Pathological Findings and Oxidative Stress Status Associated with Hydatidosis in Dromedary Camels
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals and Sampling
2.3. Hydatid Cyst Categorization
2.4. Biochemical Analysis of Cyst Fluid
2.5. Histopathological Examination
2.6. Oxidative State Evaluation
2.7. Statistical Analysis
3. Result
3.1. Prevalence of Hydatidosis in Camels
3.2. Parasitological and Biochemical Analysis of Hydatid Cyst
3.3. Antioxidant Activity
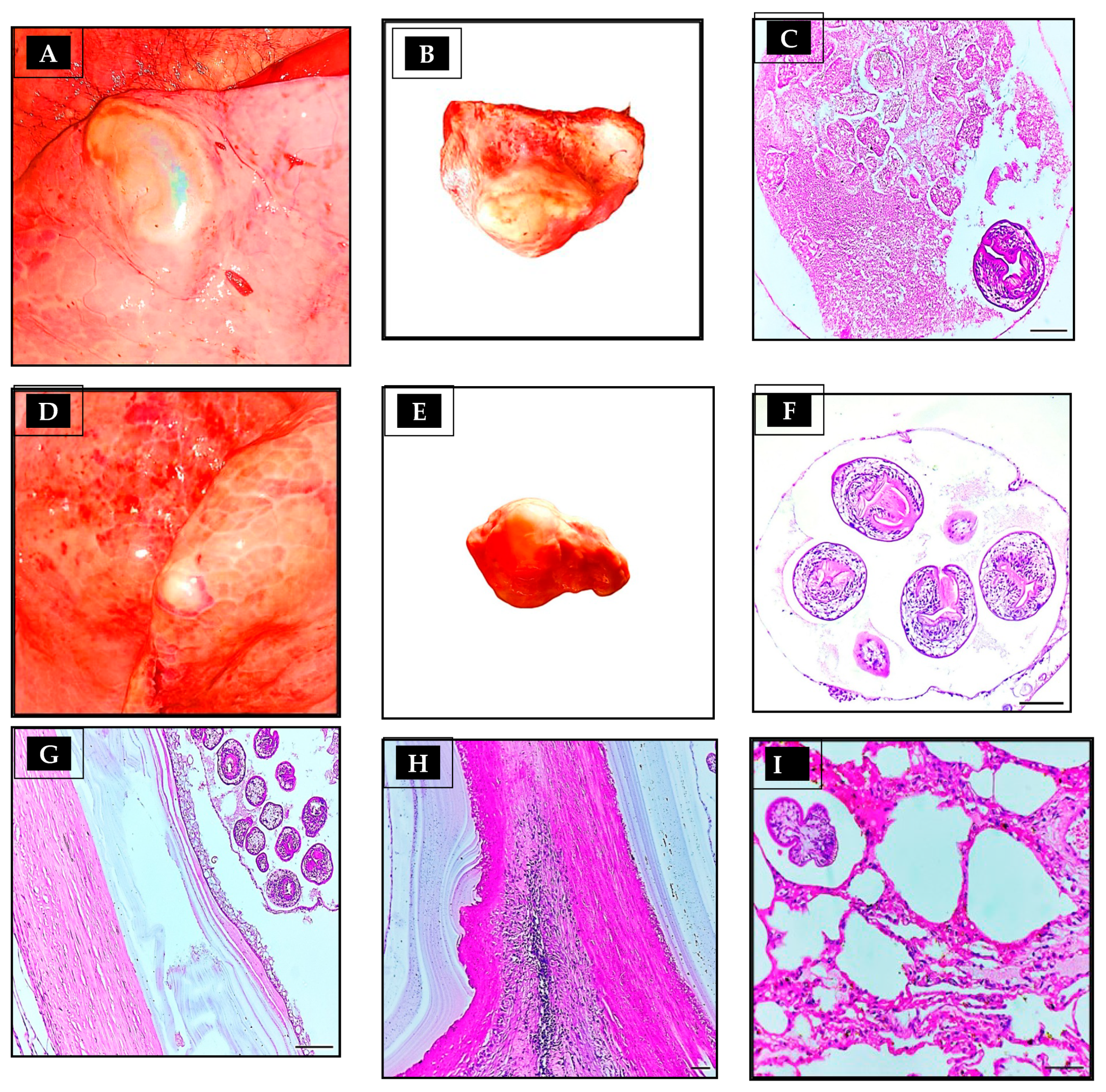
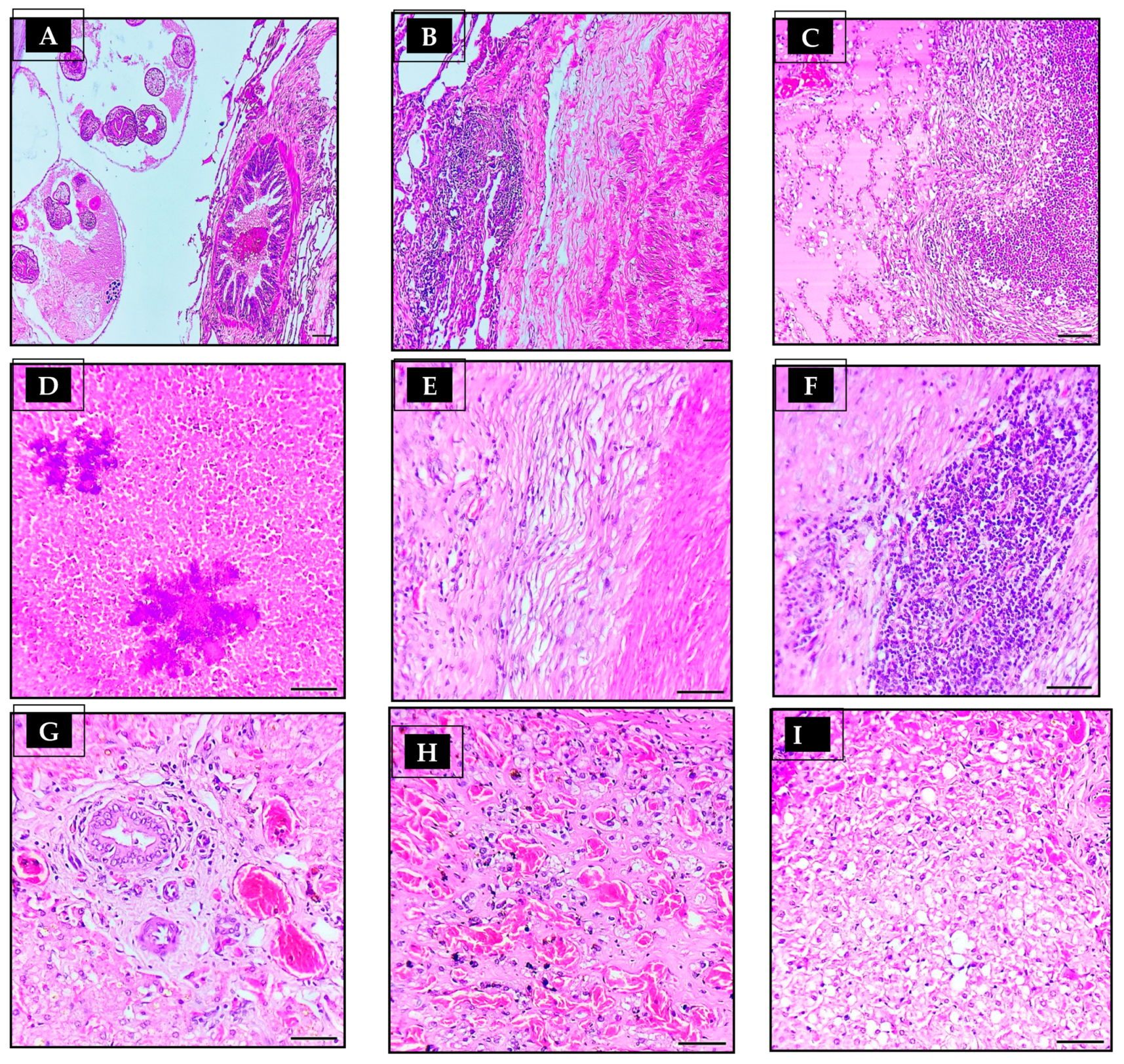
3.4. Histopathological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirkena, T.; Walelign, E.; Tewolde, N.; Gari, G.; Abebe, G.; Newman, S. Camel production systems in Ethiopia: A review of literature with notes on MERS-CoV risk factors. Pastoralism 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.; Ali, A. Seroprevalence and risk factors for C. burentii infection in camels in Egypt. Comp. Immunol. Microbiol. Infect. Dis. 2020, 68, 101402. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.; Marawan, M.A.; Ali, A.-F.; Manaa, E.; AbouelGhaut, H.A. Seroprevalence of bovine leukemia virus in cattle, buffalo, and camel in Egypt. Trop. Anim. Health Prod. 2020, 52, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.; Alsubki, R.A.; Albohairy, F.M.; Attia, K.A.; Kimiko, I. A survey of bluetongue infection in one-humped camels (Camelus dromedarius); seroprevalence and risk factors analysis. BMC Vet. Res. 2022, 18, 322. [Google Scholar] [CrossRef]
- Othman, O.E.; Abd El-Kader, H.A.; Alam, S.S.; Abd El-Aziem, S.H. Cytochrome b conservation between six camel breeds reared in Egypt. J. Genet. Eng. Biotechnol. 2017, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Babar, M.; Hussain, T.; Wajid, A.; Nawaz, A.; Nadeem, A.; Shah, S.; Shahid, M.; Ahmad, N.; Javed, K.; Abdullah, M. Mitochondrial cytochrome-b and D-loop sequence based genetic diversity in Mareecha and Bareela camel breeds of Pakistan. J. Anim. Plant Sci. 2015, 25, 591–594. [Google Scholar]
- Orazov, A.; Nadtochii, L.; Bozymov, K.; Muradova, M.; Zhumayeva, A. Role of camel husbandry in food security of the Republic of Kazakhstan. Agriculture 2021, 11, 614. [Google Scholar] [CrossRef]
- Baz, M.M.; Khater, H.F.; Baeshen, R.S.; Selim, A.; Shaheen, E.S.; El-Sayed, Y.A.; Salama, S.A.; Hegazy, M.M. Novel Pesticidal Efficacy of Araucaria heterophylla and Commiphora molmol Extracts against Camel and Cattle Blood-Sucking Ectoparasites. Plants 2022, 11, 1682. [Google Scholar] [CrossRef]
- Selim, A.; Attia, K.A.; Alsubki, R.A.; Kimiko, I.; Sayed-Ahmed, M.Z. Cross-sectional survey on Mycobacterium avium Subsp. paratuberculosis in Dromedary Camels: Seroprevalence and risk factors. Acta Trop. 2022, 226, 106261. [Google Scholar]
