Exploring the Astrovirome of Shellfish Matrices Using Nanopore Sequencing
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples
- (a)
- Oyster (Crassostrea gigas) sampling within the “European baseline survey of norovirus in oysters” [28,29] (November 2016–October 2018). Samples were taken every two months from four production areas (one class A and three class B, according to the EU Commission Implementing Regulation 2019/627) located in the northern and southern Adriatic and Thyrrenian sea, respectively (Venetian Lagoon, Apulia, Gulf of La Spezia and Sardinia). A total of 48 oyster samples were collected.
- (b)
- Bivalve shellfish (Mytilus galloprovincialis, Crassostrea gigas, Ostrea edulis) sampling in the Gulf of La Spezia (December 2020–December 2021). Samples were taken monthly from nine production areas (two class A and seven class B). A total of 86 samples was collected (Mytilus n = 43, Crassostrea n = 37, Ostrea n = 6).
2.2. RNA Extraction
2.3. RT-PCR Screening
2.4. Oxford Nanopore Technologies (ONT) Sequencing and Genome Detective Pipeline
2.5. Sequence and Phylogenetic Analyses
3. Results
3.1. RT-PCR Screening
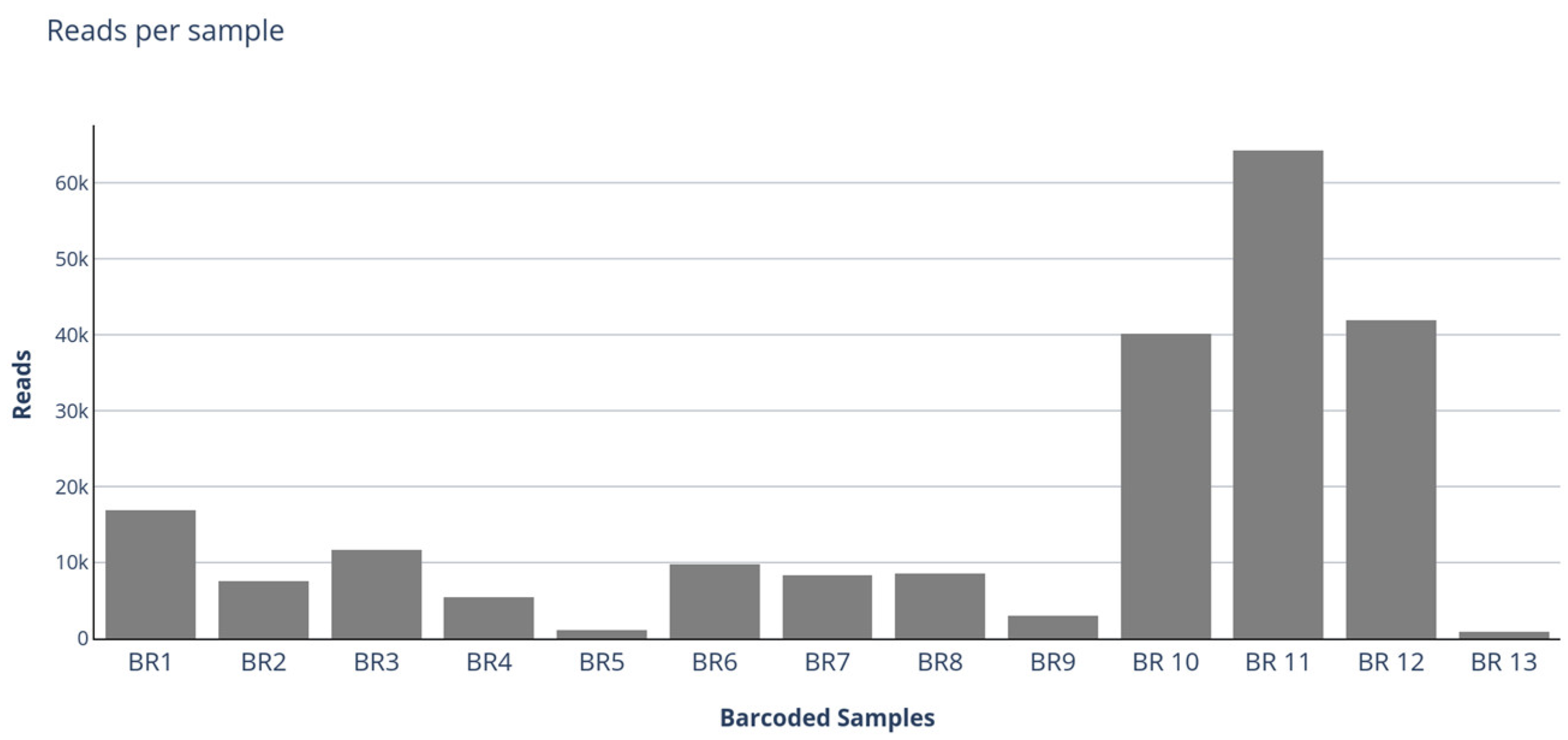
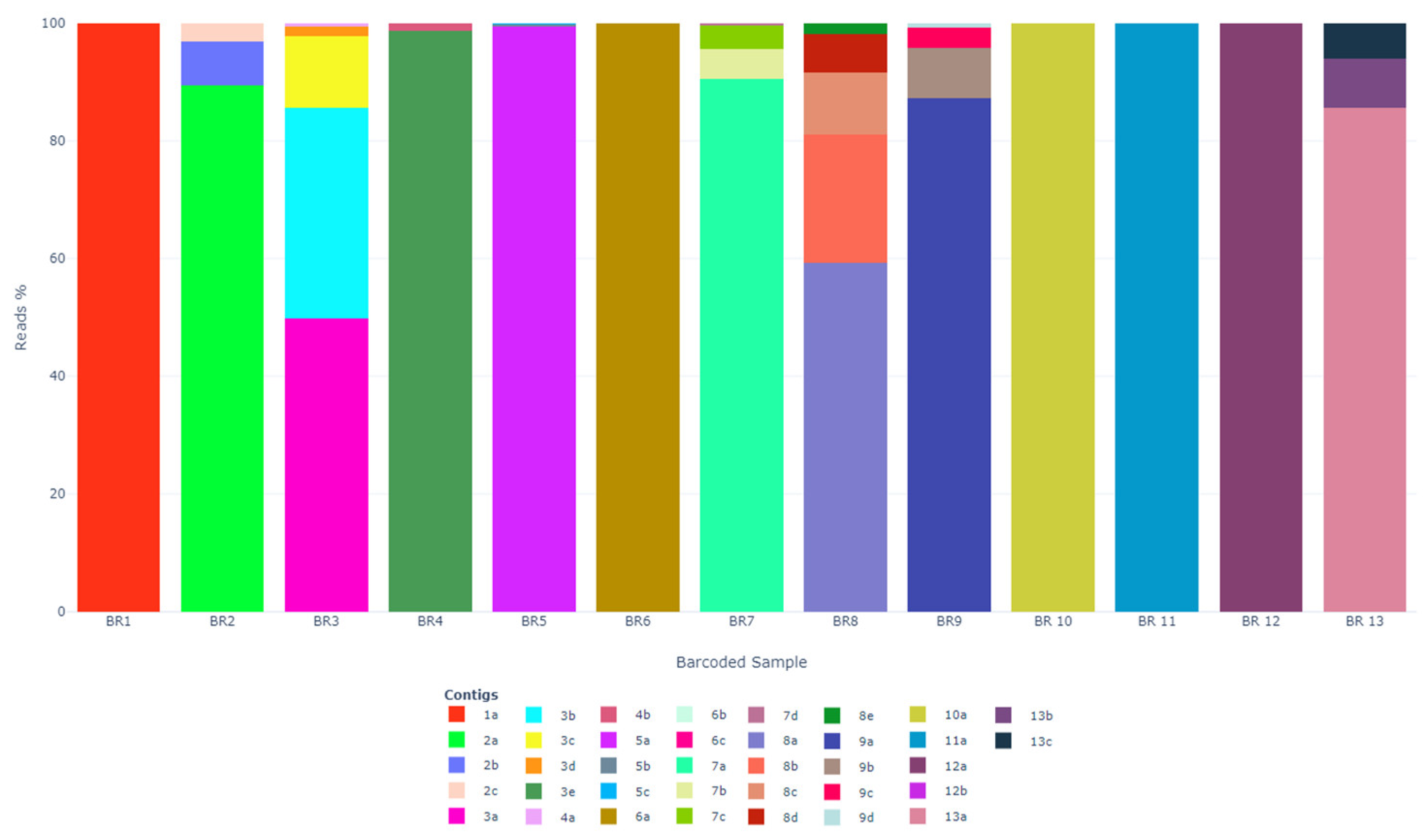
3.2. ONT Sequencing
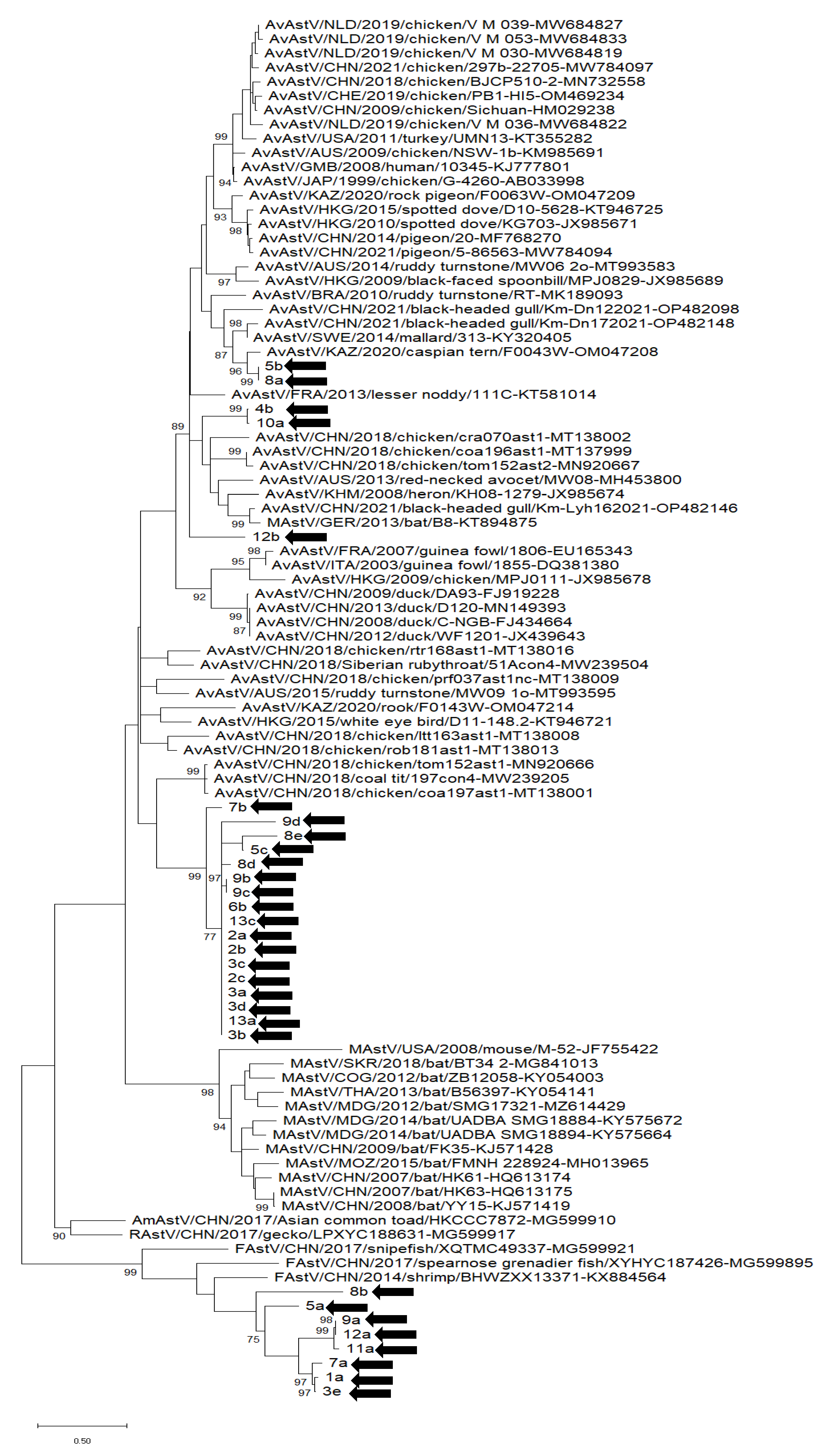
3.3. Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mendez, E.; Arias, C.F. Fields Virology, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 1. [Google Scholar]
- Appleton, H.; Higgins, P.G. Viruses and gastroenteritis in infants. Lancet 1975, 305, 1297. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.-D.; Tian, J.-H.; Chen, L.-J.; Chen, X.; Li, C.-X.; Qin, X.-C.; Li, J.; Cao, J.-P.; Eden, J.-S.; et al. Redefining the Invertebrate RNA Virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.-D.; Chen, X.; Tian, J.-H.; Chen, L.-J.; Li, K.; Wang, W.; Eden, J.-S.; Shen, J.-J.; Liu, L.; et al. The Evolutionary History of Vertebrate RNA Viruses. Nature 2018, 556, 197–202. [Google Scholar] [CrossRef] [PubMed]
