Evolution of the Prevalence of Antibiotic Resistance to Staphylococcus spp. Isolated from Horses in Florida over a 10-Year Period
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dallap Schaer, B.L.; Linton, J.K.; Aceto, H. Antimicrobial use in horses undergoing colic surgery. J. Vet. Intern. Med. 2012, 26, 1449–1456. [Google Scholar] [CrossRef]
- Loeffler, A.; Lloyd, D.H. What has changed in canine pyoderma? A narrative review. Vet. J. 2018, 235, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Murayama, N.; Nagata, M.; Terada, Y.; Shibata, S.; Fukata, T. Efficacy of a surgical scrub including 2% chlorhexidine acetate for canine superficial pyoderma. Vet. Dermatol. 2010, 21, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Normark, B.H.; Normark, S. Evolution and Spread of Antibiotic Resistance. J. Intern. Med. 2002, 252, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Peyrou, M.; Higgins, R.; Lavoie, J.P. Evolution of bacterial resistance to certain antibacterial agents in a veterinary hospital. Can. Vet. J. 2003, 44, 978–981. [Google Scholar] [PubMed]
- Malo, A.; Cluzel, C.; Labrecque, O.; Beauchamp, G.; Lavoie, J.; Leclere, M. Evolution of in vitro antimicrobial resistance in an equine hospital over 3 decades. Can. Vet. J. 2016, 57, 747–751. [Google Scholar] [PubMed]
- Melzer, M.; Eykyn, S.J.; Graunsden, W.R.; Chinn, S. Is methicillin-resistant Staphylococcus aureus more virulent than methicillin-susceptible S. aureus? A comparative cohort study of British patients with nosocomial infection and bacteremia. Clin. Infect. Dis. 2003, 37, 1453–1460. [Google Scholar] [CrossRef]
- Blot, S.I.; Vandewoude, K.H.; Hoste, E.A.; Colardyn, F.A. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch. Intern. Med. 2002, 162, 2229–2235. [Google Scholar] [CrossRef]
- Engemann, J.J.; Carmeli, Y.; Cosgrove, S.E.; Fowler, V.G.; Bronstein, M.Z.; Trivette, S.L.; Briggs, J.P.; Sexton, D.J.; Kaye, K.S. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin. Infect. Dis. 2003, 36, 592–598. [Google Scholar] [CrossRef]
- Jones, R.D.; Kania, S.A.; Rohrbach, B.W.; Frank, L.A.; Bemis, D.A. Prevalence of oxacillin- and multidrug-resistant staphylococci in clinical samples from dogs: 1772 samples (2001–2005). J. Am. Vet. Med. Assoc. 2007, 230, 221–227. [Google Scholar] [CrossRef]
- Yoon, J.W.; Lee, K.J.; Lee, S.Y.; Chae, M.J.; Park, J.K.; Yoo, J.H.; Park, H.M. Antibiotic resistance profiles of Staphylococcus pseudintermedius isolates from canine patients in Korea. J. Microbiol. Biotechnol. 2010, 20, 1764–1768. [Google Scholar]
- Penna, B.; Varges, R.; Medeiros, L.; Martins, G.M.; Martins, R.R.; Lilenbaum, W. Species distribution and antimicrobial susceptibility of staphylococci isolated from canine otitis externa. Vet. Dermatol. 2009, 21, 292–296. [Google Scholar] [CrossRef]
- Weese, J.S.; Yu, A.A. Infectious folliculitis and dermatophytosis. Vet. Clin. N. Am. Equine Pract. 2013, 29, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Schnellmann, C.; Gerber, V.; Rossano, A.; Jaquier, V.; Panchaud, Y.; Doherr, M.G.; Thomann, A.; Straub, R.; Perreten, V. Presence of new mecA and mph(C) variants conferring antibiotic resistance in Staphylococcus spp. isolated from the skin of horses before and after clinic admission. J. Clin. Microbiol. 2006, 44, 4444–4454. [Google Scholar] [CrossRef] [PubMed]
- Miragaia, M. Factors Contributing to the Evolution of mecA-Mediated β-lactam Resistance in Staphylococci: Update and New Insights from Whole Genome Sequencing (WGS). Front. Microbiol. 2018, 9, 2723. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, R.H.; Stobberingh, E.E. The molecular evolution of hospital- and community-associated methicillin-resistant Staphylococcus aureus. Curr. Mol. Med. 2009, 9, 100–115. [Google Scholar] [CrossRef]
- Foster, T. Staphylococcus . In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 12. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8448/ (accessed on 11 August 2022).