- Selim, A.; Alafari, H.A.; Attia, K.; AlKahtani, M.D.; Albohairy, F.M.; Elsohaby, I. Prevalence and animal level risk factors associated with Trypanosoma evansi infection in dromedary camels. Sci. Rep. 2022, 12, 8933. [Google Scholar] [CrossRef]
- Selim, A.; Abdelhady, A. Neosporosis among Egyptian camels and its associated risk factors. Trop. Anim. Health Prod. 2020, 52, 3381–3385. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.H.; Baz, M.M.; Ibrahim, M.; Radwan, I.T.; Selim, A.; Dawood, A.-F.D.; Taie, H.A.; Abdalla, S.; Khater, H.F. Acaricide resistance and novel photosensitizing approach as alternative acaricides against the camel tick, Hyalomma dromedarii. Photochem. Photobiol. Sci. 2022, 22, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Dyab, A.; Ahmed, M.; Abdelazeem, A. Histopathological studies on parasitic affections of lung and liver of humped Camels in Aswan slaughterhouses, Egypt. Assiut Vet. Med. J. 2019, 65, 22–26. [Google Scholar]
- Faraz, A.; Waheed, A.; Mirza, R.; Ishaq, H. Role of camel in food security: A perspective aspect. J. Fish. Livest. Prod 2019, 7, 290. [Google Scholar]
- El-Meleh, G.S.; Elmeghnawy, R.A.; Sabike, I.I.; Hassan, M.A. Parasitic affections of edible offales of slaughtered animals at El-Shohada abattoir, Monofia governorate, Egypt. Benha Vet. Med. J. 2019, 36, 117–128. [Google Scholar] [CrossRef]
- Salama, H.M.; Ahmed, N.H.; El Deeb, N.; Ahmed, R. Hepatic hydatid cysts: Sonographic follow-up after percutaneous sonographically guided aspiration. J. Clin. Ultrasound 1998, 26, 455–460. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Morsy, A.T. Liver hydatid in young age treated per-cutaneous by using Puncture-Aspiration-Injection-Reaspiration (PAIR) technique. J. Egypt. Soc. Parasitol. 2020, 50, 431–438. [Google Scholar] [CrossRef]
- Abdelbaset, A.E.; Yagi, K.; Nonaka, N.; Nakao, R. Cystic echinococcosis in humans and animals in Egypt: An epidemiological overview. Curr. Res. Parasitol. Vector Borne Dis. 2021, 1, 100061. [Google Scholar] [CrossRef]
- Latif, A.A.; Tanveer, A.; Maqbool, A.; Siddiqi, N.; Kyaw-Tanner, M.; Traub, R.J. Morphological and molecular characterisation of Echinococcus granulosus in livestock and humans in Punjab, Pakistan. Vet. Parasitol. 2010, 170, 44–49. [Google Scholar] [CrossRef]
- Hijjawi, N.S.; Al-Radaideh, A.M.; Rababah, E.M.; Al-Qaoud, K.M.; Bani-Hani, K.E. Cystic echinococcosis in Jordan: A review of causative species, previous studies, serological and radiological diagnosis. Acta Trop. 2018, 179, 10–16. [Google Scholar] [CrossRef]