- de Souza, W.M.; Fumagalli, M.J.; de Araujo, J.; Ometto, T.; Modha, S.; Thomazelli, L.M.; Durigon, E.L.; Murcia, P.R.; Figueiredo, L.T.M. Discovery of Novel Astrovirus and Calicivirus Identified in Ruddy Turnstones in Brazil. Sci. Rep. 2019, 9, 5556. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, J.L.; di Giallonardo, F.; Wille, M.; Ortiz-Baez, A.S.; Costa, V.A.; Ghaly, T.; Mifsud, J.C.O.; Turnbull, O.M.H.; Bellwood, D.R.; Williamson, J.E.; et al. Virome Composition in Marine Fish Revealed by Meta-Transcriptomics. Virus Evol. 2021, 7, veab005. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yang, S.; Wang, C.; Wang, H.; Gong, G.; Xi, Y.; Pan, J.; Wang, X.; Zeng, J.; Zhang, J.; et al. Gut Virome of the World’s Highest-Elevation Lizard Species (Phrynocephalus Erythrurus and Phrynocephalus Theobaldi) Reveals Versatile Commensal Viruses. Microbiol. Spectr. 2022, 10, e01872-21. [Google Scholar] [CrossRef] [PubMed]
- Donato, C.; Vijaykrishna, D. The Broad Host Range and Genetic Diversity of Mammalian and Avian Astroviruses. Viruses 2017, 9, 102. [Google Scholar] [CrossRef]
- Wohlgemuth, N.; Honce, R.; Schultz-Cherry, S. Astrovirus Evolution and Emergence. Infect. Genet. Evol. 2019, 69, 30–37. [Google Scholar] [CrossRef]
- Jiang, H.; Holtz, L.R.; Bauer, I.; Franz, C.J.; Zhao, G.; Bodhidatta, L.; Shrestha, S.K.; Kang, G.; Wang, D. Comparison of Novel MLB-Clade, VA-Clade and Classic Human Astroviruses Highlights Constrained Evolution of the Classic Human Astrovirus Nonstructural Genes. Virology 2013, 436, 8–14. [Google Scholar] [CrossRef]
- Kapoor, A.; Li, L.; Victoria, J.; Oderinde, B.; Mason, C.; Pandey, P.; Zaidi, S.Z.; Delwart, E. Multiple Novel Astrovirus Species in Human Stool. J. Gen. Virol. 2009, 90, 2965–2972. [Google Scholar] [CrossRef]
- Finkbeiner, S.R.; Li, Y.; Ruone, S.; Conrardy, C.; Gregoricus, N.; Toney, D.; Virgin, H.W.; Anderson, L.J.; Vinjé, J.; Wang, D.; et al. Identification of a Novel Astrovirus (Astrovirus VA1) Associated with an Outbreak of Acute Gastroenteritis. J. Virol. 2009, 83, 10836–10839. