Identification of Ameloblastin as an Amyloid Precursor Protein of Amyloid-Producing Ameloblastoma in Dogs and Cats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Information
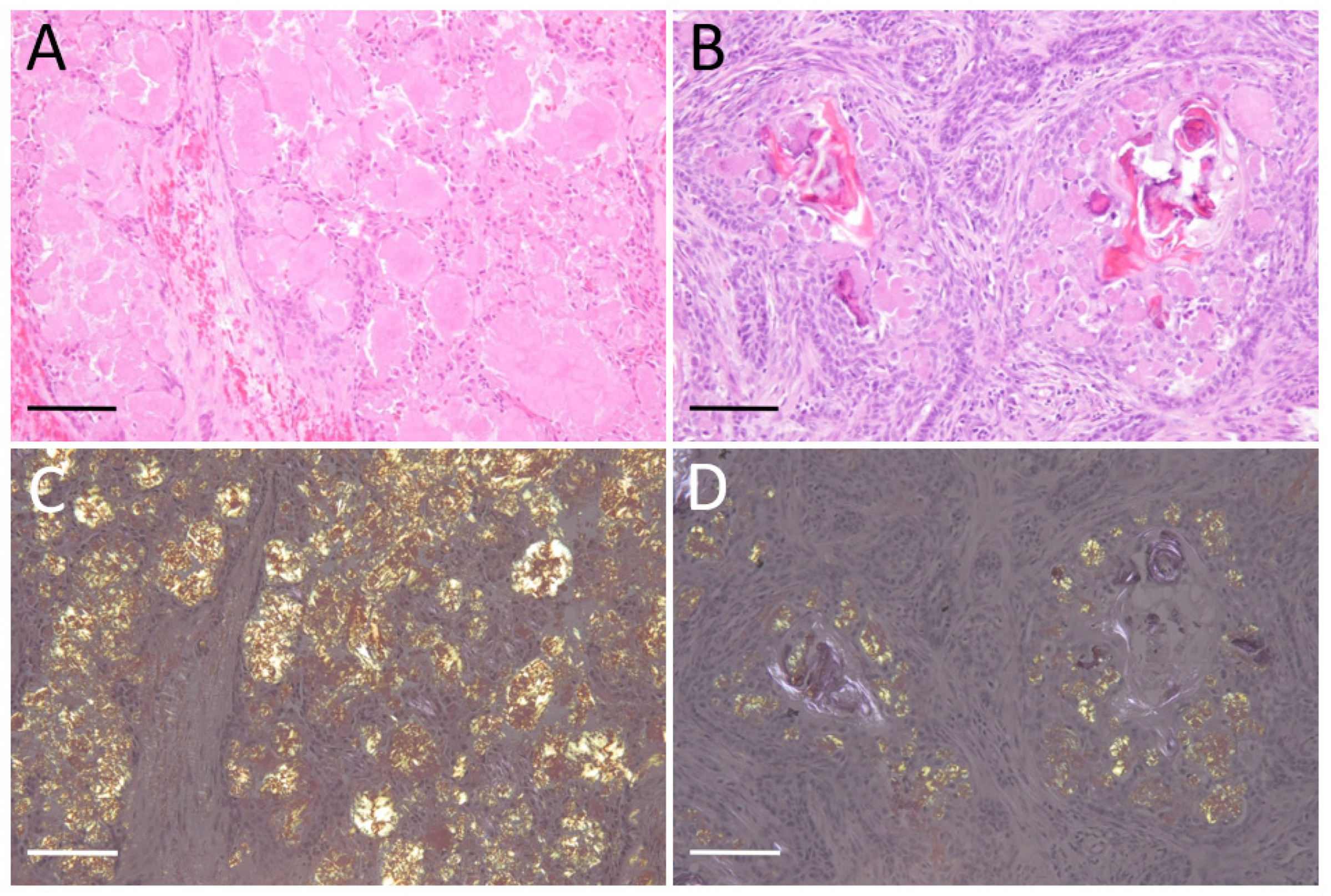
2.2. Histopathological Analysis
2.3. Mass-Spectrometry-Based Proteomic Analysis
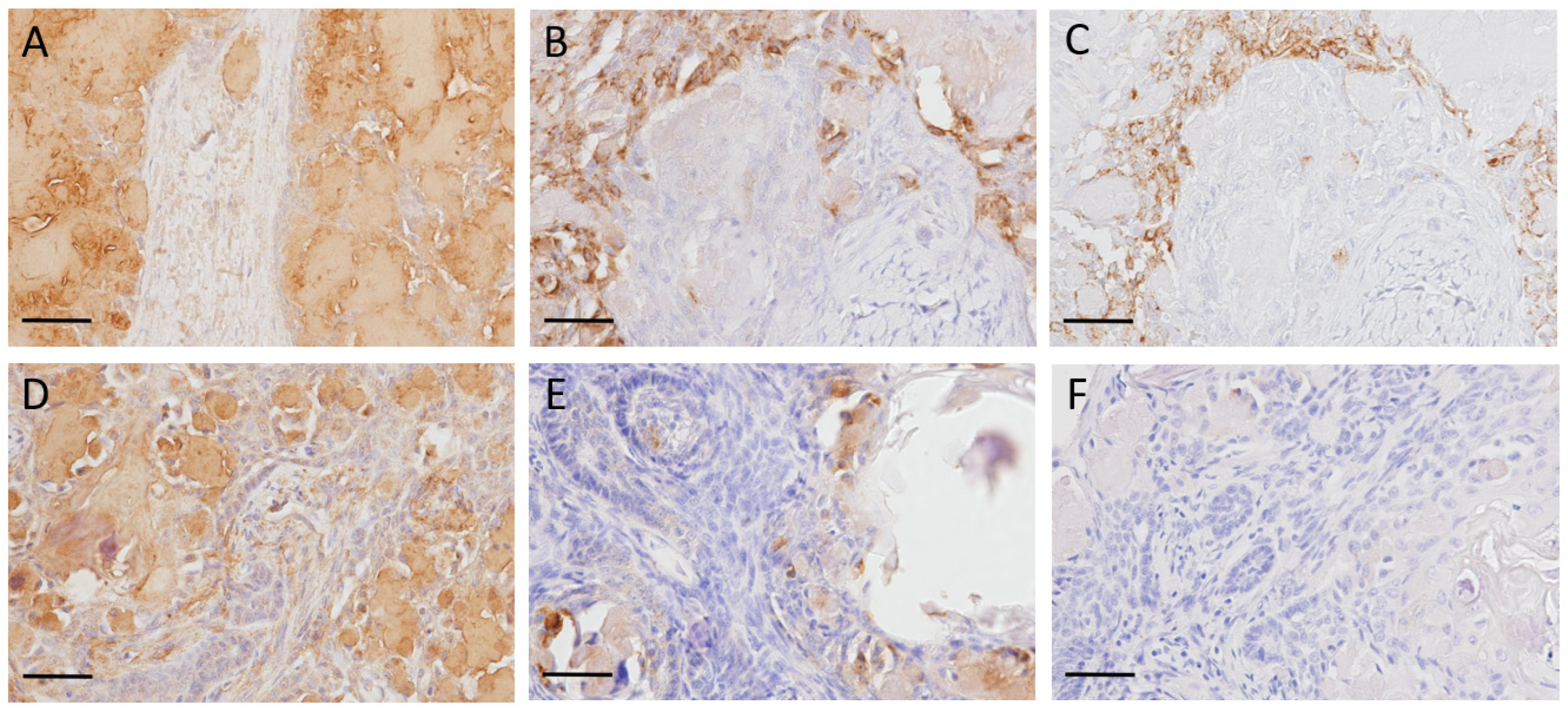
2.4. Immunohistochemistry (IHC)
3. Results
3.1. Histopathology
3.2. Proteomic Analysis
3.3. Immunohistochemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Picken, M.M. The Pathology of Amyloidosis in Classification: A Review. Acta Haematol. 2020, 143, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, J.N.; Dispenzieri, A.; Eisenberg, D.S.; Fändrich, M.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Westermark, P. Amyloid nomenclature 2022: Update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid 2022, 29, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Abbas, A.K.; Fausto, N.; Mitchell, R.N. Robbins Basic Pathology, 8th ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2007; pp. 107–172. [Google Scholar]
- Westermark, P. Aspects on human amyloid forms and their fibril polypeptides. FEBS J. 2005, 272, 5942–5949. [Google Scholar] [CrossRef] [PubMed]
- O’brien, T.D.; Westermark, P.; Johnson, K.H. Islet Amyloid Polypeptide and Calcitonin Gene-related Peptide Immunoreactivity in Amyloid and Tumor Cells of Canine Pancreatic Endocrine Tumors. Vet. Pathol. 1990, 27, 194–198. [Google Scholar] [CrossRef]
- Hirayama, K.; Kagawa, Y.; Nihtani, K.; Taniyama, H. Thyroid C-cell carcinoma with amyloid in a red fox (Vulpes vulpes schrenchki). Vet. Pathol. 1999, 36, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.G.; Dubielzig, R.R. The histopathological features of canine keratinizing ameloblastoma. J. Comp. Pathol. 1993, 109, 423–428. [Google Scholar] [CrossRef]
