Performance and Tolerance of a Protocol for Idiopathic Chronic Greasy Seborrhea in 18 Dogs Using a Shampoo and Mousse Containing Plant Extracts
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals
2.3. Design of the Study
2.4. Clinical Assessment
2.5. Skin Surface Cytology
2.6. Natural Moisturising Factor (NMF) Content
2.7. Hair Surface Lipids
2.8. Tolerance
2.9. Investigator and Owner Satisfaction
2.10. Statistical Methods
3. Results
3.1. Animal Population
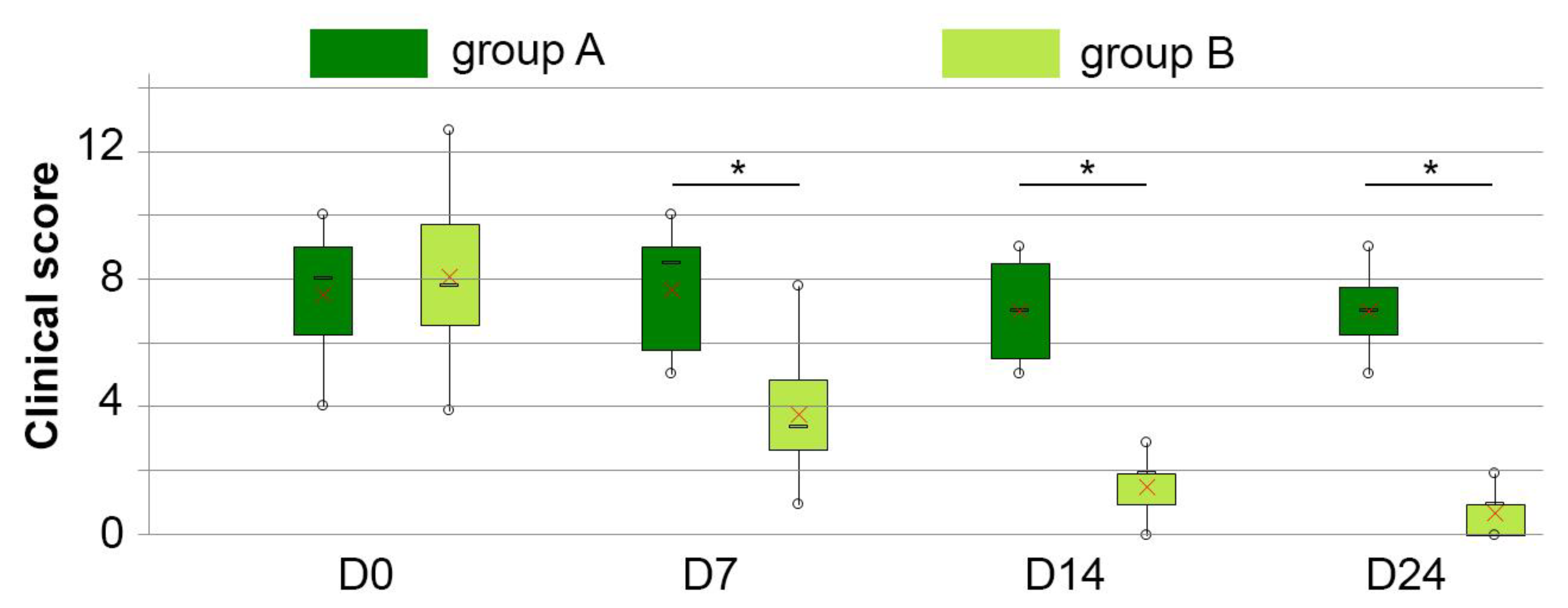
3.2. Clinical Score
3.3. Pruritus
3.4. Skin Surface Cytology
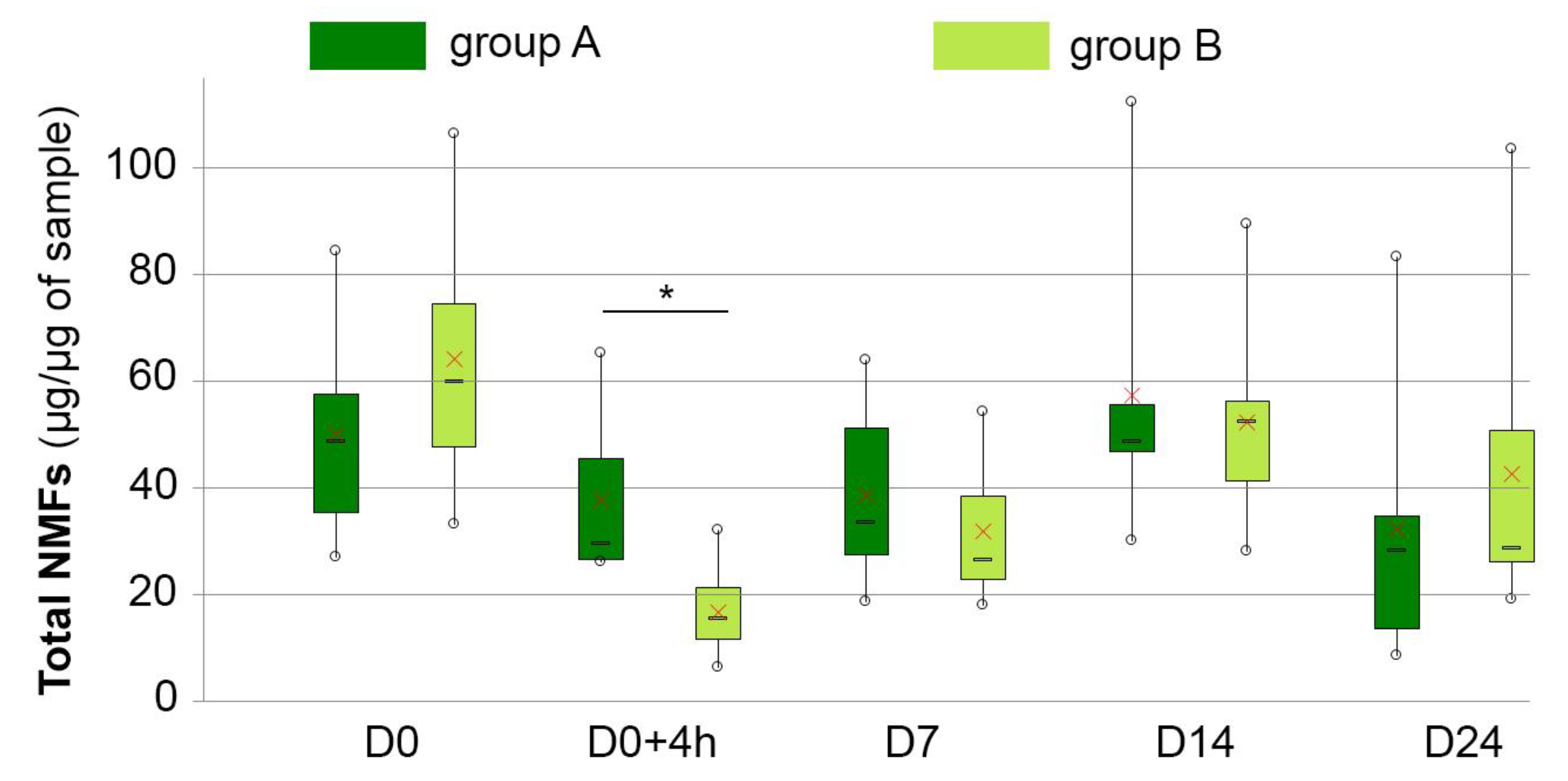
3.5. NMF Content
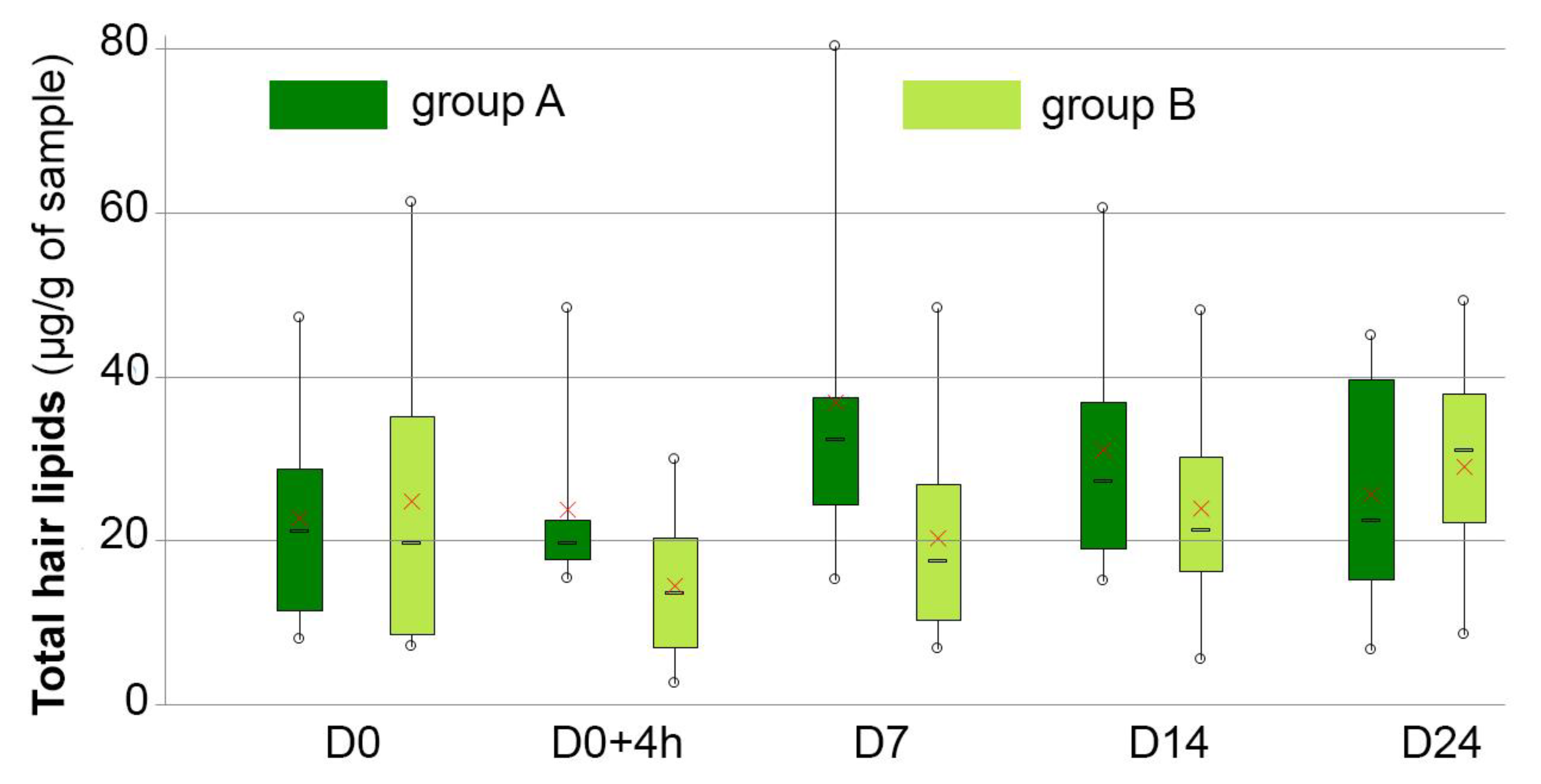
3.6. Hair Lipid Analysis
3.7. Tolerance and Feedback from the Users
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoon, J.S.; Nishifuji, K.; Ishioroshi, S.; Ide, K.; Iwasaki, T. Skin lipid profiling in normal and seborrhoeic shih tzu dogs. Vet. Dermatol. 2013, 24, 84-e22. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A. Epidermal surface lipids. Derm.-Endocrinol 2009, 1, 72–76. [Google Scholar] [CrossRef]
- Schaich, B.; Korting, H.C.; Hollmann, J. Skin lipids in seborrhea- and sebostasis-associated skin diseases. Hautarzt Z. Dermatol. Venerol. Verwandte Geb. 1993, 44, 75–80. [Google Scholar]
- Orbell, H.L.; Cave, N.J.; Parry, K.; Griffin, C.E. An explorative study comparing skin surface lipids in the West Highland white terrier dog with and without atopic dermatitis. Vet. Q. 2022, 42, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.H., Jr.; Griffin, C.E.; Campbell, K.L. Keratinization defects. In Muller and Kirk’s Small Animal Dermatology, 7th ed.; Mosby, E., Ed.; Saunders: St. Louis, MO, USA, 2012; pp. 630–646. [Google Scholar]
- Rosenkrantz, W. Practical applications of topical therapy for allergic, infectious, and seborrheic disorders. Clin. Techol. Small Anim. Pract. 2006, 21, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Loing, E.; Lamarque, E.; Borel, M. New targets in the battle against dandruff. J. Cosmet. Sci. 2017, 68, 107–113. [Google Scholar] [PubMed]
