The Surveillance of Borrelia Species in Camelus dromedarius and Associated Ticks: The First Detection of Borrelia miyamotoi in Egypt
Abstract
Simple Summary
Abstract
1. Background
2. Methods
2.1. Sample Collection and Preparation
2.2. Extraction of DNA from Blood Samples and Ticks
2.3. Molecular Identification of Tick Species
2.4. Molecular Identification of the Borrelia Species
2.5. Sequencing and Phylogenetic Analysis
3. Results
3.1. Tick Species
3.2. Prevalence of the Borrelia Species in the Camel Blood Samples and Ticks
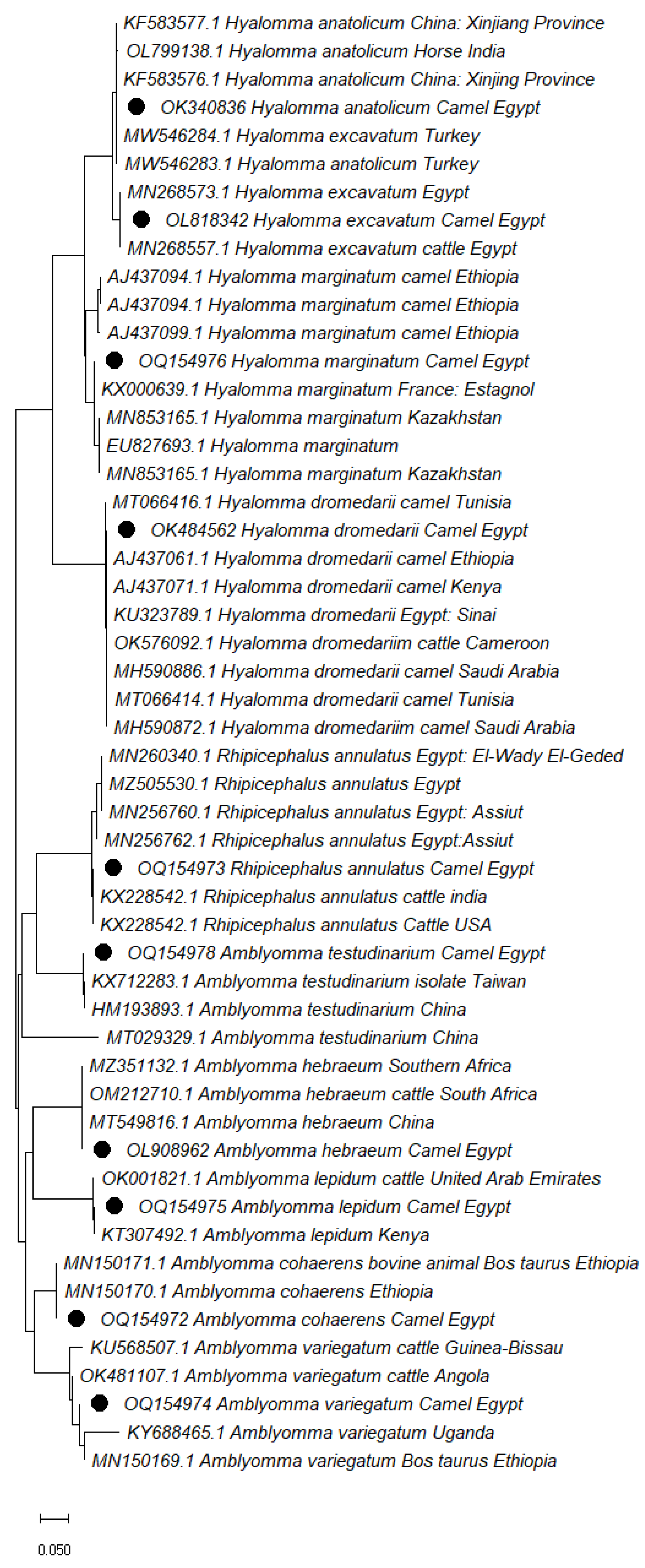
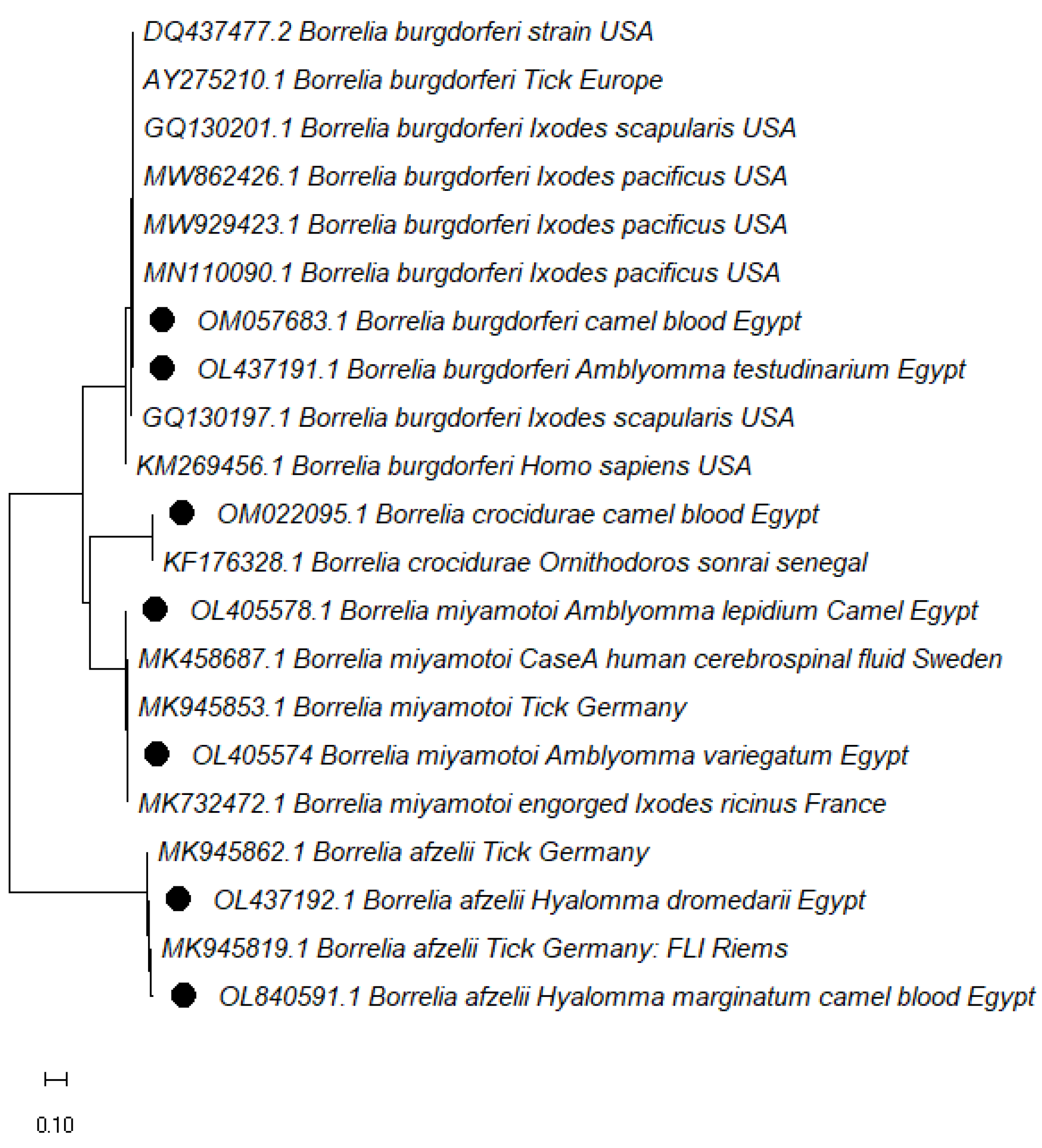
3.3. Phylogenetic analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, S.; Zimmerman, D.; Deem, S.L. A review of zoonotic pathogens of dromedary camels. Ecohealth 2019, 16, 356–377. [Google Scholar] [CrossRef] [PubMed]
- Qamar, M.; Ayaz, M.; Nazir, M. Isolation and identification of ectoparasites in single humped camels (Camelus dromedarius) of Cholistan area, Pakistan. Iraqi J. Vet. Sci. 2019, 32, 291–297. [Google Scholar] [CrossRef]
- Nasirian, H. Detailed new insights about tick infestations in domestic ruminant groups: A global systematic review and meta-analysis. J. Parasit. Dis. 2022, 46, 526–601. [Google Scholar] [CrossRef] [PubMed]
- Bellabidi, M.; Benaissa, M.H.; Bissati-Bouafia, S.; Harrat, Z.; Brahmi, K.; Kernif, T. Coxiella burnetii in camels (Camelus dromedarius) from Algeria: Seroprevalence, molecular characterization, and ticks (Acari: Ixodidae) vectors. Acta Trop. 2020, 206, 105443. [Google Scholar] [CrossRef]
- Rochlin, I.; Toledo, A. Emerging tick-borne pathogens of public health importance: A mini-review. J. Med. Microbiol. 2020, 69, 781. [Google Scholar] [CrossRef] [PubMed]
- Ghafar, A.; Cabezas-Cruz, A.; Galon, C.; Obregon, D.; Gasser, R.B.; Moutailler, S.; Jabbar, A. Bovine ticks harbour a diverse array of microorganisms in Pakistan. Parasites Vectors 2020, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Balinandi, S.; Mugisha, L.; Bbira, J.; Kabasa, W.; Nakayiki, T.; Bakkes, D.K.; Lutwama, J.J.; Chitimia-Dobler, L.; Malmberg, M. General and local morphological anomalies in Amblyomma lepidum (Acari: Ixodidae) and Rhipicephalus decoloratus infesting cattle in Uganda. J. Med. Entomol. 2019, 56, 873–877. [Google Scholar] [CrossRef]