- Murakami, T.; Kaku, T.; Tsukakoshi, K.; Iwaide, S.; Itoh, Y.; Hisada, M.; Nomura, K.; Kubo, R.; Ikebukuro, K.; Sassa-O’Brien, Y.; et al. Identification of novel amyloidosis in dogs: α-S1-casein acquires amyloidogenicity in mammary tumor by overexpression and N-terminal truncation. Vet. Pathol. 2023, in press. [CrossRef]
- Sykes, G.P.; Cooper, B.J. Caine Intestinal Carcinoids. Vet. Pathol. 1982, 19, 120–131. [Google Scholar] [CrossRef]
- Kadota, A.; Iwaide, S.; Miyazaki, S.; Mitsui, I.; Machida, N.; Murakami, T. Pathology and proteomics-based diagnosis of localized light-chain amyloidosis in dogs and cats. Vet. Pathol. 2020, 57, 658–665. [Google Scholar] [CrossRef]
- Platz, S.J.; Breuer, W.; Geisel, O.; Linke, R.P.; Hermanns, W. Identification of λ light chain amyloid in eight canine and two feline extramedullary plasmacytomas. J. Comp. Pathol. 1997, 116, 45–54. [Google Scholar] [CrossRef]
- Geisel, O.; Stiglmair-Herb, M.; Linke, R.P. Myeloma Associated with Immunoglobulin Lambda-light Chain Derived Amyloid in a Dog. Vet. Pathol. 1990, 27, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Delaney, M.A.; Singh, K.; Murphy, C.L.; Solomon, A.; Nel, S.; Boy, S.C. Immunohistochemical and Biochemical Evidence of Ameloblastic Origin of Amyloid-Producing Odontogenic Tumors in Cats. Vet. Pathol. 2013, 50, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Bock, P.; Hach, V.; Baumgärtner, W. Oral masses in two cats. Vet. Pathol. 2011, 48, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.G.; Dubielzig, R.R.; McGee, E.V. The so-called calcifying epithelial odontogenic tumour in dogs and cats (Amyloid-producing odontogenic tumour). J. Comp. Pathol. 1994, 111, 221–230. [Google Scholar] [CrossRef]
- Ohmachi, T.; Taniyama, H.; Nakade, T.; Kaji, Y.; Furuoka, H. Calcifying epithelial odontogenic tumours in small domesticated carnivores: Histological, immunohistochemical and electron microscopical studies. J. Comp. Pathol. 1996, 114, 305–314. [Google Scholar] [CrossRef]