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.T.; Bauer, I.K.; Antonio, M.; Adeyemi, M.; Saha, D.; Oundo, J.O.; Ochieng, J.B.; Omore, R.; Stine, O.C.; Wang, D.; et al. Prevalence of Classic, MLB-Clade and VA-Clade Astroviruses in Kenya and The Gambia. Virol. J. 2015, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.-L.; Bosch, A.; Pintó, R.; Guix, S. Epidemiology of Classic and Novel Human Astrovirus: Gastroenteritis and Beyond. Viruses 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Cordey, S.; Brito, F.; Vu, D.-L.; Turin, L.; Kilowoko, M.; Kyungu, E.; Genton, B.; Zdobnov, E.M.; D’Acremont, V.; Kaiser, L. Astrovirus VA1 Identified by Next-Generation Sequencing in a Nasopharyngeal Specimen of a Febrile Tanzanian Child with Acute Respiratory Disease of Unknown Etiology. Emerg. Microbes Infect. 2016, 5, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Reuter, G.; Pankovics, P.; Boros, Á. Nonsuppurative (Aseptic) Meningoencephalomyelitis Associated with Neurovirulent Astrovirus Infections in Humans and Animals. Clin. Microbiol. Rev. 2018, 31, e00040-18. [Google Scholar] [CrossRef] [PubMed]
- Koukou, G.; Niendorf, S.; Hornei, B.; Schlump, J.-U.; Jenke, A.C.; Jacobsen, S. Human Astrovirus Infection Associated with Encephalitis in an Immunocompetent Child: A Case Report. J. Med. Case Rep. 2019, 13, 341. [Google Scholar] [CrossRef]
- Schibler, M.; Brito, F.; Zanella, M.-C.; Zdobnov, E.M.; Laubscher, F.; L’Huillier, A.G.; Ambrosioni, J.; Wagner, N.; Posfay-Barbe, K.M.; Docquier, M.; et al. Viral Sequences Detection by High-Throughput Sequencing in Cerebrospinal Fluid of Individuals with and without Central Nervous System Disease. Genes 2019, 10, 625. [Google Scholar] [CrossRef]
- Quan, P.-L.; Wagner, T.A.; Briese, T.; Torgerson, T.R.; Hornig, M.; Tashmukhamedova, A.; Firth, C.; Palacios, G.; Baisre-De-Leon, A.; Paddock, C.D.; et al. Astrovirus Encephalitis in Boy with X-Linked Agammaglobulinemia. Emerg. Infect. Dis. 2010, 16, 918–925. [Google Scholar] [CrossRef]
- Drexler, J.F.; Seelen, A.; Corman, V.M.; Fumie Tateno, A.; Cottontail, V.; Melim Zerbinati, R.; Gloza-Rausch, F.; Klose, S.M.; Adu-Sarkodie, Y.; Oppong, S.K.; et al. Bats Worldwide Carry Hepatitis E Virus-Related Viruses That Form a Putative Novel Genus within the Family Hepeviridae. J. Virol. 2012, 86, 9134–9147. [Google Scholar] [CrossRef]
