The Anticoccidial Effect of Alcoholic Vitis vinifera Leaf Extracts on Eimeria papillate Oocysts Isolated in Mice In Vitro and In Vivo
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Preparation of Extract
2.3. Infrared Spectroscopy
2.4. Oocyst Sporulation
2.5. In Vitro
2.6. In Vivo
2.7. Sample Collection
2.8. Statistical Analysis
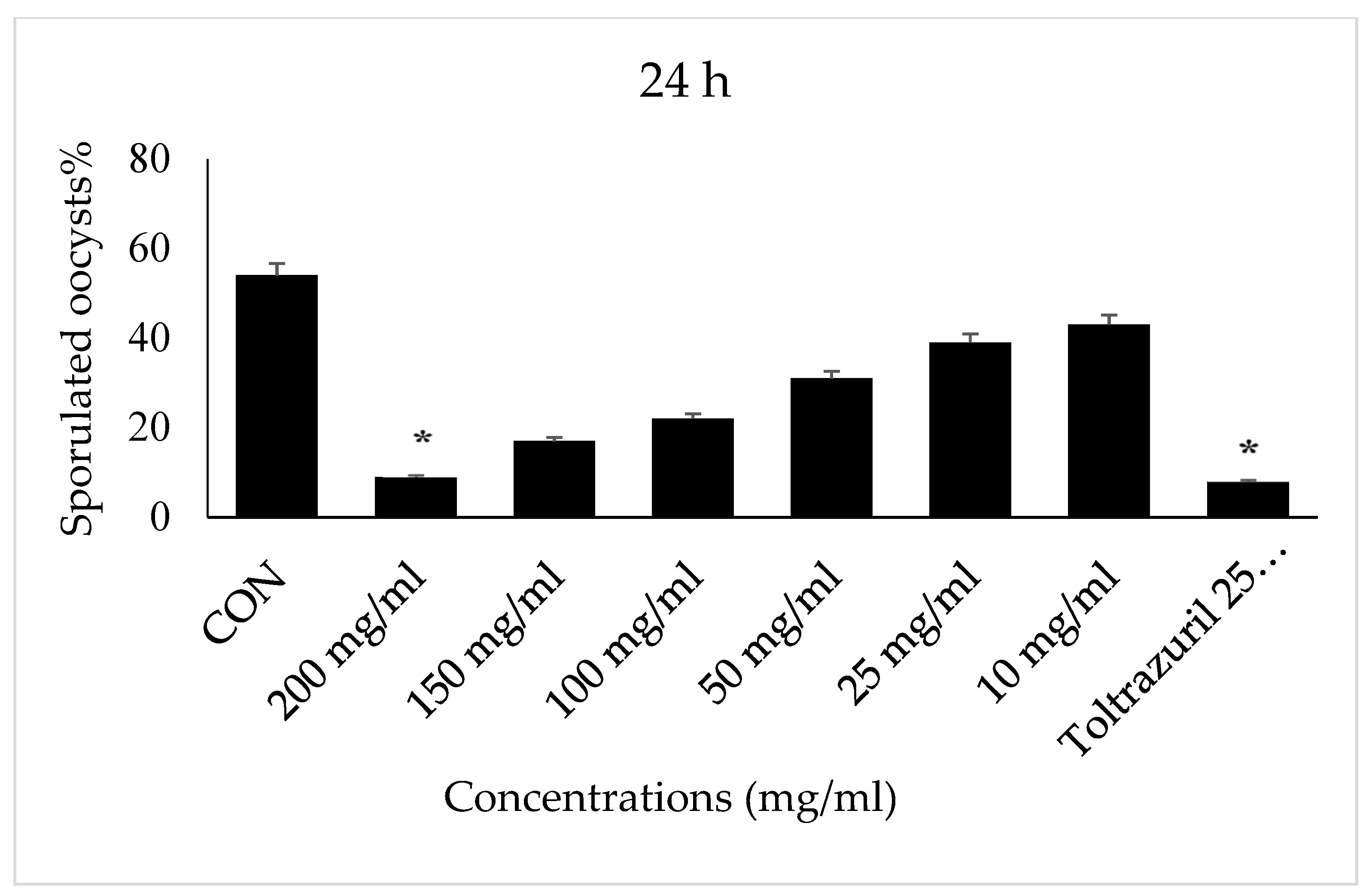
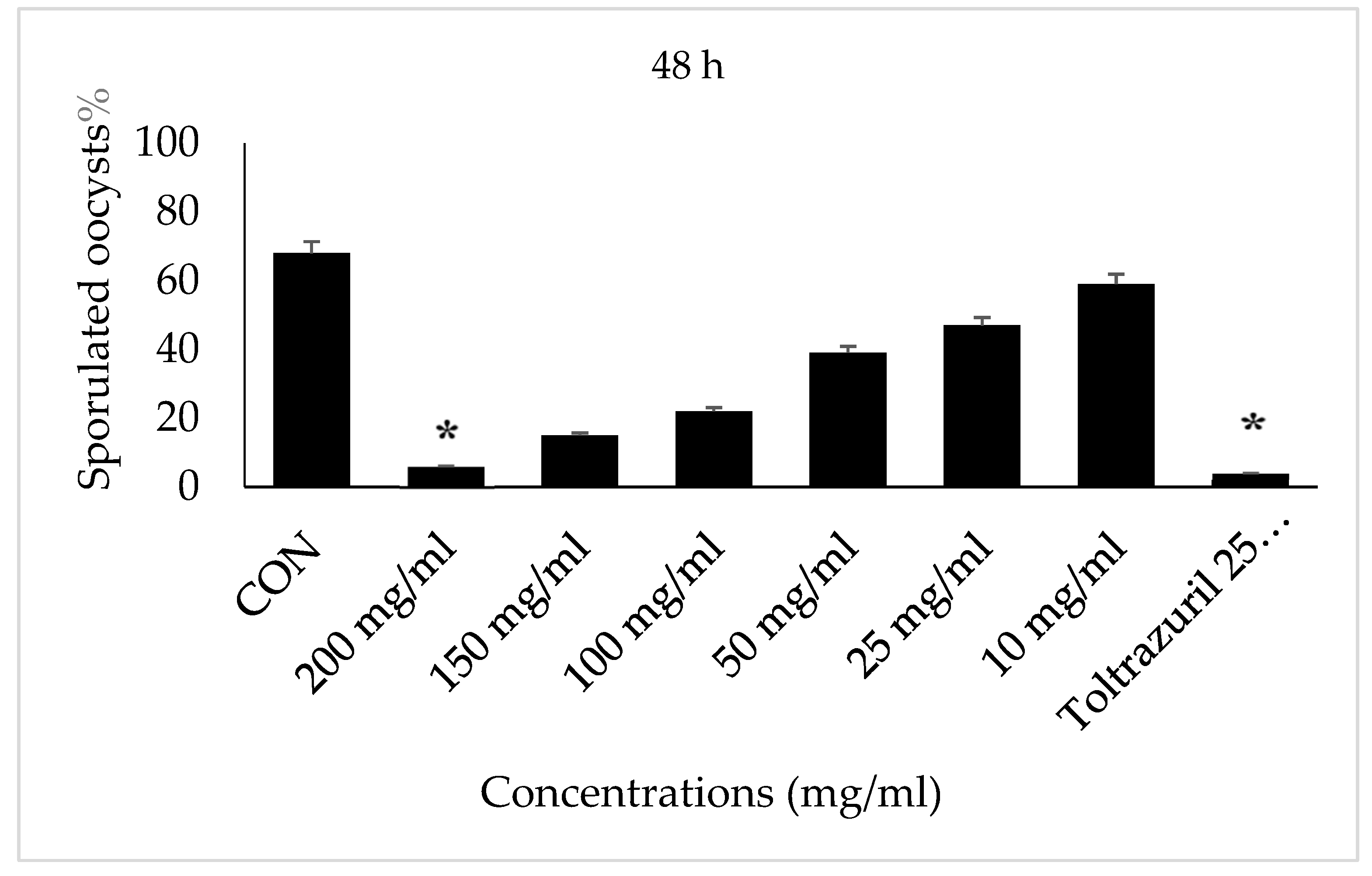
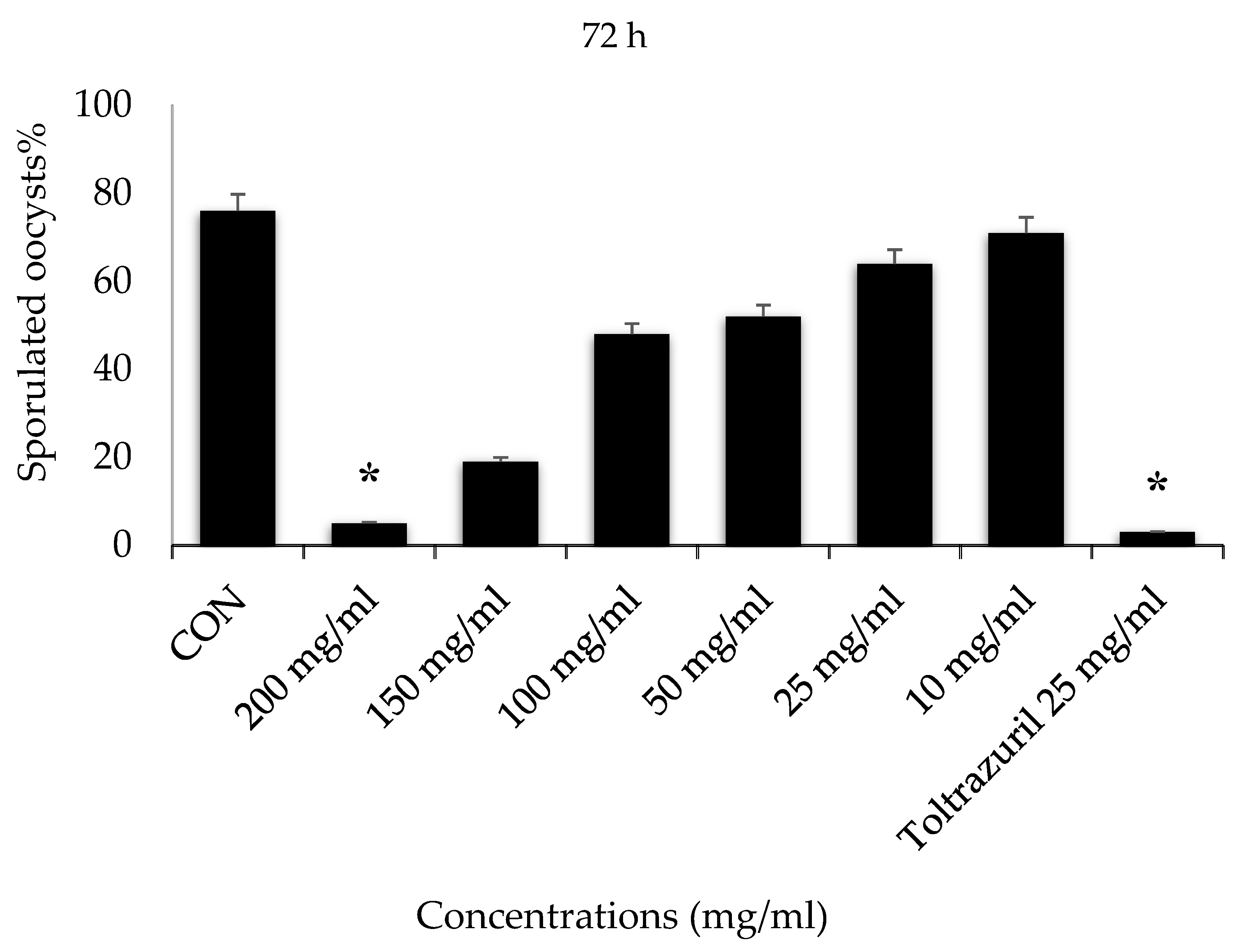
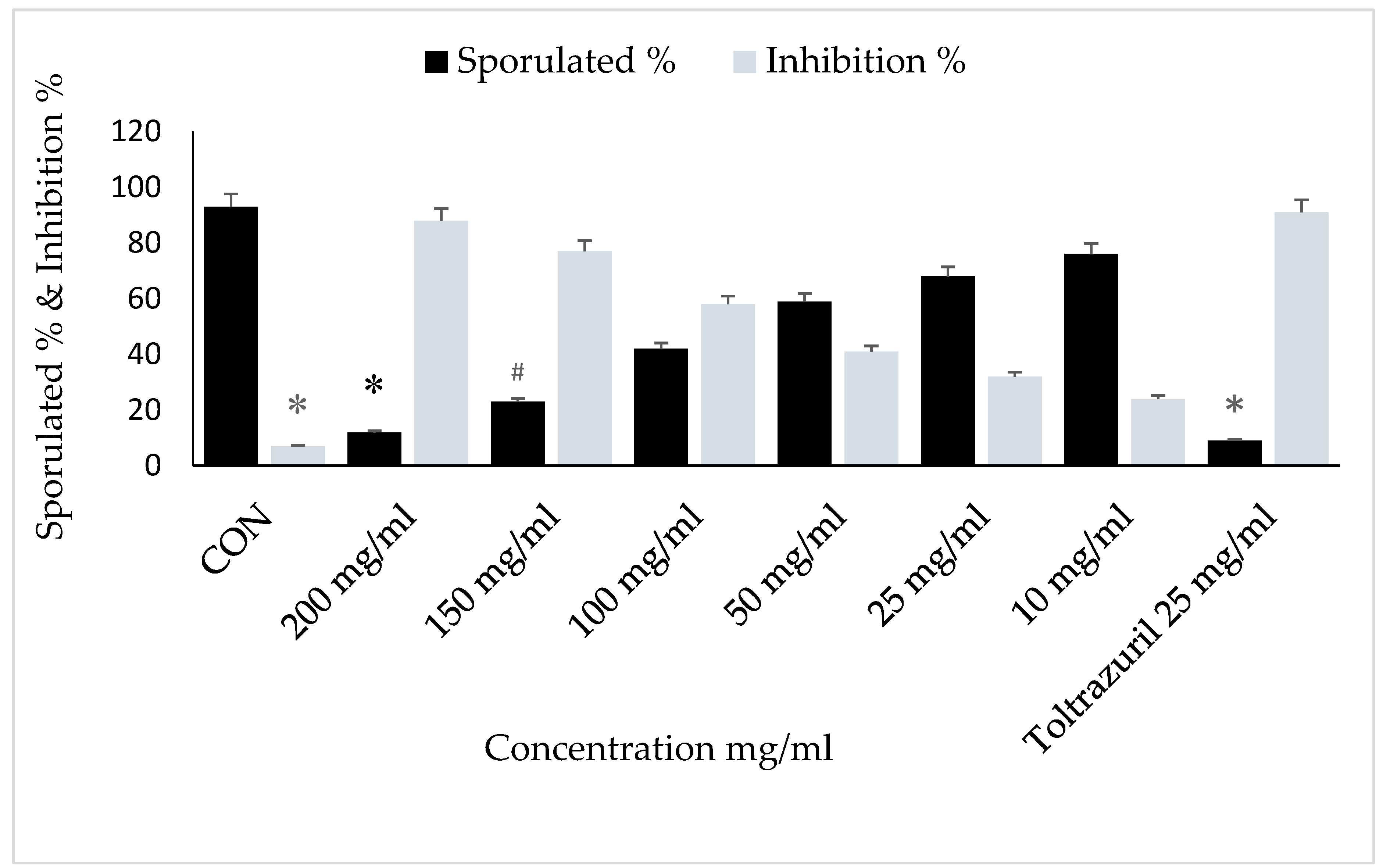
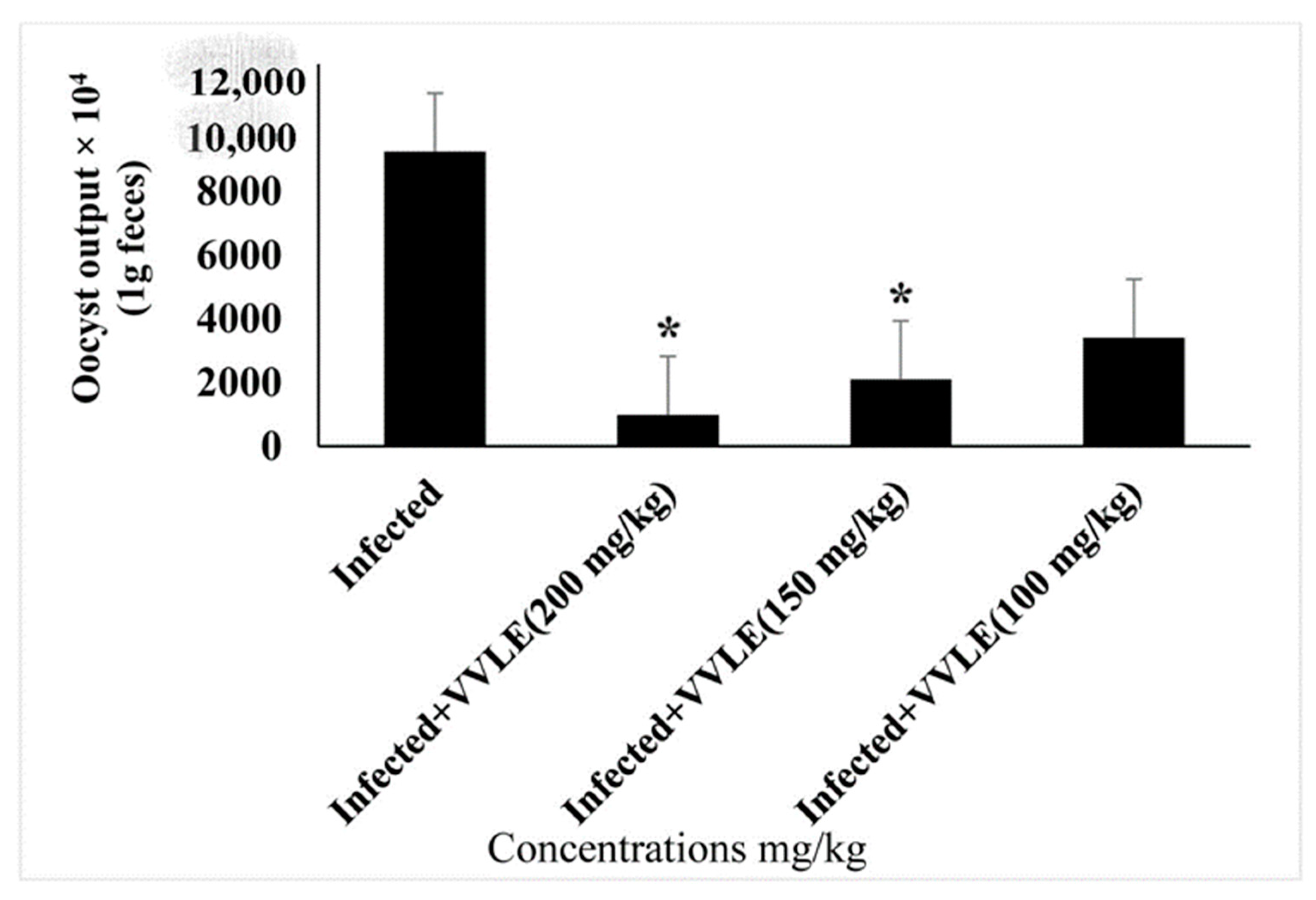
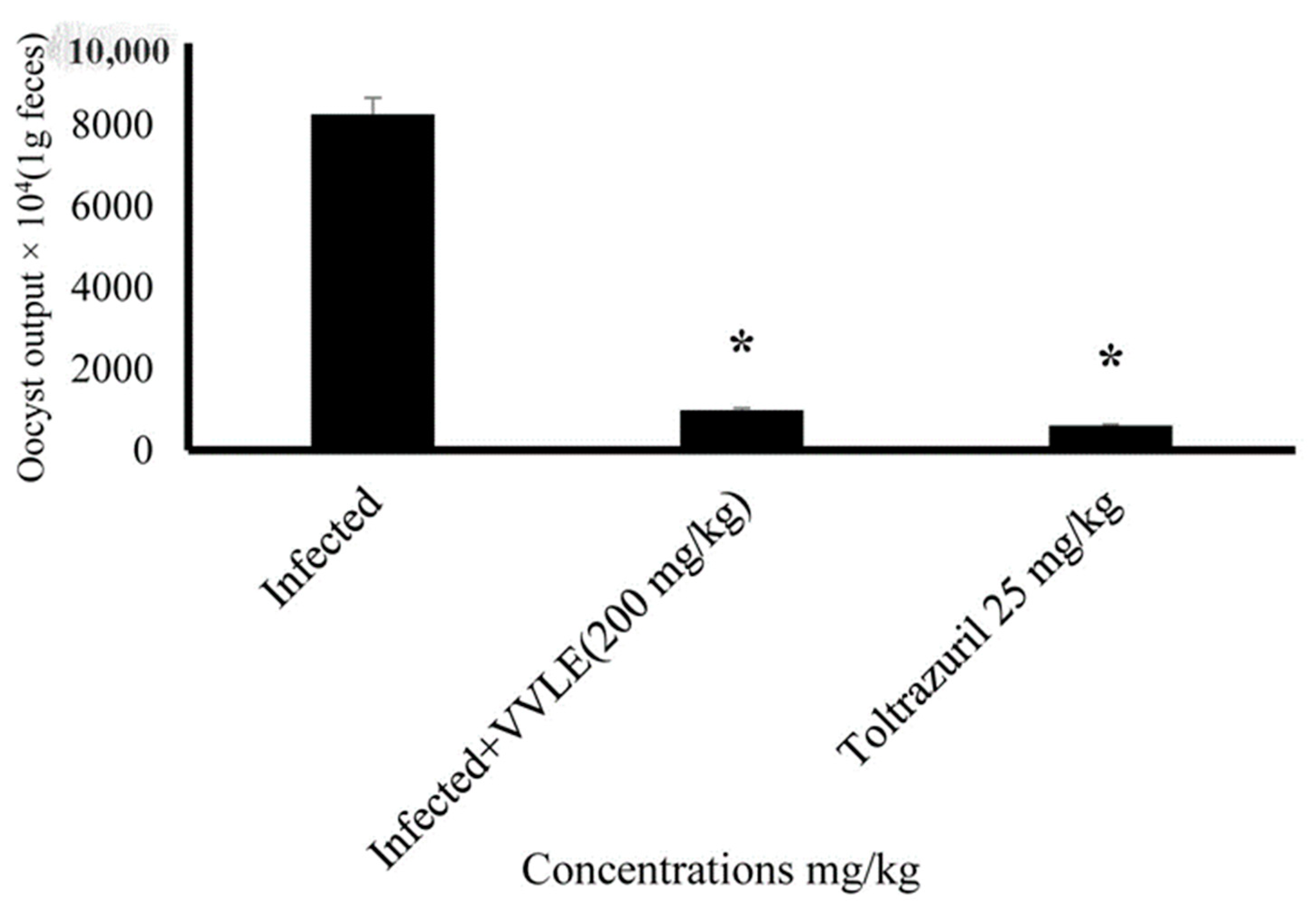
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehlhorn, H. Encyclopedic Reference of Parasitology, 6th ed.; Springer: Berlin, Germany, 2014. [Google Scholar]
- Allen, P.C.; Fetterer, R. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin. Microbiol. Rev. 2002, 15, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.R.; Taha-Abdelaziz, K.; Shojadoost, B.; Astill, J.; Sharif, S. Gastrointestinal diseases of poultry: Causes and nutritional strategies for prevention and control. In Improving Gut Health in Poultry; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 205–236. [Google Scholar]
- Collier, C.; Hofacre, C.; Payne, A.; Anderson, D.; Kaiser, P.; Mackie, R.; Gaskins, H. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008, 122, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H. Milestones in avian coccidiosis research: A review. Poult. Sci. 2014, 93, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Graat, E.; Henken, A.; Ploeger, H.; Noordhuizen, J.; Vertommen, M. Rate and course of sporulation of oocysts of Eimeria acervulina under different environmental conditions. Parasitology 1994, 108, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Mai, K.; Sharman, P.A.; Walker, R.A.; Katrib, M.; Souza, D.D.; McConville, M.J.; Wallach, M.G.; Belli, S.I.; Ferguson, D.J.; Smith, N.C. Oocyst wall formation and composition in coccidian parasites. Mem. Do Inst. Oswaldo Cruz 2009, 104, 281–289. [Google Scholar] [CrossRef] [PubMed]