- Hirayama, K.; Miyasho, T.; Ohmachi, T.; Watanabe, T.; Yokota, H.; Taniyama, H. Biochemical and immunohistochemical characterization of the amyloid in canin amyloid-producing odontogenic tumor. Vet. Pathol. 2010, 47, 915–922. [Google Scholar] [CrossRef]
- Löhr, C.V. One hundred two tumors in 100 goats (1987-2011). Vet. Pathol. 2013, 50, 668–675. [Google Scholar] [CrossRef]
- Kok, M.K.; Chambers, J.K.; Ushio, N.; Miwa, Y.; Nakayama, H.; Uchida, K. Amyloid-producing Odontoameloblastoma in a Black-tailed Prairie Dog (Cynomys ludovicianus). J. Comp. Pathol. 2018, 159, 26–30. [Google Scholar] [CrossRef]
- Kang, M.S.; Park, M.S.; Kwon, S.W.; Ma, S.A.; Cho, D.Y.; Kim, D.Y.; Kim, Y. Amyloid-producing odontogenic tumour (calcifying epithelial odontogenic tumour) in the mandible of a Bengal tiger (Panthera tigris tigris). J. Comp. Pathol. 2006, 134, 236–240. [Google Scholar] [CrossRef]
- Dittmer, K.; Roccabianca, P.; Bell, C.; Murphy, B.G.; Foster, R.A.; Scruggs, L.; Schulman, F.Y.; Thompson, D.; Avallone, G.; Kiupel, M. Surgical Pathology of Tumors of Domestic Animals, 4th ed.; Kiupel, M., Ed.; Davis-Thompson DVM Foundation: Gurnee, IL, USA, 2021; Volume 4, pp. 222–240. [Google Scholar]
- Munday, J.S.; Lohr, C.V.; Kiupel, M. Tumors of the Alimentary Tract. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2017; pp. 499–601. [Google Scholar]
- Tsai, Y.C.; Jeng, C.R.; Zhuo, Y.X.; Tsai, Y.C.; Liu, C.H.; Pang, V.F. Amyloid-producing odontogenic tumor and its immunohistochemical characterization in a Shih Tzu dog. Vet. Pathol. 2007, 44, 233–236. [Google Scholar] [CrossRef]
- Blackford Winders, C.; Bell, C.M.; Goldschmidt, S. Case Report: Amyloid-Producing Odontogenic Tumor With Pulmonary Metastasis in a Spinone Italiano-Proof of Malignant Potential. Front. Vet. Sci. 2020, 7, 576376. [Google Scholar] [CrossRef] [PubMed]