- VanDevanter, D.R.; Warrener, P.; Bennett, L.; Schultz, E.R.; Coulter, S.; Garber, R.L.; Rose, T.M. Detection and Analysis of Diverse Herpesviral Species by Consensus Primer PCR. J. Clin. Microbiol. 1996, 34, 1666–1671. [Google Scholar] [CrossRef]
- Zintz, C.; Bok, K.; Parada, E.; Barnes-Eley, M.; Berke, T.; Staat, M.A.; Azimi, P.; Jiang, X.; Matson, D.O. Prevalence and Genetic Characterization of Caliciviruses among Children Hospitalized for Acute Gastroenteritis in the United States. Infect. Genet. Evol. 2005, 5, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.W.; Poon, L.L.M.; Guan, Y.; Peiris, J.S.M. Novel Astroviruses in Insectivorous Bats. J. Virol. 2008, 82, 9107–9114. [Google Scholar] [CrossRef] [PubMed]
- Rachmadi, A.T.; Torrey, J.R.; Kitajima, M. Human Polyomavirus: Advantages and Limitations as a Human-Specific Viral Marker in Aquatic Environments. Water Res. 2016, 105, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Bonny, P.; Schaeffer, J.; Besnard, A.; Desdouits, M.; Ngang, J.J.E.; le Guyader, F.S. Human and Animal RNA Virus Diversity Detected by Metagenomics in Cameroonian Clams. Front. Microbiol. 2021, 12, 770385. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, G.; Guercio, A.; Gucciardi, F.; di Bella, S.; la Rosa, G.; Suffredini, E.; Randazzo, W.; Purpari, G. Occurrence of Human Enteric Viruses in Shellfish along the Production and Distribution Chain in Sicily, Italy. Foods 2021, 10, 1384. [Google Scholar] [CrossRef]
- Strubbia, S.; Schaeffer, J.; Oude Munnink, B.B.; Besnard, A.; Phan, M.V.T.; Nieuwenhuijse, D.F.; de Graaf, M.; Schapendonk, C.M.E.; Wacrenier, C.; Cotten, M.; et al. Metavirome Sequencing to Evaluate Norovirus Diversity in Sewage and Related Bioaccumulated Oysters. Front. Microbiol. 2019, 10, 2394. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Analysis of the European Baseline Survey of Norovirus in Oysters. EFSA J. 2019, 17, e05762. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Technical Specifications for a European Baseline Survey of Norovirus in Oysters. EFSA J. 2016, 14, e04414. [Google Scholar] [CrossRef]