- McDonald, V.; Shirley, M. Past and future: Vaccination against Eimeria. Parasitology 2009, 136, 1477–1489. [Google Scholar] [CrossRef]
- Kostadinović, L.; Lević, J. Effects of phytoadditives in poultry and pigs diseases. J. Agron. 2018, 5, 1–7. [Google Scholar]
- Sidiropoulou, E.; Skoufos, I.; Marugan-Hernandez, V.; Giannenas, I.; Bonos, E.; Aguiar-Martins, K.; Lazari, D.; Blake, D.P.; Tzora, A. In vitro anticoccidial study of oregano and garlic essential oils and effects on growth performance, fecal oocyst output, and intestinal microbiota in vivo. Front. Vet. Sci. 2020, 7, 420. [Google Scholar] [CrossRef]
- Mosaddeghi, P.; Eslami, M.; Farahmandnejad, M.; Akhavein, M.; Ranjbarfarrokhi, R.; Khorraminejad-Shirazi, M.; Shahabinezhad, F.; Taghipour, M.; Dorvash, M.; Sakhteman, A. A systems pharmacology approach to identify the autophagy-inducing effects of Traditional Persian medicinal plants. Sci. Rep. 2021, 11, 336. [Google Scholar] [CrossRef]
- Wunderlich, F.; Al-Quraishy, S.; Steinbrenner, H.; Sies, H.; Dkhil, M.A. Towards identifying novel anti-Eimeria agents: Trace elements, vitamins, and plant-based natural products. Parasitol. Res. 2014, 113, 3547–3556. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.L.; Liu, Z.; De, S. Effect of pine bark (Pious radiata) extracts on sporulation of coccidian oocysts. Folia Parasitol. 