- Izzati, U.Z.; Hidaka, Y.; Hirai, T.; Yamaguchi, R. Immunohistochemical Profile of Ameloblastic Carcinoma Arising from an Amyloid-Producing Odontogenic Tumour in a Miniature Dachshund. J. Comp. Pathol. 2019, 166, 54–58. [Google Scholar] [CrossRef]
- Hirayama, K.; Endoh, C.; Kagawa, Y.; Ohmachi, T.; Yamagami, T.; Nomura, K.; Matsuda, K.; Okamoto, M.; Taniyama, H. Amyloid-Producing Odontogenic Tumors of the Facial Skin in Three Cats. Vet. Pathol. 2017, 54, 218–221. [Google Scholar] [CrossRef]
- Palstrøm, N.B.; Rojek, A.M.; Møller, H.E.H.; Hansen, C.T.; Matthiesen, R.; Rasmussen, L.M.; Abildgaard, N.; Beck, H.C. Classification of Amyloidosis by Model-Assisted Mass Spectrometry-Based Proteomics. Int. J. Mol. Sci. 2021, 23, 319. [Google Scholar] [CrossRef]
- Donnell, R.; Murphy, C.L.; Eulitz, M.; Williams, T.K.; Macy, S.D.; Weiss, D.T.; Solomon, A. Odontogenic feline and canine tumor-associated amyloid is formed from ameloblastin. In Eleventh International Symposium on Amyloidosis; CRC Press: Boca Raton, FL, USA, 2007; pp. 56–58. [Google Scholar]
- Kuwamura, M.; Kanehara, T.; Yamate, J.; Shimada, T.; Kotani, T. Amyloid-Producing Odontogenic Tumor in a Shih-Tzu Dog. J. Vet. Med. Sci. 2000, 62, 655–657. [Google Scholar] [CrossRef]
- Kobayashi, K.; Iwaide, S.; Sakai, H.; Kametani, F.; Murakami, T. Keratinic amyloid deposition in canine hair follicle tumors. Vet. Pathol. 2022, 60, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Mikiewicz, M.; Paździor-Czapula, K.; Gesek, M.; Lemishevskyi, V.; Otrocka-Domagała, I. Canine and Feline Oral Cavity Tumours and Tumour-like Lesions: A Retrospective Study of 486 Cases (2015–2017). J. Comp. Pathol. 2019, 172, 80–87. [Google Scholar] [CrossRef] [PubMed]

| Case No. | Breed | Age, years | Sex | Region |
|---|---|---|---|---|