- Yakhchali, M.; Asri-Rezaie, S.; Samimirad, S.; Rezaie, H. The enzymes and electrolytes profiles in hydatid cyst fluid of naturally infected Iranian domestic ruminants. J. Parasit. Dis. 2017, 41, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Ziino, G.; Giuffrida, A.; Panebianco, A.; Bilei, S. Bacteria isolated from 25 hydatid cysts in sheep, cattle and goats. Vet. Rec. 2009, 165, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Razi Jalali, M.H.; Jafari, A. Evaluation of hepatic antioxidant changes in ovine dicrocoeliosis. J. Parasit. Dis. 2015, 39, 766–769. [Google Scholar] [CrossRef]
- Cinar, M.; Aydenizöz, M.; Gokpinar, S.; Çamkerten, G. Evaluation of biochemical parameters and oxidative stress in sheep naturally infected with Dicrocoelium dendriticum and hydatid cysts. Turk. J. Vet. Anim. Sci. 2018, 42, 423–428. [Google Scholar] [CrossRef]
- Gabrashanska, M.; Petkova, S.; Teodorova, S.E. The antioxidant status in Trichinella spiralis-infected rats, improved by Selenium supplementation. Open J. Chem. 2019, 5, 001–008. [Google Scholar]
- Plancarte, A.; Nava, G. Oxygen and Redox Reactions Contribute to the Protection of Free-Living and Parasite Helminths against Pathogens and/or Host Response. In Parasitic Helminths and Zoonoses-From Basic to Applied Research; IntechOpen: London, UK, 2022. [Google Scholar]
- Dakkak, A. Echinococcosis/hydatidosis: A severe threat in Mediterranean countries. Vet. Parasitol. 2010, 174, 2–11. [Google Scholar] [CrossRef]
- Budke, C.M.; Deplazes, P.; Torgerson, P.R. Global socioeconomic impact of cystic echinococcosis. Emerg. Infect. Dis. 2006, 12, 296. [Google Scholar] [CrossRef] [PubMed]
- Abegaz, S.; Mohammode, A. Crossectional study on the prevalence and economic significance of hydatidosis in slaughtered ruminants at Debrezeit Elfora export abattoir Oromia region Eastern Showa Zone, Ethiopia. Biomed. J. Sci. Tech. Res. 2018, 3, 3273–3282. [Google Scholar] [CrossRef]
- Azlaf, R.; Dakkak, A. Epidemiological study of the cystic echinococcosis in Morocco. Vet. Parasitol. 2006, 137, 83–93. [Google Scholar] [CrossRef]
- Bardonnet, K.; Benchikh-Elfegoun, M.; Bart, J.; Harraga, S.; Hannache, N.; Haddad, S.; Dumon, H.; Vuitton, D.; Piarroux, R. Cystic echinococcosis in Algeria: Cattle act as reservoirs of a sheep strain and may contribute to human contamination. Vet. Parasitol. 2003, 116, 35–44. [Google Scholar] [CrossRef]
- Kassem, H.H.; Abdel-Kader, A.-K.M.; Nass, S.A. Prevalence of hydatid cysts in slaughtered animals in Sirte, Libya. J. Egypt. Soc. Parasitol. 2013, 43, 33–40. [Google Scholar] [PubMed]
- Oudni-M’rad, M.; M’rad, S.; Babba, H. Molecular and epidemiology data on cystic echinococcosis in Tunisia. In Current Topics in Echinococcosis; IntechOpen: Rijeka, Croatia, 2015; pp. 56–74. [Google Scholar]