2009, 56, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, M.; Zhao, Z.; Yu, S. The antibiotic activity and mechanisms of sugarcane (Saccharum officinarum L.) bagasse extract against food-borne pathogens. Food Chem. 2015, 185, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Qasem, M.A.; Dkhil, M.A.; Al-Shaebi, E.M.; Murshed, M.; Mares, M.; Al-Quraishy, S. Rumex nervosus leaf extracts enhance the regulation of goblet cells and the inflammatory response during infection of chickens with Eimeria tenella. J. King Saud Univ.-Sci. 2020, 32, 1818–1823. [Google Scholar] [CrossRef]
- Qaid, M.M.; Al-Mufarrej, S.I.; Azzam, M.M.; Al-Garadi, M.A. Anticoccidial effectivity of a traditional medicinal plant, Cinnamomum verum, in broiler chickens infected with Eimeria tenella. Poult. Sci. 2021, 100, 100902. [Google Scholar] [CrossRef] [PubMed]
- Murshed, M.; Al-Quraishy, S.; Qasem, M.A. In vitro: Anti-coccidia activity of Calotropis procera leaf extract on Eimeria papillata oocysts sporulation and sporozoite. Open Chem. 2022, 20, 1057–1064. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Abbas, A.; Raza, M.A.; Khan, M.K.; Saleemi, M.K.; Saeed, Z. In vitro anticoccidial activity of Trachyspermum ammi (Ajwain) extract on oocysts of Eimeria species of Chicken. Adv. Life Sci. 2019, 7, 44–47. [Google Scholar]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive constituents: An update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef]
- Başoğlu, F.; Şahin, İ.; Korukluoğlu, M.; Uylaser, V.; Akpinar, A. A research on the effects of fermentation type and additives on quality and preservation and development of adequate technique in brined vine-leaves production. Turk. J. Agric. For. 1996, 20, 535–545. [Google Scholar]