- Moore, E.M.; Wagner, C.; Komarnytsky, S. The Enigma of Bioactivity and Toxicity of Botanical Oils for Skin Care. Front. Pharmacol. 2020, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Bi, S.-X.; Huang, Z.; Qi, J.; Yu, B.-Y. Novel steroidal saponins with cytotoxic activities from the roots of Ophiopogon japonicus (L. f.) Ker-Gawl. RSC Adv. 2018, 8, 2498–2505. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska, D.; Galanty, A.; Grabowska, K.; Makowska-Wąs, J.; Wróbel-Biedrawa, D.; Podolak, I. Saponins as cytotoxic agents: An update (2010–2018). Part I—Steroidal saponins. Phytochem. Rev. 2020, 19, 139–189. [Google Scholar] [CrossRef]
- Chen, M.H.; Chen, X.J.; Wang, M.; Lin, L.G.; Wang, Y.T. Ophiopogon japonicus—A phytochemical, ethnomedicinal and pharmacological review. J. Ethnopharmacol. 2016, 181, 193–213. [Google Scholar] [CrossRef]
- Ollivier, E.; Zemirline, C.; Amalric, N.; Rahoul, V.; Reymond, N.; Cadiergues, M.-C. Efficacy of the ingredient A97614A1 in a model of reconstructed human epidermis stressed by cytokines. In BSAVA Congress Proceedings 2019; BSAVA: Birmingham, UK, 2019; pp. 442–443. [Google Scholar]
- Ollivier, E.; Zemirline, C.; Marchand, L.; Closs, B. Effect of the ingredient A97614A1 on the adhesion and biofilm formation of Staphylococcus pseudintermedius in a model of reconstructed canine epidermis. In BSAVA Congress Proceedings 2019; BSAVA: Birmingham, UK, 2019; p. 442. [Google Scholar]
- Shaygannia, E.; Bahmani, M.; Zamanzad, B.; Rafieian-Kopaei, M. A Review Study on Punica granatum L. J. Evid. Based Complement. Altern. Med. 2016, 21, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Barathikannan, K.; Venkatadri, B.; Khusro, A.; Al-Dhabi, N.A.; Agastian, P.; Arasu, M.V.; Choi, H.S.; Kim, Y.O. Chemical analysis of Punica granatum fruit peel and its in vitro and in vivo biological properties. BMC Complement. Altern. Med. 2016, 16, 264. [Google Scholar] [CrossRef]