- El Kordy, M. On the incidence of hydatid disease in domestic animals in Egypt. J. Egypt. Med. Assoc. 1946, 29, 265–279. [Google Scholar]
- Abdou, A. Incidence and public health importance of hydatidosis in the Middle East with special reference to UAR. J. Vet. Sci. U. A. R. 1965, 2, 125–134. [Google Scholar]
- Matossian, R.; Rickard, M.; Smyth, J. Hydatidosis: A global problem of increasing importance. Bull. World Health Organ. 1977, 55, 499. [Google Scholar]
- El-Refaie, S.A.; Bassiouny, G.; Marie, N.; Moris, E. Concomitant hepatic Fasciola and hydatid infections in animals. J. Egypt. Soc. Parasitol. 1984, 14, 421–427. [Google Scholar]
- Ahmed, L. Incidence of hydatid disease in camels slaughtered at Assiut abattoir. Assiut Vet. Med. J. 1991, 24, 274–278. [Google Scholar]
- Dyab, K.A.; Hassanein, R.; Hussein, A.; Metwally, S.E.; Gaad, H.M. Hydatidosis among man and animals in Assiut and Aswan Governorates. J. Egypt. Soc. Parasitol. 2005, 35, 157–166. [Google Scholar]
- El-Dakhly, K.M.; Arafa, W.M.; El-Nahass, E.-S.N.; Shokier, K.A.; Noaman, A.F. The current prevalence and diversity of cystic echinococcosis in slaughtered animals in Egypt. J. Parasit. Dis. 2019, 43, 711–717. [Google Scholar] [CrossRef]
- Gareh, A.; Saleh, A.A.; Moustafa, S.M.; Tahoun, A.; Baty, R.S.; Khalifa, R.M.; Dyab, A.K.; Yones, D.A.; Arafa, M.I.; Abdelaziz, A.R. Epidemiological, morphometric, and molecular investigation of cystic Echinococcosis in camel and cattle from upper Egypt: Current status and zoonotic implications. Front. Vet. Sci. 2021, 8, 750640. [Google Scholar] [CrossRef]
- Gindler, E.M.; King, J.D. Rapid colorimetric determination of calcium in biologic fluids with methylthymol blue. Am. J. Clin. Pathol. 1972, 58, 376–382. [Google Scholar] [CrossRef]
- Kliching, H.; Freiburg, B. Inorganic phosphorus in serum and alkaline phosphatase in serum. Clin. Photometery Wiss. Verl. Mbh. Stutgard 1951. [Google Scholar]
- Dawborn, J.; Patalinghug, C.; Black, S. Estimation of sodium, potassium, and chloride in urine using a Technicon AutoAnalyzer. J. Clin. Pathol. 1965, 18, 684. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Woldemeskel, M.; Issa, A.; Mersie, A.; Potgieter, L. Investigation of parasitic diseases of one-humped camel (Camelus dromedarius) in eastern Ethiopia. J. Camel Pract. Res. 2001, 8, 77–81. [Google Scholar]
- Ibrahim, M.M. Study of cystic echinococcosis in slaughtered animals in Al Baha region, Saudi Arabia: Interaction between some biotic and abiotic factors. Acta Trop. 2010, 113, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Jiao, W.; Osman, I.; Qu, Q.; Wang, H. A survey of Echinococcus granulosus infection in Camelus bactrianus in north Xinjiang. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi Chin. 1998, 16, 193–196. [Google Scholar]