- Koşar, M.; Küpeli, E.; Malyer, H.; Uylaşer, V.; Türkben, C.; Başer, K.H.C. Effect of brining on biological activity of leaves of Vitis vinifera L. (Cv. Sultani Cekirdeksiz) from Turkey. J. Agric. Food Chem. 2007, 55, 4596–4603. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Oliboni, L.; Agostini, F.; Funchal, C.; Serafini, L.; Henriques, J.; Salvador, M. Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage. Toxicol. Vitr. 2010, 24, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Bombardelli, E.; Morazzoni, P.; Carini, M.; Aldini, G.; Maffei Facino, R. Biological activity of procyanidins from Vitis vinifera L. BioFactors 1997, 6, 429–431. [Google Scholar] [CrossRef]
- Walker, J.B. Evaluation of the ability of seven herbal resources to answer questions about herbal products asked in drug information centers. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2002, 22, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Ercisli, S.; Orhan, E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Manikandan, P.; Letchoumy, P.V.; Gopalakrishnan, M.; Nagini, S. Evaluation of Azadirachta indica leaf fractions for in vitro antioxidant potential and in vivo modulation of biomarkers of chemoprevention in the hamster buccal pouch carcinogenesis model. Food Chem. Toxicol. 2008, 46, 2332–2343. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.; Abdel-Baki, A.; Wunderlich, F.; Sies, H.; Al-Quraishy, S. Anticoccidial and antiinflammatory activity of garlic in murine Eimeria papillata infections. Vet. Parasitol. 2011, 175, 66–72. [Google Scholar] [CrossRef]
- Schito, M.L.; Barta, J.R.; Chobotar, B. Comparison of four murine Eimeria species in immunocompetent and immunodeficient mice. J. Parasitol. 1996, 82, 255–262. [Google Scholar] [CrossRef]
- Andrews, A. Some aspects of coccidiosis in sheep and goats. Small Rumin. Res. 2013, 110, 93–95. [Google Scholar] [CrossRef]