- Madugula, P.; Reddy, S.; Koneru, J.; Rao, A.S.; Sruthi, R.; Dalli, D.T. “Rhetoric to Reality”—Efficacy of Punica Granatum Peel Extract on Oral Candidiasis: An in vitro Study. J. Clin. Diagn. Res. 2017, 11, ZC114–ZC117. [Google Scholar] [CrossRef]
- Paul, S.; Mohanram, K.; Kannan, I. Antifungal Activity, Gas Chromatographic-Mass Spectrometric Analysis And in silico Study of Punica Granatum Peel Extracts Against Fluconazole Resistant Strains of Candida Species. Curr. Pharm. Biotechnol. 2018, 19, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Pagliarulo, C.; De Vito, V.; Picariello, G.; Colicchio, R.; Pastore, G.; Salvatore, P.; Volpe, M.G. Inhibitory effect of pomegranate (Punica granatum L.) polyphenol extracts on the bacterial growth and survival of clinical isolates of pathogenic Staphylococcus aureus and Escherichia coli. Food Chem. 2016, 190, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Betanzos-Cabrera, G.; Montes-Rubio, P.Y.; Fabela-Illescas, H.E.; Belefant-Miller, H.; Cancino-Diaz, J.C. Antibacterial activity of fresh pomegranate juice against clinical strains of Staphylococcus epidermidis. Food Nutr. Res. 2015, 59, 27620. [Google Scholar] [CrossRef]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef]
- Lee, C.J.; Chen, L.G.; Liang, W.L.; Wang, C.C. Multiple Activities of Punica granatum Linne against Acne Vulgaris. Int. J. Mol. Sci. 2017, 18, 141. [Google Scholar] [CrossRef]
- Puigdemont, A.; D’Andreano, S.; Ramio-Lluch, L.; Cusco, A.; Francino, O.; Brazis, P. Effect of an anti-inflammatory pomegranate otic treatment on the clinical evolution and microbiota profile of dogs with otitis externa. Vet. Dermatol. 2021, 32, 158-e37. [Google Scholar] [CrossRef]