- Ibrahem, M.; Craig, P. Prevalence of cystic echinococcosis in camels (Camelus dromedarius) in Libya. J. Helminthol. 1998, 72, 27–31. [Google Scholar] [CrossRef]
- Abdul-Salam, J.; Farah, M. Hydatidosis in camels in Kuwait. Parasitol. Res. 1988, 74, 267–270. [Google Scholar] [CrossRef]
- Njoroge, E.M.; Mbithi, P.M.F.; Gathuma, J.; Wachira, T.; Gathura, P.; Magambo, J.; Zeyhle, E. A study of cystic echinococcosis in slaughter animals in three selected areas of northern Turkana, Kenya. Vet. Parasitol. 2002, 104, 85–91. [Google Scholar] [CrossRef]
- Selim, A.; Manaa, E.; Khater, H. Seroprevalence and risk factors for lumpy skin disease in cattle in Northern Egypt. Trop. Anim. Health Prod. 2021, 53, 350. [Google Scholar] [CrossRef]
- Selim, A.; Almohammed, H.; Abdelhady, A.; Alouffi, A.; Alshammari, F.A. Molecular detection and risk factors for Anaplasma platys infection in dogs from Egypt. Parasit Vectors 2021, 14, 429. [Google Scholar] [CrossRef]
- Selim, A.; Megahed, A.A.; Kandeel, S.; Abdelhady, A. Risk factor analysis of bovine leukemia virus infection in dairy cattle in Egypt. Comp. Immunol. Microbiol. Infect. Dis. 2020, 72, 101517. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.B.; Ras, R.; Mahmoud, A.F.; El-Ghazaly, E.; Widmer, G.; Dahshan, H.; Elsohaby, I. Prevalence and bacterial isolation from hydatid cysts in dromedary camels (Camelus dromedarius) slaughtered at Sharkia abattoirs, Egypt. J. Parasit. Dis. 2021, 45, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Elham, M.; Hassan, B.; Ghasem, N.A.; Gholamreza, R.; Parviz, S. Epidemiological study of hydatidosis in the dromedaries (Camelus dromedarius) of different regions of Iran. Asian Pac. J. Trop. Biomed. 2014, 4, S148–S151. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Rezaei, H.; Nematollahi, A.; Ashrafihelan, J. Survey of hydatidosis infection in slaughtered camel (Camelus dromedarius) in Tabriz area, Northwest Iran. J. Parasit. Dis. 2016, 40, 444–447. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Ghamdi, M.; Gahmdi, M. Helminths community of veterinary importance of livestock in relation to some ecological and biological factors. Turk. Parazitol. Derg. 2008, 32, 42–47. [Google Scholar]
- Kebede, N.; Gebre-Egziabher, Z.; Tilahun, G.; Wossene, A. Prevalence and financial effects of hydatidosis in cattle slaughtered in Birre-Sheleko and Dangila Abattoirs, Northwestern Ethiopia. Zoonoses Public Health 2011, 58, 41–46. [Google Scholar] [CrossRef]
- Fathi, S.; Dehaghi, M.M.; Radfar, M.H. Fertility and viability rates of hydatid cysts in camels slaughtered in Kerman region, southeast of Iran. Sci. Parasitol. 2011, 12, 77–83. [Google Scholar]