- Allam, N.; El Moghazy, F.M.; Abdel-Baky, S. Molecular epidemiological updates on spotted fever rickettsioses in animal species and their hard ticks settling Egyptian desert. J. Adv. Pharm. Educ. Res. 2018, 8, 65. [Google Scholar]
- Mazyad, S.; Khalaf, S. Studies on theileria and babesia infecting live and slaughtered animals in Al Arish and El Hasanah, North Sinai Governorate, Egypt. J. Egypt. Soc. Parasitol. 2002, 32, 601–610. [Google Scholar]
- Okely, M.; Anan, R.; Gad-Allah, S.; Samy, A. Hard ticks (Acari: Ixodidae) infesting domestic animals in Egypt: Diagnostic characters and a Taxonomic key to the collected species. Med. Vet. Entomol. 2021, 35, 333–351. [Google Scholar] [CrossRef]
- Abdel-Shafy, S.; Allam, N.A.; Mediannikov, O.; Parola, P.; Raoult, D. Molecular detection of spotted fever group rickettsiae associated with ixodid ticks in Egypt. Vector-Borne Zoonotic Dis. 2012, 12, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, H.H.; Elbayoumy, M.K.; Allam, A.M.; Ashry, H.M.; Abdel-Shafy, S. Molecular epidemiology of certain vector-borne bacterial microorganisms in domestic animals and their ectoparasites in Egypt. Trop. Anim. Health Prod. 2021, 53, 484. [Google Scholar] [CrossRef] [PubMed]
- Replogle, A.J.; Sexton, C.; Young, J.; Kingry, L.C.; Schriefer, M.E.; Dolan, M.; Johnson, T.L.; Connally, N.P.; Padgett, K.A.; Petersen, J.M. Isolation of Borrelia miyamotoi and other Borreliae using a modified BSK medium. Sci. Rep. 2021, 11, 1926. [Google Scholar] [CrossRef]
- Mendoza-Roldan, J.A.; Colella, V.; Lia, R.P.; Nguyen, V.L.; Barros-Battesti, D.M.; Iatta, R.; Dantas-Torres, F.; Otranto, D. Borrelia burgdorferi (sensu lato) in ectoparasites and reptiles in southern Italy. Parasites Vectors 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Wodecka, B.; Leońska, A.; Skotarczak, B. A comparative analysis of molecular markers for the detection and identification of Borrelia spirochaetes in Ixodes ricinus. J. Med. Microbiol. 2010, 59, 309–314. [Google Scholar] [CrossRef]
- Rudenko, N.; Golovchenko, M.; Grubhoffer, L.; Oliver, J.H., Jr. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick-Borne Dis. 2011, 2, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Layzell, S.J.; Bailey, D.; Peacey, M.; Nuttall, P.A. Prevalence of Borrelia burgdorferi and Borrelia miyamotoi in questing Ixodes ricinus ticks from four sites in the UK. Ticks Tick-Borne Dis. 2018, 9, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Brisson, D.; Drecktrah, D.; Eggers, C.H.; Samuels, D.S. Genetics of Borrelia burgdorferi. Annu. Rev. Genet. 