilts were measured, and all relevant information was recorded on forms together with an ID number and the day of the birth. In total, 137 female piglets from herd A, 98 from herd B and 114 from herd C were included. The claw length measurements were performed also after farrowing in those gilts that completed their first and second farrowing. In this manuscript, we present the results only for dorsal claw length.
2.5. Claw Lesion Scoring
When gilts were in the standing-up posture in the crates during the final week of lactation both at the first and second farrowing, the medial and lateral toes of every hoof and the dew claws were separately evaluated for lesions and graded. Using three classification scales with ratings varying from 0 to 2, a modified scoring method was utilized, based on previous studies [2,16,17]; see Table 1 and Figure 2.

Table 1.
A modified scoring system for evaluation of claw and dew claw lesions, in sows of different genetic lines in three farrow-to-finish herds in Greece.
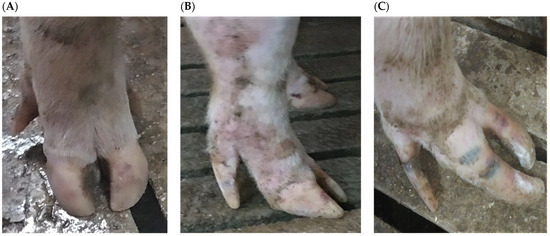
Figure 2.
Examples of lesion scoring in sows of different genetic lines in three farrow-to-finish herds in Greece: (A) Claw: 0; Wall: 0; Coronary band: 0; Dew claw: 0. (B) Claw: 1; Wall: 1; Coronary band: 0; Dew claw: 1. (C) Claw: 2; Wall: 2; Coronary band: 1; Dew claw: 2.
2.6. Back Fat Measurements
Backfat tissue length was measured at the P2 position (6.5 cm from the backbone, left and right side) with a portable ultrasound device (Renco, Minneapolis, MN, USA). Gilts were measured at insemination, while the sow entered the farrowing pen, and on the weaning day.
2.7. Mobility Scoring
Sow lameness was estimated after the completion of the lactation period. Sows were video recorded for 3–4 min, using a portable digital camera (Sony, HDR-CX240E, Handycam, Tokyo), while they walked on the concrete floor in an alley of 5–10 m and on the slatted floor of gestation pens. A person would walk beside the sows and give verbal cues or wave his hands to get them moving if necessary. Their degree of lameness was scored at a later time. Sows were provided with a score of 1 (no lame) to 4 (unable to move), using an observation movement scoring system on a 4-point scale modified from Gregoire at al. [22]:
Score 1: The sow moves with even steps, is easy to move around, feels comfortable on all four feet and requires nothing in the way of incentive.
Score 2: Uneven gait length, stiffness and fewer smooth motions are noticed, but no observable lameness is evident.
Score 3: Shortened stride, one leg is found to be lame, the caudal body swaggers and compensatory actions are all observed in the sow as she walks (head drooping, back raised)
Score 4: More than one leg is lame, the sow is hesitant to put weight on the limbs that are afflicted, she does not rest the limb on the ground, and she is hesitant and unable to move.
2.8. Reproductive Performance
Reproduction data of each herd participating in the study were kept for all reproductive parameters, such as insemination date, expected farrowing date, farrowing date, total born piglets, born alive piglets, fostering between sows, lactation length, weaned piglets and age at the first farrowing.
2.9. Statistical Analysis
The Statistical Package for the Social Sciences (SPSS) program was used to examine the data (SPSS 25.0 Version, Chicago, IL, USA). The threshold for statistical significance was set at p = 0.05. With herds acting as a fixed factor in the one-way analysis of variance of the general linear model procedure of SPSS, differences among herds in terms of IRT, claw length, claw lesion, anisodactylia, backfat thickness and reproductive performance measurements were studied. Kolmogorov–Smirnov and Shapiro–Wilk tests were performed in order to evaluate the normal distribution of data, and post-hoc assessments among herds were then done using Tukey’s test. The data are displayed as mean standard deviation (SD).
3. Results
3.1. IRT Recordings
Table 2 displays the highest temperatures recorded during IRT recordings (mean SD) in the four lower-foot body sites of each sow at the first farrowing. The maximum temperature in IRT1, IRT2, IRT3 and IRT4 of almost all rear feet, with the exception of IRT4 in the rear right foot, were lower in herd A and higher in herd B (p < 0.001) at the first farrowing. All recorded temperatures values, in all three herds, at the first farrowing were numerically higher in rear feet than in the front.