- Hensel, P.; Santoro, D.; Favrot, C.; Hill, P.; Griffin, C. Canine atopic dermatitis: Detailed guidelines for diagnosis and allergen identification. BMC Vet. Res. 2015, 11, 196. [Google Scholar] [CrossRef]
- Urbaniak, G.C.; Plous, S. Research Randomizer (Version 4.0). Available online: https://www.randomizer.org (accessed on 13 April 2019).
- Viaud, S.; Maynard, L.; Sanquer, A. Comparison of two shampoos as sole treatment for canine bacterial overgrowth syndrome. Vet. Rec. 2012, 170, 675. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.B.; Lau, P.; Rybnicek, J. Development of an owner-assessed scale to measure the severity of pruritus in dogs. Vet. Dermatol. 2007, 18, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Bouassiba, C.; Osthold, W.; Mueller, R.S. Comparison of four different staining methods for ear cytology of dogs with otitis externa. Tierarztl. Praxis. Ausg. K Kleintiere Heimtiere 2013, 41, 7–15. [Google Scholar]
- Angelova-Fischer, I.; Dapic, I.; Hoek, A.K.; Jakasa, I.; Fischer, T.W.; Zillikens, D.; Kezic, S. Skin barrier integrity and natural moisturising factor levels after cumulative dermal exposure to alkaline agents in atopic dermatitis. Acta Derm. Venereol. 2014, 94, 640–644. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Nuttall, T. Topical therapy in canine atopic dermatitis: New products. Companion Anim. 2020, 25, 76–82. [Google Scholar] [CrossRef]
- Gatellet, M.; Åhman, S.; Bruet, V.; Cadot, P.; Mueller, R.; Noli, C.; Nuttall, T.; Ollivier, E.; Blondel, T.; Savelli, N. Performance of a combined application of Ophytrium-containing shampoo and mousse in dogs with sensitive skin: A European field trial. Vet. Dermatol. 2020, 31, 37. [Google Scholar] [CrossRef]
- Bensignor, E.J.; Fabries, L.J. Use of antipruritic and rehydrating foams on localized lesions of atopic dermatitis in dogs: A small-scale pilot and comparative double-blinded study. Vet. Dermatol. 2018, 29, 446-e150. [Google Scholar] [CrossRef]