2012, 46, 515–536. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, S.A.; Bormane, A.; Dinnis, R.E.; Seelig, F.; Dobson, A.D.; Aanensen, D.M.; James, M.C.; Donaghy, M.; Randolph, S.E.; Feil, E.J. Host migration impacts on the phylogeography of Lyme Borreliosis spirochaete species in Europe. Environ. Microbiol. 2011, 13, 184–192. [Google Scholar] [CrossRef]
- Zhai, B.; Niu, Q.; Liu, Z.; Yang, J.; Pan, Y.; Li, Y.; Zhao, H.; Luo, J.; Yin, H. First detection and molecular identification of Borrelia species in Bactrian camel (Camelus bactrianus) from Northwest China. Infect. Genet. Evol. 2018, 64, 149–155. [Google Scholar] [CrossRef]
- Krause, P.J.; Narasimhan, S.; Wormser, G.P.; Rollend, L.; Fikrig, E.; Lepore, T.; Barbour, A.; Fish, D. Human Borrelia miyamotoi infection in the United States. N. Engl. J. Med. 2013, 368, 291. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Fish, D.; Narasimhan, S.; Barbour, A.G. Borrelia miyamotoi infection in nature and in humans. Clin. Microbiol. Infect. 2015, 21, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Boyer, P.H.; Koetsveld, J.; Zilliox, L.; Sprong, H.; Talagrand-Reboul, É.; Hansmann, Y.; de Martino, S.J.; Boulanger, N.; Hovius, J.W.; Jaulhac, B. Assessment of Borrelia miyamotoi in febrile patients and ticks in Alsace, an endemic area for Lyme borreliosis in France. Parasites Vectors 2020, 13, 1–7. [Google Scholar] [CrossRef]
- Wagemakers, A.; Staarink, P.J.; Sprong, H.; Hovius, J.W. Borrelia miyamotoi: A widespread tick-borne relapsing fever spirochete. Trends Parasitol. 2015, 31, 260–269. [Google Scholar] [CrossRef]
- Page, S.; Daschkin, C.; Anniko, S.; Krey, V.; Nicolaus, C.; Maxeiner, H.-G. First report of Borrelia miyamotoi in an Ixodes ricinus tick in Augsburg, Germany. Exp. Appl. Acarol. 2018, 74, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Pukhovskaya, N.M.; Morozova, O.V.; Vysochina, N.P.; Belozerova, N.B.; Ivanov, L.I. Prevalence of Borrelia burgdorferi sensu lato and Borrelia miyamotoi in ixodid ticks in the Far East of Russia. Int. J. Parasitol. Parasites Wildl. 2019, 8, 192–202. [Google Scholar] [CrossRef]