- Vilsker, M.; Moosa, Y.; Nooij, S.; Fonseca, V.; Ghysens, Y.; Dumon, K.; Pauwels, R.; Alcantara, L.C.; vanden Eynden, E.; Vandamme, A.-M.; et al. Genome Detective: An Automated System for Virus Identification from High-Throughput Sequencing Data. Bioinformatics 2019, 35, 871–873. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and Sensitive Protein Alignment Using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Deforche, K. An Alignment Method for Nucleic Acid Sequences against Annotated Genomes. bioRxiv 2017. [Google Scholar] [CrossRef]
- Katoh, K. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic. Acids. Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Conceição-Neto, N.; Yinda, K.C.; van Ranst, M.; Matthijnssens, J. NetoVIR: Modular Approach to Customize Sample Preparation Procedures for Viral Metagenomics. In The Human Virome: Methods and Protocols; Humana Press: New York, NY, USA, 2018; pp. 85–95. [Google Scholar]
- Briese, T.; Kapoor, A.; Mishra, N.; Jain, K.; Kumar, A.; Jabado, O.J.; Lipkin, W.I. Virome Capture Sequencing Enables Sensitive Viral Diagnosis and Comprehensive Virome Analysis. MBio 2015, 6, e01491-15. [Google Scholar] [CrossRef]
- Quick, J.; Grubaugh, N.D.; Pullan, S.T.; Claro, I.M.; Smith, A.D.; Gangavarapu, K.; Oliveira, G.; Robles-Sikisaka, R.; Rogers, T.F.; Beutler, N.A.; et al. Multiplex PCR Method for MinION and Illumina Sequencing of Zika and Other Virus Genomes Directly from Clinical Samples. Nat. Protoc. 2017, 12, 1261–1276. [Google Scholar] [CrossRef]
- Ollivier, J.; Lowther, J.; Desdouits, M.; Schaeffer, J.; Wacrenier, C.; Oude Munnink, B.B.; Besnard, A.; Mota Batista, F.; Stapleton, T.; Schultz, A.C.; et al. Application of Next Generation Sequencing on Norovirus-contaminated Oyster Samples. EFSA Support. Publ. 2022, 19, 7348E. [Google Scholar] [CrossRef]