- Pin, D.; Bekrich, M.; Fantini, O.; Noel, G.; Videmont, E. An emulsion restores the skin barrier by decreasing the skin pH and inflammation in a canine experimental model. J. Comp. Pathol. 2014, 151, 244–254. [Google Scholar] [CrossRef]
- Ramos, S.J.; Woodward, M.; Hoppers, S.M.; Liu, C.C.; Pucheu-Haston, C.M.; Mitchell, M.S. Residual antibacterial activity of canine hair treated with five mousse products against Staphylococcus pseudintermedius in vitro. Vet. Dermatol. 2019, 30, 183-e57. [Google Scholar] [CrossRef]
- Gatellet, M.; Åhman, S.; Bruet, V.; Cadot, P.; Mueller, R.; Noli, C.; Nuttall, T.; Ollivier, E.; Blondel, T.; Savelli, N. Performance of combined shampoo and mousse applications containing Ophytrium and Seboliance in dogs with scaling disorders: A European field trial. Vet. Dermatol. 2020, 31, 33. [Google Scholar]
- Maynard, L.; Reme, C.A.; Viaud, S. Comparison of two shampoos for the treatment of canine Malassezia dermatitis: A randomised controlled trial. J. Small Anim. Pract. 2011, 52, 566–572. [Google Scholar] [CrossRef]
- Budgin, J.B.; Flaherty, M.J. Alternative therapies in veterinary dermatology. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Meason-Smith, C.; Older, C.E.; Ocana, R.; Dominguez, B.; Lawhon, S.D.; Wu, J.; Patterson, A.P.; Rodrigues Hoffmann, A. Novel association of Psychrobacter and Pseudomonas with malodour in bloodhound dogs, and the effects of a topical product composed of essential oils and plant-derived essential fatty acids in a randomized, blinded, placebo-controlled study. Vet. Dermatol. 2018, 29, 465-e158. [Google Scholar] [CrossRef] [PubMed]
- Bensignor, E.; Fabries, L.; Bailleux, L. A split-body, randomized, blinded study to evaluate the efficacy of a topical spray composed of essential oils and essential fatty acids from plant extracts with antimicrobial properties. Vet. Dermatol. 2016, 27, 464-e123. [Google Scholar] [CrossRef] [PubMed]