- Rich, M. Staphylococci in animals: Prevalence, identification and antimicrobial susceptibility, with an emphasis on methicillin-resistant Staphylococcus aureus. Br. J. Biomed. Sci. 2005, 62, 98–105. [Google Scholar] [CrossRef]
- Panchaud, Y.; Gerber, V.; Rossano, A.; Perreten, V. Bacterial infections in horses: A retrospective study at the University Equine Clinic of Bern. Schweizer Arch. Tierheilkd. 2010, 152, 176–182. [Google Scholar] [CrossRef]
- Sangiorgio, D.B.; Hilty, M.; Kaiser-Thom, S.; Epper, P.G.; Ramseyer, A.A.; Overesch, G.; Gerber, V.M. The influence of clinical severity and topical antimicrobial treatment on bacteriological culture and the microbiota of equine pastern dermatitis. Vet. Dermatol. 2021, 32, 173-e141. [Google Scholar] [CrossRef]
- de Martino, L.; Lucido, M.; Mallardo, K.; Facello, B.; Mallardo, M.; Iovane, G.; Pagnini, U.; Tufano, M.A.; Catalanotti, P. Methicillin-resistant staphylococci isolated from healthy horses and horse personnel in Italy. J. Vet. Diagn. Investig. 2010, 22, 77–82. [Google Scholar] [CrossRef]
- Huber, H.; Ziegler, D.; Pflüger, V.; Vogel, G.; Zweifel, C.; Stephan, R. Prevalence and characteristics of methicillin-resistant coagulase-negative staphylococci from livestock, chicken carcasses, bulk tank milk, minced meat, and contact persons. BMC Vet. Res. 2011, 7, 6. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 1st ed.; CLSI Supplement VET01S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2004. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 1st ed.; CLSI Supplement VET01S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 1st ed.; CLSI Supplement VET01S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- The Ohio State University College of Veterinary Medicine. OSU VMC Antimicrobial Use Guidelines; The Ohio State University College of Veterinary Medicine: Columbus, OH, USA, 2018; Available online: https://ohiostate.pressbooks.pub/osuvmcabxuse/ (accessed on 26 June 2022).
- Riviere, J.E.; Papich, M.G. Veterinary Pharmacology and Therapeutics, 10th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Marsella, R. Manual of Equine Dermatology; CABI International: Oxfordshire, UK, 2019; pp. 108–147. [Google Scholar]
- Scott, D.W.; Miller, W.H. Equine Dermatology, 2nd ed.; Bacterial skin diseases; Elsevier Saunders: St. Louis, MO, USA, 2011; pp. 130–170. [Google Scholar]
- Bourély, C.; Cazeau, G.; Jarrige, N.; Haenni, M.; Gay, E.; Leblond, A. Antimicrobial resistance in bacteria isolated from diseased horses in France. Equine Vet. J. 2020, 52, 112–119. [Google Scholar] [CrossRef]
- Frank, L.A.; Loeffler, A. Meticillin-resistant Staphylococcus pseudintermedius: Clinical challenge and treatment options. Vet. Dermatol. 2012, 23, 283–291.e56. [Google Scholar] [CrossRef]
- Cain, C.L.; Morris, D.O.; Rankin, S.C. Clinical characterization of Staphylococcus schleiferi infections and identification of risk factors for acquisition of oxacillin-resistant strains in dogs: 225 cases (2003–2009). J. Am. Vet. Med. Assoc. 2011, 239, 1566–1573. [Google Scholar] [CrossRef]
- Griffeth, G.C.; Morris, D.O.; Abraham, J.L.; Shofer, F.S.; Rankin, S.C. Screening for skin carriage of methicillin-resistant coagulase-positive staphylococci and Staphylococcus schleiferi in dogs with healthy and inflamed skin. Vet. Dermatol. 2008, 19, 142–149. [Google Scholar] [CrossRef]