- Ahmadi, N. Hydatidosis in camels (Camelus dromedarius) and their potential role in the epidemiology of Echinococcus granulosus in Iran. J. Helminthol. 2005, 79, 119–125. [Google Scholar] [CrossRef]
- Frayha, G.J.; Haddad, R. Comparative chemical composition of protoscolices and hydatid cyst fluid of Echinococcus granulosus (Cestoda). Int. J. Parasitol. 1980, 10, 359–364. [Google Scholar] [CrossRef]
- Chowdhury, N.; Singh, R. Distribution of some elements in hydatid cysts of Echinococcus granulosus from buffalo (Bubalus bubalis). J. Helminthol. 1993, 67, 112–114. [Google Scholar] [CrossRef]
- Sharif, M.; Keyghobadi, M.; Ziaei, H.; Izadi, J.; Gholami, S.; Khaliliyan, A. Measurement of biochemical components of liver hydatid cyst fluids in human, sheep, goat, cattle and camel Mazandaran 2004. J. Arak Univ. Med. Sci. 2005, 8, 24–31. [Google Scholar]
- Değer, S.; Değer, Y.; Ertekin, A.; Gül, A.; Biçek, K.; Ozdal, N. Determination of the status of lipid peroxidation and antioxidants in cattle infected with Dictyocaulus viviparus. Turk. Parazitolojii Derg. (Article in Turkish) 2008, 32, 234–237. [Google Scholar]
- Morel, F.; Doussiere, J.; Vignais, P.V. The superoxide-generating oxidase of phagocytic cells: Physiological, molecular and pathological aspects. Eur. J. Biochem. 1991, 201, 523–546. [Google Scholar] [CrossRef]
- Dede, S.; Deger, Y.; Deger, S.; Alkan, M. Bazı endoparazitlerle (Fasciola sp. + Trichostrongylidae sp. + Eimeria sp.) enfekte koyunlarda lipit peroksidasyonu ve antioksidan durumunun saptanması. Türkiye Parazitol. Derg. (Article in Turkish) 2000, 24, 190–193. [Google Scholar]
- Öz, N.; Kurtoğlu, F. Serbest radikaller ile antioksidan sistemler ve hastalıklarla ilişkileri. Veterinarium 2002, 13, 21–31. [Google Scholar]
- Samadieh, H.; Mohammadi, G.-R.; Maleki, M.; Borji, H.; Azizzadeh, M.; Heidarpour, M. Relationships between oxidative stress, liver, and erythrocyte injury, trace elements and parasite burden in sheep naturally infected with Dicrocoelium dendriticum. Iran. J. Parasitol. 2017, 12, 46–55. [Google Scholar] [PubMed]
- Sarin, K.; Kumar, A.; Prakash, A.; Sharma, A. before and after Chloroquine Treatment. Indian J. Malariolooy 1993, 30, 127–133. [Google Scholar]
- Shiferaw, F.; Bekele, W.; Giro, B.; Mequanint, Y. Epidemiology and economic importance of hydatidosis in domestic animal and human in Ethiopia-A Review. J. Vet. Sci. Technol. 2018, 9, 563. [Google Scholar]
- Haridy, F.M.; Ibrahim, B.B.; Elshazly, A.M.; Awad, S.E.; Sultan, D.M.; El-Sherbini, G.T.; Morsy, T.A. Hydatidosis granulosus in Egyptian slaughtered animals in the years 2000–2005. J. Egypt. Soc. Parasitol. 2006, 36, 1087–1100. [Google Scholar]