- Pakandl, M. Selection of a precocious line of the rabbit coccidium Eimeria flavescens Marotel and Guilhon (1941) and characterisation of its endogenous cycle. Parasitol. Res. 2005, 97, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Williams, R. A compartmentalised model for the estimation of the cost of coccidiosis to the world's chicken production industry. Int. J. Parasitol. 1999, 29, 1209–1229. [Google Scholar] [CrossRef] [PubMed]
- Pieri, F.; Silva, V.; Vargas, F.; Veiga Junior, V.; Moreira, M. Antimicrobial activity of Copaifera langsdorffii oil and evaluation of its most bioactive fraction against bacteria of dog’s dental plaque. Pak. Vet. J. 2014, 34. [Google Scholar]
- Xiao, C.; Ji, Q.; Rajput, Z.; Wei, Q.; Liu, Y.; Bao, G. Antifungal Efficacy of Phellodendron amurense Ethanol Extract against Trichophyton mentagrophytes in Rabbits. Pak. Vet. J. 2014, 34, 165–169. [Google Scholar]
- Fornazier, R.F.; Ferreira, R.R.; Vitoria, A.; Molina, S.; Lea, P.; Azevedo, R. Effects of cadmium on antioxidant enzyme activities in sugar cane. Biol. Plant. 2002, 45, 91–97. [Google Scholar] [CrossRef]
- El-Abasy, M.; Motobu, M.; Sameshima, T.; Koge, K.; Onodera, T.; Hirota, Y. Adjuvant effects of sugar cane extracts (SCE) in chickens. J. Vet. Med. Sci. 2003, 65, 117–119. [Google Scholar] [CrossRef]
- Hikosaka, K.; El-Abasy, M.; Koyama, Y.; Motobu, M.; Koge, K.; Isobe, T.; Kang, C.B.; Hayashidani, H.; Onodera, T.; Wang, P.C. Immunostimulating effects of the polyphenol-rich fraction of sugar cane (Saccharum officinarum L.) extract in chickens. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gong, T.; Chen, R.Y. Two new prenylflavonoids from Morus nigra L. Chin. Chem. Lett. 2009, 20, 1469. [Google Scholar] [CrossRef]
- Molan, A.; Sivakumaran, S.; Spencer, P.; Meagher, L. Green tea flavan-3-ols and oligomeric proanthocyanidins inhibit the motility of infective larvae of Teladorsagia circumcincta and Trichostrongylus colubriformis in vitro. Res. Vet. Sci. 2004, 77, 239–243. [Google Scholar] [CrossRef]