- Khanal, S.; Boonyayatra, S.; Awaiwanont, N. Prevalence of Methicillin-Resistant Staphylococcus aureus in Dairy Farms: A Systematic Review and Meta-Analysis. Front. Vet. Sci. 2022, 9, 947154. [Google Scholar] [CrossRef]
- Dorado-García, A.; Dohmen, W.; Bos, M.E.; Verstappen, K.M.; Houben, M.; Wagenaar, J.A.; Heederik, D.J. Dose-response relationship between antimicrobial drugs and livestock-associated MRSA in pig farming. Emerg. Infect. Dis. 2015, 21, 950–959. [Google Scholar] [CrossRef]
- Fishovitz, J.; Hermoso, J.A.; Chang, M.; Mobashery, S. Penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. IUBMB Life 2014, 66, 572–577. [Google Scholar] [CrossRef]
- Schnepf, A.; Bienert-Zeit, A.; Ertugrul, H.; Wagels, R.; Werner, N.; Hartmann, M.; Feige, K.; Kreienbrock, L. Antimicrobial Usage in Horses: The Use of Electronic Data, Data Curation, and First Results. Front. Vet. Sci. 2020, 7, 216. [Google Scholar] [CrossRef]
- Zur, G.; Gurevich, B.; Elad, D. Prior antimicrobial use as a risk factor for resistance in selected Staphylococcus pseudintermedius isolates from the skin and ears of dogs. Vet. Dermatol. 2016, 27, 468-e125. [Google Scholar] [CrossRef]
- Weber, S.G.; Gold, H.S.; Hooper, D.C.; Karchmer, A.W.; Carmeli, Y. Fluoroquinolones and the risk for methicillin-resistant Staphylococcus aureus in hospitalized patients. Emerg. Infect. Dis. 2003, 9, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, L.; Pépin, J.; Toulouse, K.; Ouellette, M.F.; Coulombe, M.A.; Corriveau, M.P.; Alary, M.E. Fluoroquinolones and risk for methicillin-resistant Staphylococcus aureus, Canada. Emerg. Infect. Dis. 2006, 12, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- John, J.F.; Harvin, A.M. History and evolution of antibiotic resistance in coagulase-negative staphylococci: Susceptibility profiles of new anti-staphylococcal agents. Ther. Clin. Risk Manag. 2007, 3, 1143–1152. [Google Scholar] [PubMed]
| Pyoderma | Superficial Wounds | Abscesses | Surgical Incision Sites | Nose | Foot | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Erythromycin | 28/36 | 77.8 | 8/10 | 80 | 13/15 | 86.7 | 3/5 | 60 | 6/8 | 75 | 1/3 | 33.3 |
| Gentamicin | 24/36 | 66.7 | 7/10 | 70 | 15/15 | 100 | 1/5 | 20 | 7/8 | 87.5 | 2/3 | 66.7 |
| Imipenem | 30/36 | 83.3 | 8/10 | 80 | 12/15 | 80 | 3/5 | 60 | 5/8 | 62.5 | 1/3 | 33.3 |
| Oxacillin | 25/30 | 83.3 | 7/9 | 77.8 | 11/14 | 78.6 | 3/5 | 60 | 5/8 | 62.5 | 1/3 | 33.3 |
| Rifampin | 36/36 | 100 | 9/10 | 90 | 14/15 | 93.3 | 5/5 | 100 | 8/8 | 100 | 3/3 | 100 |
| Amikacin | 23/28 | 82.1 | 8/10 | 80 | 13/14 | 92.9 | 1/2 | 50 | 6/6 | 100 | 2/2 | 100 |
| Ampicillin | 20/32 | 62.5 | 5/10 | 50 | 12/15 | 80 | 2/4 | 50 | 3/6 | 50 | 1/1 | 100 |
| Azithromycin | 29/36 | 80.6 | 9/10 | 90 | 12/14 | 85.7 | 3/5 | 60 | 6/8 | 75 | 1/3 | 33.3 |
| Cefazolin | 15/35 | 42.9 | 3/10 | 30 | 8/15 | 53.3 | 2/5 | 40 | 4/7 | 57.1 | 1/3 | 33.3 |
| Chloramphenicol | 34/36 | 94.4 | 8/10 | 80 | 15/15 | 100 | 5/5 | 100 | 7/7 | 100 | 2/3 | 66.7 |
| Clarithromycin | 22/28 | 78.6 | 8/9 | 88.9 | 11/13 | 84.6 | 1/3 | 33.3 | 4/6 | 66.7 | 1/1 | 100 |
| Doxycycline | 21/22 | 95.5 | 5/6 | 83.3 | 13/14 | 92.9 | 2/2 | 100 | 5/6 | 83.3 | 1/1 | 100 |