- Rahman, M.; Sokkar, S.; Dahab, S. Comparative studies on hydatidosis in farm animals in Egypt. DTW. Dtsch. Tierarztl. Wochenschr. 1992, 99, 438–440. [Google Scholar]
- Heath, D. The migration of oncospheres of Taenia pisiformis, T. serialis and Echinococcus granulosus within the intermediate host. Int. J. Parasitol. 1971, 1, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Ghada, M.; Osama, H. Seroprevalence of hydatidosis in camels of Assuit Province, Egypt. Madr. J. Vaccine 2017, 1, 1–4. [Google Scholar]
- Al-Khayat, F.A.A.-M. Prevalence and public health importance of hydatidosis in sheep slaughtered by unlicensed ways. Biomed. Pharmacol. J. 2019, 12, 399–402. [Google Scholar] [CrossRef]
- Saad, M.; Magzoub, M. Hydatidosis in sheep and goats in the Sudan. Sud. J. Vet. Sci. Anim. Husb 1989, 28, 33–37. [Google Scholar]
| Variable | Total Examined Camels | No. of Positive | % | p Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 82 | 15 | 18.29 | 0.269 * |
| Female | 70 | 18 | 25.71 | |
| Age | ||||
| <4 years | 120 | 27 | 22.50 | 0.648 * |
| >4 years | 32 | 6 | 18.75 | |
| Total | 152 | 33 | 21.7 |
| Animal | Organ | Cyst Status | Fluid Status | Fluid Color | Size (cm) | Fluid Amount (mL) | Sodium (mmol/L) | Potassium (mmol/L) | Calcium (mg/100 mL) | Phosphorus (mg/100 mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lungs | Sterile | Watery | Clear | 4 | 9 | 61.4 | 2 | 42 | 0.7 |
| 2 | Lungs | Fertile | Thick | Turbid | 6 | 10 | 60.3 | 0.3 | 33 | 1.3 |
| 3 | Lungs | Fertile | Watery | Clear | 4 | 8 | 35.9 | 1.8 | 9 | 4.5 |
| 4 | Liver | Sterile | Watery | Clear | 3 | 7 | 55 | 1.4 | 13 | 1 |
| 5 | lungs | Sterile | Watery | Clear | 8 | 15 | 44.4 | 1.8 | 66 | 1.4 |
| 6 | Lungs | Fertile | Thick | Turbid | 7 | 12 | 64.5 | 0.6 | 39 | 1.5 |
| 7 | Lungs | Sterile | Watery | Clear | 7 | 11 | 59 | 0.9 | 55 | 1.8 |
| 8 | Liver | Sterile | Watery | Clear | 2 | 5 | 50.1 | 1.4 | 13 | 0.9 |
| 9 | Lungs | Fertile | Watery | Clear | 6 | 12 | 40.5 | 1.5 | 71 | 2.5 |
| 10 | Lungs | Fertile | Watery | Clear | 7 | 16 | 34.8 | 1.7 | 62 | 1.3 |
| 11 | Lungs | Fertile | Watery | Clear | 8 | 16 | 48 | 1.8 | 60 | 1.2 |
| 12 | Lungs | Fertile | Thick | Turbid | 7 | 10 | 67.3 | 0.8 | 40 | 1.7 |
| 13 | Liver | Sterile | Watery | Clear | 3 | 6 | 52.7 | 1.3 | 11 | 1.1 |
| 14 | Lungs | Fertile | Watery | Clear | 8 | 15 | 40 | 1.6 | 60 | 1.3 |
| 15 | Lungs | Fertile | Watery | Clear | 5 | 10 | 72 | 2.2 | 50 | 0.8 |
| 16 | Lungs | Fertile | Thick | Turbid | 7 | 9 | 66.2 | 0.7 | 38 | 1.7 |
| 17 | Lungs | Fertile | Watery | Clear | 5 | 9 | 35.5 | 1.1 | 84 | 2 |
| 18 | Lungs | Sterile | Watery | Clear | 3.5 | 8 | 68.1 | 2.1 | 44 | 0.4 |