- Baumer, W.; Jacobs, M.; Tamamoto-Mochizuki, C. Efficacy study of a topical treatment with a plant extract with antibiofilm activities using an in vivo model of canine superficial pyoderma. Vet. Dermatol. 2020, 31, 86–89. [Google Scholar] [CrossRef]
- Catarino, M.; Combarros-Garcia, D.; Mimouni, P.; Pressanti, C.; Cadiergues, M.C. Control of canine idiopathic nasal hyperkeratosis with a natural skin restorative balm: A randomized double-blind placebo-controlled study. Vet. Dermatol. 2018, 29, 134-e53. [Google Scholar] [CrossRef]
- Bensignor, E.; Videmont, E. Weekly topical therapy based on plant extracts combined with lokivetmab in canine atopic dermatitis. Vet. Dermatol. 2021, 33, 68-e22. [Google Scholar] [CrossRef] [PubMed]
- Ananthapadmanabhan, K.P.; Moore, D.J.; Subramanyan, K.; Misra, M.; Meyer, F. Cleansing without compromise: The impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol. Ther. 2004, 17 (Suppl. S1), 16–25. [Google Scholar] [CrossRef] [PubMed]
- Barba, C.; Semenzato, A.; Baratto, G.; Coderch, L. Action of surfactants on the mammal epidermal skin barrier. G. Ital. Dermatol. Venereol. 2019, 154, 405–412. [Google Scholar] [CrossRef]
- Goffin, V.; Fontaine, J.; Pierard, G.E. Comparative surfactant reactivity of canine and human stratum corneum: A plea for the use of the corneosurfametry bioassay. Altern. Lab. Anim. 1999, 27, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.R.; Rawlings, A.V. 18 Effects of Natural Moisturizing Factor and Lactic Acid Isomers on Skin Function. In Dry Skin Moisturizers; CRC Press: Boca Raton, FL, USA, 2000; Volume 187. [Google Scholar]
- Haftek, M.; McAleer, M.A.; Jakasa, I.; McLean, W.I.; Kezic, S.; Irvine, A.D. Changes in nano-mechanical properties of human epidermal cornified cells in children with atopic dermatitis. Wellcome Open Res. 2020, 5, 97. [Google Scholar] [CrossRef] [PubMed]
| Parameter | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| malodour | absence | low | mild | high |