| Penicillin | 18/32 | 56.3 | 5/10 | 50 | 11/14 | 78.6 | 2/4 | 50 | 3/6 | 50 | 1/1 | 100 |
| Tetracycline | 29/36 | 80.6 | 6/9 | 66.7 | 13/14 | 92.9 | 3/5 | 60 | 7/8 | 87.5 | 3/3 | 100 |
| TMS | 26/36 | 72.2 | 6/9 | 66.7 | 13/15 | 86.7 | 3/5 | 60 | 5/8 | 62.5 | 2/3 | 66.7 |
| Variable | by Variable | Spearman ρ | Prob > |ρ| | Difference Plot |
|---|---|---|---|---|
| Date | Amikacin | –0.1582 | 0.2234 |  |
| Date | Ampicillin | 0.2649 | 0.0290 |  |
| Date | Azithromycin | –0.0212 | 0.8559 | 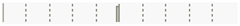 |
| Date | Cefazolin | 0.3012 | 0.0086 |  |
| Date | Chloramphenicol | –0.1176 | 0.3116 |  |
| Date | Clarithromycin | 0.1034 | 0.4316 |  |
| Date | Doxycycline | –0.0842 | 0.5567 |  |
| Date | Erythromycin | 0.0135 | 0.9070 | 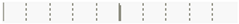 |
| Date | Gentamycin | 0.0635 | 0.5831 |  |
| Date | Imipenem | –0.1567 | 0.1735 |  |
| Date | Oxacillin | –0.1013 | 0.4074 |  |
| Date | Penicillin | 0.2541 | 0.0380 |  |
| Date | Rifampin | –0.0404 | 0.7271 | 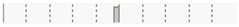 |
| Date | Tetracycline | 0.0379 | 0.7466 | 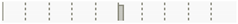 |
| Date | TMS | 0.1697 | 0.1428 |  |
| Ampicillin | ||||||||
| IG | -IG | Score Mean Difference | Std Err Dif | Z | p-Value | Lower CL | Upper CL | Difference Plot |
| 3 | 1 | 13.9167 | 4.871794 | 2.85658 | 0.0348 * | 0.000 | 1.000 |  |
| 4 | 1 | 0.0000 | 4.565305 | 0.00000 | 1.000 | –1.0000 | 1.000 | 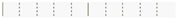 |
| Azithromycin | ||||||||
| IG | -IG | Score Mean Difference | Std Err Dif | Z | p-Value | Lower CL | Upper CL | Difference Plot |
| 2 | 1 | 13.0952 | 4.160561 | 3.14747 | 0.0142 * | 0.000 | 1.000 |  |
| 3 | 1 | 11.3021 | 3.968627 | 2.84786 | 0.0356 * | 0.000 | 1.000 |  |
| 5 | 1 | 1.6875 | 3.712311 | 0.45457 | 0.9912 | 0.000 | 0.500 |  |
| 3 | 2 | –0.4018 | 2.004459 | –0.20045 | 0.9996 | –1.000 | 1.000 |  |
| 5 | 4 | –0.6190 | 1.585838 | –0.39036 | 0.9951 | –1.000 | 1.000 | 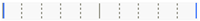 |
| 4 | 3 | –1.4732 | 1.968340 | –0.74846 | 0.9450 | –1.000 | 1.000 | 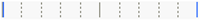 |
| Cefazolin | ||||||||
| IG | -IG | Score Mean Difference | Std Err Dif | Z | p-Value | Lower CL | Upper CL | Difference Plot |
| 3 | 1 | 18.0652 | 5.672817 | 3.18451 | 0.0126 * | 0.000 | 1.000 |  |
| 5 | 3 | –5.6875 | 2.075498 | –2.74031 | 0.0483 * | –1.000 | 0.000 |  |
| Clarithromycin | ||||||||
| IG | -IG | Score Mean Difference | Std Err Dif | Z | p-Value | Lower CL | Upper CL | Difference Plot |
| 2 | 1 | 11.8611 | 3.500000 | 3.38889 | 0.0063 * | 0.000 | 1.000 |  |
| 5 | 4 | 0.0000 | 1.610235 | 0.00000 | 1.0000 | . | . | 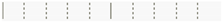 |
| 5 | 3 | –0.7857 | 1.739253 | –0.45175 | 0.9914 | . | . |  |
| 4 | 3 | –0.8571 | 1.860521 | –0.46070 | 0.9907 | –1.000 | 1.000 |  |
| 3 | 2 | –0.9286 | 1.947220 | –0.47687 | 0.9895 | –1.000 | 1.000 |  |
| Erythromycin | ||||||||
| IG | -IG | Score Mean Difference | Std Err Dif | Z | p-Value | Lower CL | Upper CL | Difference Plot |
| 2 | 1 | 14.5104 | 4.132282 | 3.51148 | 0.0041 * | 0.000 | 1.000 |  |
| 3 | 1 | 14.1458 | 4.139495 | 3.41729 | 0.0057 * | 0.000 | 1.000 |  |
| 5 | 1 | 1.5938 | 3.708735 | 0.42973 | 0.9929 | 0.000 | 0.000 |  |