| 19 | Lungs | Fertile | Watery | Clear | 6 | 13 | 45.4 | 1.5 | 25 | 1.4 |
| 20 | Lungs | Fertile | Watery | Clear | 6 | 14 | 35.3 | 1.3 | 38 | 1.3 |
| 21 | Liver | Sterile | Watery | Clear | 2.5 | 5 | 43.7 | 1.7 | 19 | 1 |
| 22 | Liver | Sterile | Watery | Clear | 2 | 5 | 40.2 | 1.7 | 22 | 0.9 |
| 23 | Lungs | Fertile | Watery | Clear | 4 | 9 | 38.5 | 1.7 | 10 | 4.8 |
| 24 | Lungs | Sterile | Watery | Clear | 4.5 | 10 | 51 | 1.5 | 59 | 0.6 |
| 25 | Liver | Sterile | Watery | Clear | 2 | 4 | 69 | 0.7 | 39 | 0.4 |
| 26 | Lungs | Fertile | Thick | Turbid | 4 | 4 | 66.1 | 0.5 | 40 | 1.4 |
| 27 | Lungs | Fertile | Thick | Turbid | 8 | 6 | 62 | 0.4 | 33 | 1.3 |
| 28 | Lungs | Sterile | Watery | Clear | 5 | 11 | 48.3 | 1.7 | 40 | 0.9 |
| 29 | Lungs | Fertile | Thick | Turbid | 7 | 11 | 45.8 | 0.5 | 30 | 1.1 |
| 30 | Lungs | Fertile | Watery | Clear | 5 | 8 | 33.2 | 1.6 | 15 | 1.9 |
| 31 | lungs | Sterile | Watery | Clear | 5 | 12 | 40.1 | 1.5 | 57 | 1.5 |
| 32 | Lungs | Fertile | Thick | Turbid | 6 | 9 | 60.1 | 0.9 | 40 | 1.4 |
| 33 | Lungs | Sterile | Watery | Clear | 7 | 13 | 53.2 | 1.3 | 51 | 1.6 |
| Animal | MDA (nmol/mL) | GSH (U/mL) | SOD (U/mL) | CAT (mM/L) |
|---|---|---|---|---|
| Infected | 6.61 ± 19 | 3.32 ± 0.13 | 78.07 ± 0.96 | 113.52 ± 6.84 |
| Control | 1.96 ± 0.06 | 7.04 ± 0.12 | 122.64 ± 1.65 | 157.77 ± 1.48 |
| p value | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoulah, S.A.; Gaballa, M.M.S.; Marawan, M.A.; Saqr, S.A.; Abdelhady, A.; Alzahrani, H.A.; Wakid, M.H.; Al-Jabr, O.A.; Selim, A. Pathological Findings and Oxidative Stress Status Associated with Hydatidosis in Dromedary Camels. Vet. Sci. 2023, 10, 74. https://doi.org/10.3390/vetsci10020074
Shoulah SA, Gaballa MMS, Marawan MA, Saqr SA, Abdelhady A, Alzahrani HA, Wakid MH, Al-Jabr OA, Selim A. Pathological Findings and Oxidative Stress Status Associated with Hydatidosis in Dromedary Camels. Veterinary Sciences. 2023; 10(2):74. https://doi.org/10.3390/vetsci10020074
Chicago/Turabian StyleShoulah, Salma A., Mohamed M. S. Gaballa, Marawan A. Marawan, Sayed A. Saqr, Abdelhamed Abdelhady, Hayat Ali Alzahrani, Majed H. Wakid, Omar A. Al-Jabr, and Abdelfattah Selim. 2023. "Pathological Findings and Oxidative Stress Status Associated with Hydatidosis in Dromedary Camels" Veterinary Sciences 10, no. 2: 74. https://doi.org/10.3390/vetsci10020074
APA StyleShoulah, S. A., Gaballa, M. M. S., Marawan, M. A., Saqr, S. A., Abdelhady, A., Alzahrani, H. A., Wakid, M. H., Al-Jabr, O. A., & Selim, A. (2023). Pathological Findings and Oxidative Stress Status Associated with Hydatidosis in Dromedary Camels. Veterinary Sciences, 10(2), 74. https://doi.org/10.3390/vetsci10020074