- Narsih, K.S.; Wignyanto, W.S. Identification of aloin and saponin and chemical composition of volatile constituents from Aloe vera (L.) Peel. J. Agric. Food Tech. 2012, 2, 79–84. [Google Scholar]
- Abbas, R.; Abbas, A.; Iqbal, Z.; Raza, M.; Hussain, K.; Ahmed, T.; Shafi, M. In vitro anticoccidial activity of Vitis vinifera extract on oocysts of different Eimeria species of broiler chicken. J. Hell. Vet. Med. Soc. 2020, 71, 2267–2272. [Google Scholar] [CrossRef]
- Olaku, O.O.; Ojukwu, M.O.; Zia, F.Z.; White, J.D. The role of grape seed extract in the treatment of chemo/radiotherapy induced toxicity: A systematic review of preclinical studies. Nutr. Cancer 2015, 67, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Al-Quraishy, S.; Abdel Moneim, A.E.; Delic, D. Protective effect of Azadirachta indica extract against Eimeria papillata-induced coccidiosis. Parasitol. Res. 2013, 112, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Amer, O.S.; Dkhil, M.A.; Hikal, W.M.; Al-Quraishy, S. Antioxidant and anti-inflammatory activities of pomegranate (Punica granatum) on Eimeria papillata-induced infection in mice. BioMed Res. Int. 2015, 2015, 219670. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, F.; Al-Shaebi, E.M.; Dkhil, M.A.; Al-Quraishy, S. In vivo anti-eimeria and in vitro anthelmintic activity of Ziziphus spina-christi leaf extracts. Pak. J. Zool. 2016, 48. [Google Scholar]
- Thagfan, F.A.; Al-Megrin, W.A.; Al-Quraishy, S.; Dkhil, M.A.M. Mulberry extract as an ecofriendly anticoccidial agent: In vitro and in vivo application. Rev. Bras. De Parasitol. Veterinária 2020, 29, e009820. [Google Scholar] [CrossRef] [PubMed]