| 3 | 2 | –0.5000 | 2.091650 | –0.23905 | 0.9993 | –1.000 | 1.000 |  |
| 5 | 4 | –0.6190 | 1.585838 | –0.39036 | 0.9951 | –1.000 | 1.000 |  |
| Imipenem | ||||||||
| IG | -IG | Score Mean Difference | Std Err Dif | Z | p-Value | Lower CL | Upper CL | Difference Plot |
| 3 | 1 | 17.4271 | 4.427189 | 3.93638 | 0.0008 * | 0.000 | 1.0000 |  |
| Oxacillin | ||||||||
| IG | -IG | Score Mean Difference | Std Err Dif | Z | p-Value | Lower CL | Upper CL | Difference Plot |
| 3 | 1 | 20.6250 | 4.491474 | 4.59203 | <0.0001 * | 0 | 1.000 |  |
| 2 | 1 | 18.3292 | 4.720480 | 3.88290 | 0.0010 * | 0 | 1.000 |  |
| 3 | 2 | 0.1714 | 1.367527 | 0.12536 | 0.9999 | . | . |  |
| 4 | 2 | –1.1250 | 1.509346 | –0.74536 | 0.9458 | . | . |  |
| 5 | 1 | –2.6500 | 3.674889 | –0.72111 | 0.9517 | 0 | 0.000 |  |
| Penicillin | ||||||||
| IG | -IG | Score Mean Difference | Std Err Dif | Z | p-Value | Lower CL | Upper CL | Difference Plot |
| 3 | 1 | 17.4167 | 4.923900 | 3.53717 | 0.0037 * | 0.000 | 1.000 |  |
| 4 | 1 | 0.0000 | 4.565305 | 0.00000 | 1.0000 | –1.000 | 1.000 |  |
| 5 | 4 | –0.3429 | 1.588923 | –0.21578 | 0.9995 | . | . |  |
| 5 | 1 | –1.9024 | 5.026786 | –0.37845 | 0.9957 | –1.000 | 1.000 |  |
| Antibiotic | Staphylococcus aureus | Non-Hemolytic Staphylococcus | MG | Staphylococcus pseudintermedius | Beta-Hemolytic Staphylococcus | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |
| Oxacillin | 5/48 | 10.4 | 4/5 | 80 | 6/7 | 85.7 | 2/4 | 50 | 0/5 | 0 |
| Penicillin | 12/42 | 28.6 | 5/6 | 83.3 | 7/7 | 100 | 2/7 | 28.6 | 1/5 | 20 |
| Ampicillin | 12/42 | 28.6 | 4/7 | 57.1 | 6/7 | 85.7 | 2/7 | 28.6 | 1/5 | 20 |
| TMS | 12/47 | 25.5 | 3/8 | 37.5 | 3/8 | 37.5 | 2/7 | 28.6 | 1/6 | 16.7 |
| Cefazolin | 5/47 | 10.6 | 4/8 | 50 | 6/8 | 75 | 1/6 | 16.7 | 0/6 | 0 |
| Tetracycline | 10/48 | 20.8 | 1/6 | 16.7 | 1/8 | 12.5 | 1/7 | 14.3 | 1/6 | 16.7 |
| Rifampin | 1/48 | 2.1 | 0/8 | 0 | 0/8 | 0 | 0/7 | 0 | 1/6 | 16.7 |
| Antibiotic | Staphylococcus aureus | Non-Hemolytic Staphylococcus | MG | Staphylococcus pseudintermedius | Beta-Hemolytic Staphylococcus | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |
| Cefazolin | 18/47 | 38.3 | 3/8 | 37.5 | 1/8 | 12.5 | 3/6 | 50 | 1/6 | 16.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marshall, K.; Marsella, R. Evolution of the Prevalence of Antibiotic Resistance to Staphylococcus spp. Isolated from Horses in Florida over a 10-Year Period. Vet. Sci. 2023, 10, 71. https://doi.org/10.3390/vetsci10020071
Marshall K, Marsella R. Evolution of the Prevalence of Antibiotic Resistance to Staphylococcus spp. Isolated from Horses in Florida over a 10-Year Period. Veterinary Sciences. 2023; 10(2):71. https://doi.org/10.3390/vetsci10020071
Chicago/Turabian StyleMarshall, Kalie, and Rosanna Marsella. 2023. "Evolution of the Prevalence of Antibiotic Resistance to Staphylococcus spp. Isolated from Horses in Florida over a 10-Year Period" Veterinary Sciences 10, no. 2: 71. https://doi.org/10.3390/vetsci10020071
APA StyleMarshall, K., & Marsella, R. (2023). Evolution of the Prevalence of Antibiotic Resistance to Staphylococcus spp. Isolated from Horses in Florida over a 10-Year Period. Veterinary Sciences, 10(2), 71. https://doi.org/10.3390/vetsci10020071