| scaling | absence | low | mild | high |
| greasiness | absence | low | mild | high |
| haircoat quality | normal | slightly altered | mildly altered | highly altered |
| extension (%body surface) | <20% | 20–50% | 50–75% | >75% |
| Group | Breed | Gender | Age (Years) Median (Min–Max) | Weight (kg) Median (Min–Max) | Length of Haircoat |
|---|---|---|---|---|---|
| A | 5 Griffon | 4 male, 1 female | 4 (4–5) | 27 (24–33) | longhaired |
| 1 Bruno du Jura | 1 male | 2 | 33 | shorthaired | |
| B | 6 Griffon | 6 male | 5.5 (1–7) | 28 (25–28) | longhaired |
| 3 Bruno du Jura | 3 male | 6 (2–8) | 28 (24–29) | shorthaired | |
| 3 Bleu de Gascogne | 1 male, 2 female | 3 (3–6) | 24 (20–25) | longhaired |
| Parameter | Group | D0 | D0 + 4 h | D7 | D14 | D24 |
|---|---|---|---|---|---|---|
| Clinical score (/15) | A | 7.5 ± 2.3 | 7.7 ± 2.2 | 7.0 ± 1.8 | 7.0 ± 1.4 | |
| B | 8.3 ± 2.6 | 3.9 ± 2.0 | 1.6 ± 0.9 | 0.8 ± 0.8 | ||
| Pruritus (/10) | A | 0.6 ± 0.3 | 0.0 ± 0.0 | 0.2 ± 0.1 | 0.0 ± 0.0 | |
| B | 0.5 ± 0.2 | 0.0 ± 0.1 | 0.2 ± 0.1 | 0.0 ± 0.0 | ||
| NMFs (µg/sample) | A | 49.96 ± 20.72 | 37.72 ± 16.29 | 38.58 ± 17.76 | 57.20 ± 28.38 | 32.17 ± 27.49 |
| B | 64.83 ± 25.36 | 17.43 ± 7.51 | 32.41 ± 12.15 | 53.02 ± 16.70 | 43.30 ± 28.11 | |
| Hair lipids (μg of total lipids per mg of hair fibers) | A | 22.80 ± 14.86 | 23.85 ± 12.29 | 36.85 ± 22.93 | 31.05 ± 16.98 | 25.74 ± 15.88 |
| B | 25.32 ± 18.27 | 15.25 ± 8.05 | 20.93 ± 11.94 | 24.43 ± 13.40 | 29.47 ± 12.52 |
| Clinical Parameter | Group | D0 | D7 | D14 | D24 |
|---|---|---|---|---|---|
| malodour | A | 1.2 ± 0.8 | 1.3 ± 0.5 → | 1.8 ± 0.8 ↑ | 1.8 ± 0.4 ↑ |
| B | 1.2 ± 0.7 | 0.1 ± 0.3 ↓ | 0.0 ± 0.0 ↓ | 0.0 ± 0.0 ↓ | |
| scaling | A | 1.0 ± 0.9 | 1.5 ± 0.5 ↑ | 2.2 ± 0.4 ↑ | 1.2 ± 1.0 → |
| B | 1.6 ± 0.8 | 0.9 ± 1.0 ↓ | 0.8 ± 0.6 ↓ | 0.4 ± 0.5 ↓ | |
| greasiness | A | 2.0 ± 0.6 | 1.7 ± 0.5 → | 1.8 ± 0.4 → | 1.7 ± 0.5 → |