- Alcicek, A.; Bozkurt, M.; Çabuk, M. The effect of a mixture of herbal essential oils, an organic acid or a probiotic on broiler performance. S. Afr. J. Anim. Sci. 2004, 34, 217–222. [Google Scholar]
- Gracia, M.; Millán, C.; Sánchez, J.; Guyard-Nicodeme, M.; Mayot, J.; Carre, Y.; Csorbai, A.; Chemaly, M.; Medel, P. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period: Part B. Poult. Sci. 2016, 95, 886–892. [Google Scholar] [CrossRef]
- Abdullateef, S.; Taib Tahir, M.; A Hasso, R. The effect of nandrolone decanoate on liver of rabbitsusing histological and ultrasound methods. Ann. Coll. Med. Mosul 2009, 35, 160–166. [Google Scholar] [CrossRef]
- Marizvikuru, M.; Patrick, J.M. Point prevalence study of gastro-intestinal parasites in village chickens of Centane district, South Africa. Afr. J. Agric. Res. 2011, 6, 2033–2038. [Google Scholar]
- Onan, K.S.; Toure, A.; Ouattara, K.; Djaman, A.J.; Nrsquo; Guessan, J.D. In vitro anticoccidial activity of Thonningia sanguinea extract on Eimeria tenella and Eimeria necatrix sporozoites cell. Afr. J. Microbiol. Res. 2012, 6, 6247–6251. [Google Scholar]
- Otu, M.O.; Lawal, I.A.; George, B.D.; Abubakar, M.S.; Adeyinka, I.A.; Abeke, F.O.; Sekoni, A.A.; Nwagu, B.I.; Adejoh-Ubani, E.O.; Oluntunmogun, A.K.; et al. Effects of N-Butanol and Aqueous Fractions of Khaya senegalensis, Guiera senegalensis and Tamarindus Indica Leaves Extracts on Eimeria tenella Oocyst Sporulation in Vitro. Niger. Vet. J. 2020, 41, 175–191. [Google Scholar] [CrossRef]
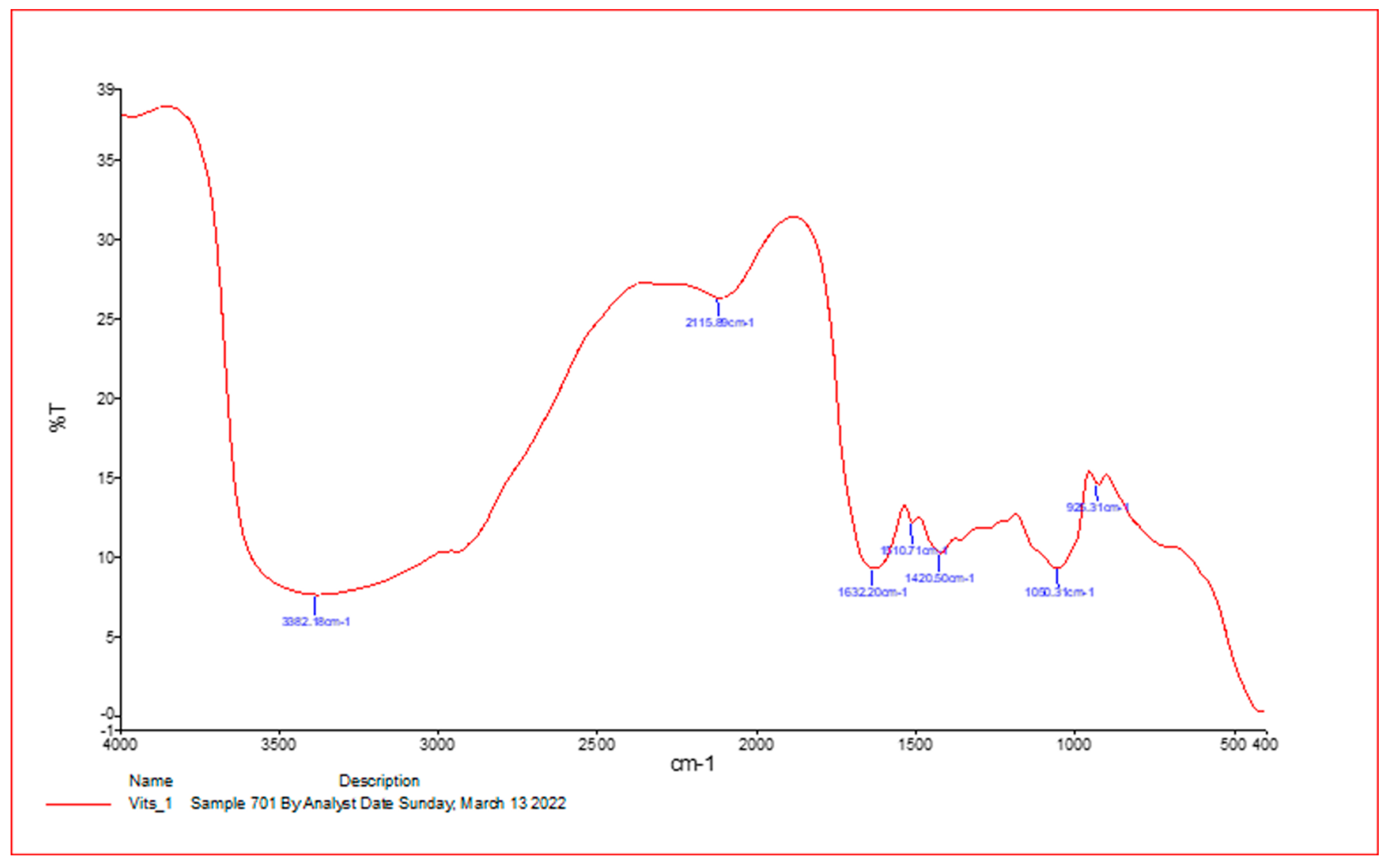


| Absorption (cm−1) | Appearance | Transmittance (%) | Group | Compound Class |
|---|---|---|---|---|
| 3425.53 | medium | 12 | N-H stretching | Aliphatic primary amine |
| 2093.10 | strong | 47 | N=C=S stretchy | Isothiocyanate |
| 1641.41 | strong | 25 | C=C stretching | Alkene |
| 1209.12 | strong | 37 | C-O stretching tertiary | alcohol |