Table 2.
Results of maximum temperature (mean ± SD) in each foot at four areas in replacement gilts of different genetic lines in three farrow-to-finish herds in Greece at first farrowing.
Maximum IRT temperatures in both the front and rear feet for all regions estimated (IRT1, IRT2, IRT3 and IRT4) were different among herds, being the lowest in herd B compared to A and C (p < 0.001) Table 3. All recorded temperature values, in all three herds, at the second farrowing were numerically higher in rear feet than in the front.

Table 3.
Results of maximum temperature (mean ± SD) in each foot at four areas in replacement gilts of different genetic lines in three farrow-to-finish herds in Greece at second farrowing.
3.2. Claw Length Measurements and Anisodactylia
The mean values (±SD) of the dorsal claw lengths of the medial and lateral claw of the front and rear feet at the weaning stage and at the first and second farrowing are shown in Table 4. At weaning, the dorsal claw lengths in all four feet statistically were lower in herd B than in A and C (p < 0.001). At the first farrowing, the claw lengths in both the front and rear feet were lower in herd C compared to A and B (p < 0.001). At the second farrowing, the dorsal claw lengths were also lower in herd C compared to A and B (p < 0.001).

Table 4.
Results of dorsal claw length (mm) (mean ± SD) at weaning and at first and second farrowing in replacement gilts of three different genetic lines in three farrow-to-finish herds in Greece.
In Table 5, the results regarding anisodactylia in each one of the four feet of the replacement gilts on the weaning day and at the first and second farrowing in the three herds are presented. Anisodactylia was lower in herd A at weaning than in herds B and C (p < 0.001). At the first farrowing, herd B had higher front feet than herds A and C (p < 0.001), and herd A had higher rear feet than herds B and C (p < 0.001). At the second farrowing, anisodactylia in the front left foot and both rear feet was higher in herd A (p < 0.001).

Table 5.
Results of anisodactylia (mm) (mean ± SD) in replacement gilts of different genetic lines in three farrow-to-finish herds in Greece, at weaning age and at first and second farrowing.
3.3. Claw Lesion Score
The total claw lesion score in each foot, the count of lesion scoring in the region of interest (claw, wall, coronary band and dew claw) in all four feet, was the highest in herd A (p < 0.001) at the first farrowing. In addition, at both feet, the medial and lateral claw lesions were significantly higher in herd A compared to B and C (p < 0.001). At the second farrowing, the total feet lesion score was the highest in herd A (p < 0.001), and for the front-rear feet, the medial-lateral claw lesion scores were greater in herd B compared to A and C (p < 0.001) Table 6.

Table 6.
Results of claw lesions (mean ± SD) in replacement gilts of different genetic lines in three farrow-to-finish herds in Greece, at first and second farrowing.
3.4. Backfat Measurement and Backfat Loss
Table 7 presents the backfat thickness at the farrowing and weaning day, as well as the backfat loss and the backfat loss % at the first and second farrowing of sows. At the first farrowing, the backfat thickness, backfat loss and backfat loss % significantly differ among herds, being higher in herd B than in herd A and C (p < 0.001). At the second farrowing, herd B is the herd with the statistically highest values for all measured backfat parameters among all three herds (p < 0.05).

Table 7.
Results of backfat thickness (mm), backfat loss (mm) and backfat loss % (mean ± SD) of replacement gilts of different genetic lines in three farrow-to-finish herds in Greece, at farrowing and weaning, and at first and second farrowing.
3.5. Mobility Score
The mobility score of sows was analyzed as the mean score of the three assessment scores. Mobility scoring was higher in herd A compared to B and C (p < 0.001 in the first farrowing; p = 0.002 in the second farrowing) Table 8.

Table 8.
Results of mobility score (mean ± SD) of replacement gilts of different genetic lines in three farrow-to-finish herds in Greece, at first and second farrowing.
3.6. Reproductive Performance
Table 9 shows the total born, born alive and weaned piglets for the first and second farrowing. At the first farrowing, the litter size parameters were greater in herd B than in A and C (p < 0.001). At the second farrowing, the reproductive parameters differed among the three herds, with herd B showing the best performance indexes (p < 0.001).

Table 9.
Average values of total born, born alive and weaned piglets at first and second farrowing in sows of different genetic lines in three farrow-to-finish herds in Greece.