| B | 1.7 ± 0.7 | 0.8 ± 0.6 ↓ | 0.8 ± 0.5 ↓ | 0.1 ± 0.3 ↓ | |
| haircoat quality | A | 1.7 ± 0.5 | 1.7 ± 0.5 → | 0.8 ± 0.8 ↓ | 1.3 ± 0.8 ↓ |
| B | 2.0 ± 0.7 | 1.3 ± 0.5 ↓ | 0.0 ± 0.0 ↓ | 0.2 ± 0.4 ↓ | |
| Extension (%body surface) | A | 1.7 ± 0.5 | 1.5 ± 0.8 → | 0.3 ± 0.5 ↓ | 1.0 ± 0.6 ↓ |
| B | 1.9 ± 0.7 | 0.8 ± 0.5 ↓ | 0.0 ± 0.0 ↓ | 0.1 ± 0.3 ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondratjeva, J.; Brun, J.; Amalric, N.; Moog, F.; Combarros, D.; Pressanti, C.; Zemirline, C.; Maubert, N.; Ollivier, E.; Gatellet, M.; et al. Performance and Tolerance of a Protocol for Idiopathic Chronic Greasy Seborrhea in 18 Dogs Using a Shampoo and Mousse Containing Plant Extracts. Vet. Sci. 2023, 10, 95. https://doi.org/10.3390/vetsci10020095
Kondratjeva J, Brun J, Amalric N, Moog F, Combarros D, Pressanti C, Zemirline C, Maubert N, Ollivier E, Gatellet M, et al. Performance and Tolerance of a Protocol for Idiopathic Chronic Greasy Seborrhea in 18 Dogs Using a Shampoo and Mousse Containing Plant Extracts. Veterinary Sciences. 2023; 10(2):95. https://doi.org/10.3390/vetsci10020095
Chicago/Turabian StyleKondratjeva, Jevgenija, Jessie Brun, Nicolas Amalric, Fabien Moog, Daniel Combarros, Charline Pressanti, Claudine Zemirline, Nadège Maubert, Elodie Ollivier, Marina Gatellet, and et al. 2023. "Performance and Tolerance of a Protocol for Idiopathic Chronic Greasy Seborrhea in 18 Dogs Using a Shampoo and Mousse Containing Plant Extracts" Veterinary Sciences 10, no. 2: 95. https://doi.org/10.3390/vetsci10020095
APA StyleKondratjeva, J., Brun, J., Amalric, N., Moog, F., Combarros, D., Pressanti, C., Zemirline, C., Maubert, N., Ollivier, E., Gatellet, M., & Cadiergues, M. C. (2023). Performance and Tolerance of a Protocol for Idiopathic Chronic Greasy Seborrhea in 18 Dogs Using a Shampoo and Mousse Containing Plant Extracts. Veterinary Sciences, 10(2), 95. https://doi.org/10.3390/vetsci10020095