- Wormser, G.P.; McKenna, D.; Scavarda, C.; Cooper, D.; El Khoury, M.Y.; Nowakowski, J.; Sudhindra, P.; Ladenheim, A.; Wang, G.; Karmen, C.L. Co-infections in persons with early Lyme disease, New York, USA. Emerg. Infect. Dis. 2019, 25, 748. [Google Scholar] [CrossRef]
- Gao, Y.; Lv, X.-L.; Han, S.-Z.; Wang, W.; Liu, Q.; Song, M. First detection of Borrelia miyamotoi infections in ticks and humans from the northeast of Inner Mongolia, China. Acta Trop. 2021, 217, 105857. [Google Scholar] [CrossRef]
- Siński, E.; Welc-Falęciak, R.; Zajkowska, J. Borrelia miyamotoi: A human tick-borne relapsing fever spirochete in Europe and its potential impact on public health. Adv. Med. Sci. 2016, 61, 255–260. [Google Scholar] [CrossRef]
- Iwabu-Itoh, Y.; Bazartseren, B.; Naranbaatar, O.; Yondonjamts, E.; Furuno, K.; Lee, K.; Sato, K.; Kawabata, H.; Takada, N.; Andoh, M. Tick surveillance for Borrelia miyamotoi and phylogenetic analysis of isolates in Mongolia and Japan. Ticks Tick-Borne Dis. 2017, 8, 850–857. [Google Scholar] [CrossRef]
- Cutler, S.; Vayssier-Taussat, M.; Estrada-Peña, A.; Potkonjak, A.; Mihalca, A.D.; Zeller, H. A new Borrelia on the block: Borrelia miyamotoi—A human health risk? Eurosurveillance 2019, 24, 1800170. [Google Scholar] [CrossRef] [PubMed]
- Trape, J.-F.; Diatta, G.; Arnathau, C.; Bitam, I.; Sarih, M.h.; Belghyti, D.; Bouattour, A.; Elguero, E.; Vial, L.; Mane, Y. The epidemiology and geographic distribution of relapsing fever borreliosis in West and North Africa, with a review of the Ornithodoros erraticus complex (Acari: Ixodida). PLoS ONE 2013, 8, e78473. [Google Scholar] [CrossRef] [PubMed]
- Talagrand-Reboul, E.; Boyer, P.H.; Bergström, S.; Vial, L.; Boulanger, N. Relapsing fevers: Neglected tick-borne diseases. Front. Cell. Infect. Microbiol. 2018, 8, 98. [Google Scholar] [CrossRef]
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging tick-borne diseases. Clin. Microbiol. Rev. 2020, 33, e00083-00018. [Google Scholar] [CrossRef]
- Fomenko, N.; Livanova, N.; Borgoyakov, V.Y.; Kozlova, I.; Shulaykina, I.; Pukhovskaya, N.; Tokarevich, K.; Livanov, S.; Doroschenko, E.; Ivanov, L. Detection of Borrelia miyamotoi in ticks Ixodes persulcatus from Russia. Parasitology 2010, 44, 201–211. [Google Scholar]