4. Discussion
The annual replacement rate of the sow reproduction population within each herd may reach up to 40%, originating from replacement gilts. In our previous investigation [21], we have shown that significant differences occurred among purebred sows of different genetic lines regarding the claw lengths, lower feet infrared temperature distribution and occurrence of anisodactylia. Based on these findings, we investigated the hypothesis if the replacement gilts originating from the same purebred sows would show similar claw abnormalities, starting from their selection at weaning age up to the completion of their second farrowing. The results showed that those gilts exhibiting claw length differences and higher lesion scores were prone to greater mobility issues. Meanwhile, reproductive performance indexes, such as backfat tissue mobilization during lactation and litter size at birth and at weaning, were collected. To our knowledge, this is the first study evaluating the claw condition in replacements and its effects on reproductive performance in the first two reproductive cycles. The results indicate that selection of a genetic line should not be focused only on litter size characteristics, but also on claw health condition.
In our investigation, other anatomical areas besides phalanges may be more vascularized, and hence, IRT may be able to show changes in the local metabolic rate and blood flow more clearly [18,23]. There was a substantial difference among the analyzed genetic lines in both the first and second farrowing, according to the measured IRT temperature distribution in the replacement gilts from three distinct herds. The IRT distribution in gilts at the first and second farrowing were in accordance with our findings in purebred sows’ feet temperature evaluation in our recent study [21]. It should be highlighted that in the four sites of focus, sows’ highest IRT temperatures were numerically higher in the posterior than the front feet (IRT1, IRT2, IRT3 and IRT4), which is consistent with previous reports from purebred sows [21]. It is possible that variations in the distribution of weight bearing are to blame for the greater IRT temperatures found in sows’ rear feet as opposed to their front feet. The formation of claw lesions in various feet in sows is greatly influenced by their weight distribution [24,25]. Interestingly, the rear feet lesions score was numerically higher than the front feet lesions score in the first and second farrowing. Thus, it can be suggested that differences in weight distribution exist even from the first farrowing in the replacement gilts, and differences in lesion scores are evident among different genetic lines.
Our findings for claw lengths at weaning are the first published data showing a range of mean values for claw lengths between 10.43 mm and 26.89 mm for front feet and 9.59 mm and 27.27 mm for rear feet. At the first and second farrowing, the lowest claw lengths were observed in herd C compared to A and B. Fick (2014) [26] reported sow lateral and medial dorsal claw length measurements that were shorter than ours. In terms of anisodactylia, there were also numerical differences in favor of the rear foot. This is consistent with earlier discoveries [27]. In contrast to our findings on the second farrowing, for the rear legs, Newman et al. [28] showed that enlarged and normal claw lengths ranged from 51 mm to 79 mm and 38 mm to 50 mm, respectively. Sasaki et al. [4] reported toe size measurements for the medial and lateral claws that ranged from 45.0 mm to 45.1 mm and 46.20 mm to 46.60 mm, respectively, in research that only examined the rear feet of gestating sows. These results are different from our findings at the first (55.16 mm and 58.10 mm for the medial and lateral rear feet claw, respectively) and second farrowing (62.92 mm and 66.52 mm for the medial and lateral rear feet claw, respectively). Sows with a toe length higher than 63 mm were categorized as having overgrowth in a study by Fitzgerald et al. [29]. Our data suggest that gilts from herds B and C, particularly when compared to those from herd A, have enlarged claws.
According to recent studies, the average increase of claws ranged from 0.21 mm/day for gilts to 0.39 mm/day for sows [30,31], and while the wear rate decreased to 0.17 mm/day, the inevitable claw length increased steadily and got shorter with age [31]. When compared to Yorkshire sows, the lateral toes of Duroc and Crossbred (Duroc Yorkshire) sows grow more quickly; the dorsal toes of sows at parity two grow the fastest, followed by those at parity one and three [31]. In our study, the mean growth rate (growth minus wear) for dorsal claw length was about 0.114 mm/day, 0.109 mm/day and 0.096 mm/day for herd A, B and C, respectively, slightly different to the findings of the latter studies for the first (weaning to first farrowing) and second (first to second farrowing) evaluated periods. These results may be indicative of a genetic influence in claw horn growth, which warrants further investigation.