- Chu, D.K.W.; Leung, C.Y.H.; Perera, H.K.K.; Ng, E.M.; Gilbert, M.; Joyner, P.H.; Grioni, A.; Ades, G.; Guan, Y.; Peiris, J.S.M.; et al. A Novel Group of Avian Astroviruses in Wild Aquatic Birds. J. Virol. 2012, 86, 13772–13778. [Google Scholar] [CrossRef]
- Biđin, M.; Lojkić, I.; Tišljar, M.; Biđin, Z.; Majnarić, D. Astroviruses Associated with Stunting and Pre-Hatching Mortality in Duck and Goose Embryos. Avian Pathol. 2012, 41, 91–97. [Google Scholar] [CrossRef]
- Fernández-Correa, I.; Truchado, D.A.; Gomez-Lucia, E.; Doménech, A.; Pérez-Tris, J.; Schmidt-Chanasit, J.; Cadar, D.; Benítez, L. A Novel Group of Avian Astroviruses from Neotropical Passerine Birds Broaden the Diversity and Host Range of Astroviridae. Sci. Rep. 2019, 9, 9513. [Google Scholar] [CrossRef] [PubMed]
- Meliopoulos, V.A.; Kayali, G.; Burnham, A.; Oshansky, C.M.; Thomas, P.G.; Gray, G.C.; Beck, M.A.; Schultz-Cherry, S. Detection of Antibodies against Turkey Astrovirus in Humans. PLoS ONE 2014, 9, e96934. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Yang, Y.; Wang, G.-S.; Shao, X.-Q.; Zhang, S.-Q.; Wang, F.-X.; Tan, B.; Tian, F.-L.; Cheng, S.-P.; Wen, Y.-J. Detection and Characterization of Avastrovirus Associated with Diarrhea Isolated from Minks in China. Food Environ. Virol. 2014, 6, 169–174. [Google Scholar] [CrossRef] [PubMed]
| Barcode | Nr Contig | Reads | Depth of Coverage | Identity to Reference Strains | |||
|---|---|---|---|---|---|---|---|
| Genus | Accession nr | FASTA Nucleotide | nt identity % | ||||
| BR1 | 1a | 16,846 | 1870 | Unclassified | KX884564 | FAstV/CHN/2014/shrimp/BHWZXX13371 | 60.1 |
| BR2 | 2a | 6733 | 5255.6 | Avastrovirus | KT946721 | AAstV/HKG/2015/white eye bird/D11-148.2 | 64.4 |
| 2b | 566 | 482.6 | Avastrovirus | KT946721 | AAstV/HKG/2015/white eye bird/D11-148.2 | 63.5 | |
| 2c | 228 | 221.4 | Avastrovirus | KT946721 | AAstV/HKG/2015/white eye bird/D11-148.2 | 63.8 | |
| BR3 | 3a | 5831 | 489.8 | Avastrovirus | KT946721 | AAstV/HKG/2015/white eye bird/D11-148.2 | 64.3 |
| 3b | 4208 | 3625.2 | Avastrovirus | KT946721 | AAstV/HKG/2015/white eye bird/D11-148.2 | 64.1 | |
| 3c | 1413 | 1151.6 | Avastrovirus | KT946721 | AAstV/HKG/2015/white eye bird/D11-148.2 | 64.6 | |