| Dog No. 1 | Toy poodle | 10 | Female | Left mandibular gingival mass |
| Dog No.2 | Shih-Tzu | Unknown | Female | Left mandibular mass |
| Dog No.3 | Miniature schnauzer | 11 | Male | Right mandibular mass |
| Cat No.1 | Mix | 8 | Female | Right maxillary gingival mass |
| Cat No.2 | Unknown | 18 | Female | Left maxillary mass |
| Case | Ameloblastin | Keratin 5 | Keratin 14 | ApoA-I | ApoA-IV | Vitronectin | Clusterin |
|---|---|---|---|---|---|---|---|
| Dog No.1 | 844 | 838 | 1274 | - | 65 | 38 | - |
| Dog No.2 | 795 | 386 | 408 | 57 | - | - | - |
| Dog No.3 | 735 | 822 | 1333 | - | 51 | 197 | 124 |
| Cat No.1 | 105 | - | - | - | - | 95 | - |
| Cat No.2 | 112 | 399 | 578 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masoud, N.S.; Iwaide, S.; Itoh, Y.; Hisada, M.; Harada, T.; Murakami, T. Identification of Ameloblastin as an Amyloid Precursor Protein of Amyloid-Producing Ameloblastoma in Dogs and Cats. Vet. Sci. 2023, 10, 166. https://doi.org/10.3390/vetsci10020166
Masoud NS, Iwaide S, Itoh Y, Hisada M, Harada T, Murakami T. Identification of Ameloblastin as an Amyloid Precursor Protein of Amyloid-Producing Ameloblastoma in Dogs and Cats. Veterinary Sciences. 2023; 10(2):166. https://doi.org/10.3390/vetsci10020166
Chicago/Turabian StyleMasoud, Niki Sedghi, Susumu Iwaide, Yoshiyuki Itoh, Miki Hisada, Tomoyuki Harada, and Tomoaki Murakami. 2023. "Identification of Ameloblastin as an Amyloid Precursor Protein of Amyloid-Producing Ameloblastoma in Dogs and Cats" Veterinary Sciences 10, no. 2: 166. https://doi.org/10.3390/vetsci10020166
APA StyleMasoud, N. S., Iwaide, S., Itoh, Y., Hisada, M., Harada, T., & Murakami, T. (2023). Identification of Ameloblastin as an Amyloid Precursor Protein of Amyloid-Producing Ameloblastoma in Dogs and Cats. Veterinary Sciences, 10(2), 166. https://doi.org/10.3390/vetsci10020166