Claws that are too long may cause sows to dedicate less time to eating [29]. It is known that feed consumption should be maintained at high levels during lactation to avoid excessive body weight and backfat loss due to increased milk production. It has been described that lame sows may have impaired body condition compared to non-lame ones consuming the same amount of feed [32]. Furthermore, cytokines derived from inflammatory procedures may not only be involved in connective tissue degradation, which worsens lameness, but may also influence the action of hormones involved in reproduction [32]. Significant inflammation and epithelial alterations may be linked to claw overgrowth [27,28]. Claw lesions have also been linked to poor litter performance [3,29]. As revealed by our findings, those gilts that showed higher backfat tissue loss during both lactation periods originated from herd B. The same gilts showed a higher lesion score at the rear and lateral claws at the second farrowing, but did not exhibit higher anisodactylia or IRT measurements compared to those from herds A and C. It should be noted that gilts from herd B had the best piglet performance indexes. Thus, it could be hypothesized that gilts may mobilize backfat tissue during lactation to support litter growth independently of claw length differences or overall feet condition. Nevertheless, this field warrants further investigation analyzing the relationships among the aforementioned parameters.
Lameness prevalence in all three herds at the first farrowing were 18.9% of total gilts and at the second, 48%. To estimate the prevalence of lameness, gilts with a locomotor value of greater than two were labeled as lame. The gilts originating from herd A had simultaneously greater mobility problems, a greater total lesion score and anisodactylia at both the first and second farrowing. It can be suggested that gilts with overgrown claws may be more prone compromised mobility, even at the stage of the first farrowing. This phenomenon remains persistent in the completion of the second farrowing. Further research is required due to the nature of the interactions among these parameters.
The housing, management practices and environmental factors were similar among the participating herds. In brief, the floor type was similar, weaning took place in all farms at 28 days, the gilts and sows were introduced to the gestation pen grouping at approximately 30 days after weaning, and the gestation pen groups were static and not dynamic, while the space allowance in the gestation pens was similar on all farms. According to Hallowel and Pierdon [33], such housing and environmental approaches may be connected to sow lameness. The usage of dynamic groups was suggested to increase the likelihood of lameness, along with increasing sow density in the latter trial. Overall, their influence on the analyzed parameters may not have been significant due to the three farms’ similar housing and management techniques. Yet, more research has to be done in this area.
5. Conclusions
Monitoring claw health and reproductive performance evolution from weaning age to the second parturition of replacement individuals is the main topic of the study. Our findings show that the IRT temperature pattern of lower feet varies, as well as the claw lengths, anisodactylia, mobility pattern and reproductive performance within replacement gilts of different genetic lines reared under almost similar housing, flooring conditions and management practices. Based on the results, it appears that gilts prioritize backfat tissue mobilization to support litter growth during lactation, independently of the extent of claw abnormalities. Still, those gilts with compromised mobility at the first and second farrowing were those with a great anisodactylia incidence and higher IRT measurements and claw lesion scoring. Yet, the nature of the associations among these parameters warrants further investigation.
Author Contributions
Conceptualization, L.L., P.F. and G.A.P.; methodology, V.S., L.L., P.F. and G.A.P.; software, G.A.P. and V.S.; validation, G.A.P. and P.F.; formal analysis, G.A.P. and V.S.; investigation, F.G.K. and G.A.P.; resources, P.F.; data curation, G.A.P.; writing—original draft preparation, F.G.K.; writing—review and editing, F.G.K. and G.A.P.; visualization, F.G.K.; supervision, P.F.; project administration, P.F.; funding acquisition, L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH–CREATE–INNOVATE project T1EDK-02073-FITSOW.
Institutional Review Board Statement
The study protocol acquired institutional approval from the Research Committee of the Aristotle University of Thessaloniki (Protocol No. 106674/6-9-2018).
Informed Consent Statement
The collaborating farms were participants, together with the academic units of the “T1EDK-02073-FITSOW” research project investigating the longevity and welfare of sows in commercial Greek farms (financed by the European Regional Development Fund of the European Union and by Greek national funds). The research project was under the control of the Ministry of Rural Development of Greece, and the owners of the participating herds have signed an agreement with the Ministry.