| 3d | 208 | 235.3 | Avastrovirus | KT946721 | AAstV/HKG/2015/white eye bird/D11-148.2 | 64.9 | |
| 3e | 57 | 51.6 | Unclassified | KX884564 | FAstV/CHN/2014/shrimp/BHWZXX13371 | 58.0 | |
| BR4 | 4a | 5417 | 13,488.9 | Unclassified | MG599895 | FAstV/CHN/2017/spearnose grenadier fish/XYHYC187426 | 60.2 |
| 4b | 67 | 142.8 | Mamastrovirus | KT894875 | MAstV/GER/2013/bat/B8 | 72.2 | |
| BR5 | 5a | 1147 | 1474.7 | Unclassified | KX884564 | FAstV/CHN/2014/shrimp/BHWZXX13371 | 59.9 |
| 5b | 3 | 5.9 | Avastrovirus | KY320405 | AAstV/SWE/2014/mallard/313 | 81.9 | |
| 5c | 3 | 4.9 | Avastrovirus | JX985678 | AAstV/HKG/2009/chicken/MPJ0111 | 63.7 | |
| BR6 | 6a | 9727 | 19,702.3 | Unclassified | KX884564 | FAstV/CHN/2014/shrimp/BHWZXX13371 | 59.7 |
| 6b | 5 | 6 | Avastrovirus | KT946721 | AAstV/HKG/2015/white eye bird/D11-148.2 | 62.2 | |
| 6c | 1 | 2 | Avastrovirus | KY320405 | AAstV/SWE/2014/mallard/313 | 80.1 | |
| BR7 | 7a | 7566 | 809 | Unclassified | KX884564 | FAstV/CHN/2014/shrimp/BHWZXX13371 | 57.6 |
| 7b | 425 | 450.3 | Avastrovirus | KT946721 | AAstV/HKG/2015/white eye bird/D11-148.2 | 67.5 | |
| 7c | 326 | 669.4 | Avastrovirus | KY320405 | AAstV/SWE/2014/mallard/313 | 92.5 | |
| 7d | 31 | 59.4 | Unclassified | MG599917 | RAstV/CHN/2017/gecko/LPXYC188631 | 69.3 | |
| BR8 | 8a | 5065 | 540.5 | Avastrovirus | KY320405 | AAstV/SWE/2014/mallard/313 | 82.2 |
| 8b | 1861 | 1861 | Unclassified | KX884564 | FAstV/CHN/2014/shrimp/BHWZXX13371 | 59.9 | |
| 8c | 897 | 1224.9 | Unclassified | MG599917 | RAstV/CHN/2017/gecko/LPXYC188631 | 67.9 | |
| 8d | 561 | 474 | Avastrovirus | KT946721 | AAstV/HKG/2015/white eye bird/D11-148.2 | 62.1 | |
| 8e | 149 | 161.1 | Avastrovirus | KT946721 | AAstV/HKG/2015/white eye bird/D11-148.2 | 64.2 | |
| BR9 | 9a | 2659 | 3935 | Unclassified | KX884564 | FAstV/CHN/2014/shrimp/BHWZXX13371 | 58.3 |
| 9b | 261 | 286.8 | Avastrovirus | KT946721 | AAstV/HKG/2015/white eye bird/D11-148.2 | 63.6 | |
| 9c | 105 | 120.1 | Avastrovirus | KT946721 | AAstV/HKG/2015/white eye bird/D11-148.2 | 63.1 | |