| 1045.64 | Strong broad | 35 | CO-O-CO stretching | Anhydride |
| 410.42 | strong | 3 | C-H bending | 1,2-disubstituted |
| Weight of Mice (g) | Oocyst Output/g Feces | ||
|---|---|---|---|
| Day 0 | Day 5 | ||
| Noninfected (−vitis) | 28.19 ± 1.4 | 28.70 ± 1.4 | 0 |
| Noninfected (+vitis) | 26.9 ± 1.16 | 27.06 ± 0.98 | 0 |
| Infected (−vitis) | 26.5 ± 1.4 | 26.3 ± 1.1 | 828.75 ± 54.668 |
| Infected (+vitis) | 26.51 ± 0.74 | 26.83 ± 0.746 | 28.808 ± 6.785 * |
| Toltrazuril 25 mg/mL | 26.17 ± 1.15 | 26.98 ± 1.5 | 26.743 ± 6.321 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murshed, M.; Al-Quraishy, S.; Alghamdi, J.; Aljawdah, H.M.A.; Mares, M.M. The Anticoccidial Effect of Alcoholic Vitis vinifera Leaf Extracts on Eimeria papillate Oocysts Isolated in Mice In Vitro and In Vivo. Vet. Sci. 2023, 10, 97. https://doi.org/10.3390/vetsci10020097
Murshed M, Al-Quraishy S, Alghamdi J, Aljawdah HMA, Mares MM. The Anticoccidial Effect of Alcoholic Vitis vinifera Leaf Extracts on Eimeria papillate Oocysts Isolated in Mice In Vitro and In Vivo. Veterinary Sciences. 2023; 10(2):97. https://doi.org/10.3390/vetsci10020097
Chicago/Turabian StyleMurshed, Mutee, Saleh Al-Quraishy, Jawahir Alghamdi, Hossam M. A. Aljawdah, and Mohammed M. Mares. 2023. "The Anticoccidial Effect of Alcoholic Vitis vinifera Leaf Extracts on Eimeria papillate Oocysts Isolated in Mice In Vitro and In Vivo" Veterinary Sciences 10, no. 2: 97. https://doi.org/10.3390/vetsci10020097
APA StyleMurshed, M., Al-Quraishy, S., Alghamdi, J., Aljawdah, H. M. A., & Mares, M. M. (2023). The Anticoccidial Effect of Alcoholic Vitis vinifera Leaf Extracts on Eimeria papillate Oocysts Isolated in Mice In Vitro and In Vivo. Veterinary Sciences, 10(2), 97. https://doi.org/10.3390/vetsci10020097