Data Availability Statement
Data are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Nalon, E.; Maes, D.; Piepers, S.; van Riet, M.M.J.; Janssens, G.P.J.; Millet, S.; Tuyttens, F.A.M. Mechanical Nociception Thresholds in Lame Sows: Evidence of Hyperalgesia as Measured by Two Different Methods. Vet. J. 2013, 198, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Lisgara, M.; Skampardonis, V.; Angelidou, E.; Kouroupides, S.; Leontides, L. Associations between Claw Lesions and Reproductive Performance of Sows in Three Greek Herds. Vet. Med. 2015, 60, 415–422. [Google Scholar] [CrossRef]
- Pluym, L.; Nuffel, A.V.; Maes, D. Treatment and Prevention of Lameness with Special Emphasis on Claw Disorders in Group-Housed Sows. Livest. Sci. 2013, 156, 36–43. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ushijima, R.; Sueyoshi, M. Field Study of Hind Limb Claw Lesions and Claw Measures in Sows. Anim. Sci. J. 2015, 86, 351–357. [Google Scholar] [CrossRef]
- Amezcua, R.; Walsh, S.; Luimes, P.H.; Friendship, R.M. Infrared Thermography to Evaluate Lameness in Pregnant Sows. Can. Vet. J. 2014, 55, 268–272. [Google Scholar] [PubMed]
- Soroko, M.; Howell, K. Infrared Thermography: Current Applications in Equine Medicine. J. Equine Vet. Sci. 2018, 60, 90–96. [Google Scholar] [CrossRef]
- Martins, R.F.S.; do Prado Paim, T.; de Abreu Cardoso, C.; Stéfano Lima Dallago, B.; de Melo, C.B.; Louvandini, H.; McManus, C. Mastitis Detection in Sheep by Infrared Thermography. Res. Vet. Sci. 2013, 94, 722–724. [Google Scholar] [CrossRef]
- Schaefer, A.L.; Cook, N.J.; Bench, C.; Chabot, J.B.; Colyn, J.; Liu, T.; Okine, E.K.; Stewart, M.; Webster, J.R. The Non-Invasive and Automated Detection of Bovine Respiratory Disease Onset in Receiver Calves Using Infrared Thermography. Res. Vet. Sci. 2012, 93, 928–935. [Google Scholar] [CrossRef]
- McCafferty, D.J. The Value of Infrared Thermography for Research on Mammals: Previous Applications and Future Directions. Mamm. Rev. 2007, 37, 207–223. [Google Scholar] [CrossRef]
- Stokes, J.E.; Leach, K.A.; Main, D.C.J.; Whay, H.R. An Investigation into the Use of Infrared Thermography (IRT) as a Rapid Diagnostic Tool for Foot Lesions in Dairy Cattle. Vet. J. 2012, 193, 674–678. [Google Scholar] [CrossRef]
- Van Hoogmoed, L.M.; Snyder, J.R. Use of Infrared Thermography to Detect Injections and Palmar Digital Neurectomy in Horses. Vet. J. 2002, 164, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Head, M.J.; Dyson, S. Talking the Temperature of Equine Thermography. Vet. J. 2001, 162, 166–167. [Google Scholar] [CrossRef]
- Fabbri, G.; Gianesella, M.; Tessari, R.; Bassini, A.; Morgante, M.; Contiero, B.; Faillace, V.; Fiore, E. Thermographic Screening of Beef Cattle Metatarsal Growth Plate Lesions. Animals 2022, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Gianesella, M.; Arfuso, F.; Fiore, E.; Giambelluca, S.; Giudice, E.; Armato, L.; Piccione, G. Infrared Thermography as a Rapid and Non-Invasive Diagnostic Tool to Detect Inflammatory Foot Diseases in Dairy Cows. Pol. J. Vet. Sci. 2018, 21, 299–305. [Google Scholar] [CrossRef]
- Gjein, H.; Larssen, R.B. The Effect of Claw Lesions and Claw Infections on Lameness in Loose Housing of Pregnant Sows. Acta Vet. Scand. 1995, 36, 451–459. [Google Scholar] [CrossRef]
- Pluym, L.; Van Nuffel, A.; Dewulf, J.; Cools, A.; Vangroenweghe, F.; Van Hoorebeke, S.; Maes, D. Prevalence and Risk Factors of Claw Lesions and Lameness in Pregnant Sows in Two Types of Group Housing. Vet. Med. 2011, 56, 101–109. [Google Scholar] [CrossRef]
- Foot First Team 2010: Foot First Swine Claw Lesion Identification. Available online: http://www.zinpro.com/lameness/swine/lesion-identification (accessed on 20 March 2013).