| 9d | 24 | 38.6 | Mamastrovirus | KY575664 | MAstV/MDG/2014/bat/UADBA SMG18894 | 68.4 | |
| BR 10 | 10a | 40,150 | 6633.5 | Avastrovirus | MK189093 | AAstV/BRA/2010/ruddy turnstone/RT | 66.7 |
| BR 11 | 11a | 64,214 | 67,410.2 | Unclassified | KX884564 | FAstV/CHN/2014/shrimp/BHWZXX13371 | 58.2 |
| BR 12 | 12a | 41,948 | 6639 | Unclassified | KX884564 | FAstV/CHN/2014/shrimp/BHWZXX13371 | 58.2 |
| 12b | 17 | 49.4 | Avastrovirus | HM029238 | AAstV/CHN/2009/chicken/Sichuan | 75.0 | |
| BR 13 | 13a | 723 | 93.1 | Avastrovirus | KT946721 | AAstV/HKG/2015/white eye bird/D11-148.2 | 64.7 |
| 13b | 72 | 100.2 | Avastrovirus | KY320405 | AAstV/SWE/2014/mallard/313 | 80.2 | |
| 13c | 50 | 52.8 | Avastrovirus | KT946721 | AAstV/HKG/2015/white eye bird/D11-148.2 | 63.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beikpour, F.; Pellegrini, F.; Lanave, G.; Camero, M.; Catella, C.; Di Martino, B.; Di Profio, F.; Masotti, C.; Battistini, R.; Serracca, L.; et al. Exploring the Astrovirome of Shellfish Matrices Using Nanopore Sequencing. Vet. Sci. 2023, 10, 175. https://doi.org/10.3390/vetsci10030175
Beikpour F, Pellegrini F, Lanave G, Camero M, Catella C, Di Martino B, Di Profio F, Masotti C, Battistini R, Serracca L, et al. Exploring the Astrovirome of Shellfish Matrices Using Nanopore Sequencing. Veterinary Sciences. 2023; 10(3):175. https://doi.org/10.3390/vetsci10030175
Chicago/Turabian StyleBeikpour, Farzad, Francesco Pellegrini, Gianvito Lanave, Michele Camero, Cristiana Catella, Barbara Di Martino, Federica Di Profio, Chiara Masotti, Roberta Battistini, Laura Serracca, and et al. 2023. "Exploring the Astrovirome of Shellfish Matrices Using Nanopore Sequencing" Veterinary Sciences 10, no. 3: 175. https://doi.org/10.3390/vetsci10030175
APA StyleBeikpour, F., Pellegrini, F., Lanave, G., Camero, M., Catella, C., Di Martino, B., Di Profio, F., Masotti, C., Battistini, R., Serracca, L., La Rosa, G., Martella, V., & Suffredini, E. (2023). Exploring the Astrovirome of Shellfish Matrices Using Nanopore Sequencing. Veterinary Sciences, 10(3), 175. https://doi.org/10.3390/vetsci10030175