- Jørgensen, B. Osteochondrosis / Osteoarthrosis and Claw Disorders in Sows, Associated with Leg Weakness. Acta Vet. Scand. 2000, 41, 123–138. [Google Scholar] [CrossRef]
- Hultén, F.; Lundeheim, N.; Dalin, A.M.; Einarsson, S. A Field Study on Group Housing of Lactating Sows with Special Reference to Sow Health at Weaning. Acta Vet. Scand. 1995, 36, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Calderón Díaz, J.A.; Stienezen, I.M.J.; Leonard, F.C.; Boyle, L.A. The Effect of Overgrown Claws on Behaviour and Claw Crates. Appl. Anim. Behav. Sci. 2015, 166, 44–51. [Google Scholar] [CrossRef]
- Kroustallas, F.G.; Papadopoulos, G.A.; Chalvatzi, S.; Skampardonis, V.; Leontides, L.; Fortomaris, P. Infrared Thermography Evaluation of Feet Temperature and Its Association with Claw Lengths and Anisodactylia in Purebred Sows of Three Greek Herds. Vet. Sci. 2021, 8, 309. [Google Scholar] [CrossRef]
- Grégoire, J.; Bergeron, R.; D’Allaire, S.; Meunier-Salaün, M.C.; Devilles, N. Assessment of lameness in sows using gait, footprints, postural behaviour and foot lesion analysis. Animal 2013, 7, 1163–1173. [Google Scholar] [CrossRef]
- Alsaaod, M.; Syring, C.; Dietrich, J.; Doherr, M.G.; Gujan, T.; Steiner, A. A Field Trial of Infrared Thermography as a Non-Invasive Diagnostic Tool for Early Detection of Digital Dermatitis in Dairy Cows. Vet. J. 2014, 199, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Anil, S.S.; Anil, L.; Deen, J. Factors Associated with Claw Lesions in Gestating Sows. J. Swine Health Prod. 2007, 15, 78–83. [Google Scholar]
- Kroneman, A.; Vellenga, L.; van der Wilt, F.J.; Vermeer, H.M. Review of Health Problems in Group-Housed Sows, with Special Emphasis on Lameness. Vet. Q. 1993, 15, 26–29. [Google Scholar] [CrossRef]
- Megan Elizabeth, F. Anatomical Characterization of the Porcine Hoof Capsule. Master’s Thesis, Iowa State University, Ames, IO, USA, 1 January 2014. [Google Scholar] [CrossRef]
- Papadopoulos, G.A.; Chalvatzi, S.; Kroustallas, F.; Skampardonis, V.; Cernat, M.; Marouda, C.; Psychas, V.; Poutahidis, T.; Leontides, L.; Fortomaris, P. Claw Characteristics of Culled Sows from Three Farrow-to-Finish Greek Farms. Part 1: Claw Length Measurements, Lesion Scores and Their Association. Vet. Sci. 2021, 8, 126. [Google Scholar] [CrossRef]
- Newman, S.J.; Acvp, D.; Rohrbach, B.W.; Wilson, M.E.; Torrison, J.; Sarel Van Amstel, A. Characterization of Histopathologic Lesions among Pigs with Overgrown Claws. J. Swine Health Prod. 2015, 23, 91–96. [Google Scholar]
- Fitzgerald, R.F.; Stalder, K.J.; Karriker, L.A.; Sadler, L.J.; Hill, H.T.; Kaisand, J.; Johnson, A.K. The Effect of Hoof Abnormalities on Sow Behavior and Performance. Livest. Sci. 2012, 145, 230–238. [Google Scholar] [CrossRef]
- van Amstel, S.; Doherty, T. Claw horn growth and wear rates, toe length, and claw size in commercial pigs: A pilot study. J. Swine Health. Prod. 2010, 18, 239–243. [Google Scholar]
- Johnson, A.; Garcia, A.; Karriker, L.A.; Stalder, K.J. Sow lateral toe growth and lesion presence on hooves when housed in gestation stalls. J. Anim. Sci. Livest. Prod. 2020, 4, 2–7. [Google Scholar] [CrossRef]
- Calderón Díaz, J.A.; Fahey, A.G.; Kilbride, A.L.; Green, L.E.; Boyle, L.A. Longitudinal study of the effect of rubber slat mats on locomotory ability, body, limb and claw lesions, and dirtiness of group housed sows. J. Anim. Sci. 2013, 91, 3940–3954. [Google Scholar] [CrossRef]
- Hallowell, A.; Pierdon, M. Effects of lameness on productivity and longevity for sows in pen gestation. J. Swine Health Prod. 2022, 30, 223–229. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).