- Reiter, M.; Schötta, A.-M.; Müller, A.; Stockinger, H.; Stanek, G. A newly established real-time PCR for detection of Borrelia miyamotoi in Ixodes ricinus ticks. Ticks Tick-Borne Dis. 2015, 6, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A. Ticks of Domestic Animals in the Mediterranean Region: A Guide to Identification of Species; University of Zaragoza: Zaragoza, Spain, 2004. [Google Scholar]
- Abdullah, H.H.; El-Molla, A.; Salib, F.A.; Allam, N.A.; Ghazy, A.A.; Abdel-Shafy, S. Morphological and molecular identification of the brown dog tick Rhipicephalus sanguineus and the camel tick Hyalomma dromedarii (Acari: Ixodidae) vectors of Rickettsioses in Egypt. Vet. World 2016, 9, 1087. [Google Scholar] [CrossRef]
- Marconi, R.T.; Garon, C.F. Identification of a third genomic group of Borrelia burgdorferi through signature nucleotide analysis and 16S rRNA sequence determination. Microbiology 1992, 138, 533–536. [Google Scholar] [CrossRef]
- Bunikis, J.; Garpmo, U.; Tsao, J.; Berglund, J.; Fish, D.; Barbour, A.G. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology 2004, 150, 1741–1755. [Google Scholar] [CrossRef]
- Gabriele-Rivet, V.; Arsenault, J.; Badcock, J.; Cheng, A.; Edsall, J.; Goltz, J.; Kennedy, J.; Lindsay, L.R.; Pelcat, Y.; Ogden, N.H. Different ecological niches for ticks of public health significance in Canada. PLoS ONE 2015, 10, e0131282. [Google Scholar] [CrossRef]
- Nazifi, S.; Tamadon, A.; Behzadi, M.-A.; Haddadi, S.; Raayat-Jahromi, A.-R. One-humped camels (Camelus dromedaries) hard ticks infestation in Qeshm Island, Iran. Vet. Res. Forum 2011, 2, 135–138. [Google Scholar]
- Ganjali, M.; Dabirzadeh, M.; Sargolzaie, M. Species diversity and distribution of ticks (Acari: Ixodidae) in Zabol County, eastern Iran. J. Arthropod-Borne Dis. 2014, 8, 219. [Google Scholar] [PubMed]
- Li, L.-H.; Zhang, Y.; Wang, J.-Z.; Li, X.-S.; Yin, S.-Q.; Zhu, D.; Xue, J.-B.; Li, S.-G. High genetic diversity in hard ticks from a China-Myanmar border county. Parasites Vectors 2018, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wu, S.; Zhang, Y.; Chen, Y.; Feng, C.; Yuan, X.; Jia, G.; Deng, J.; Wang, C.; Wang, Q. Assessment of four DNA fragments (COI, 16S rDNA, ITS2, 12S rDNA) for species identification of the Ixodida (Acari: Ixodida). Parasites Vectors 2014, 7, 1–11. [Google Scholar] [CrossRef]
- Barghash, S.; Hafez, A.; Darwish, A.; El-Naga, T. Molecular detection of pathogens in ticks infesting camels in Matrouh Governorate, Egypt. J. Bacteriol. Parasitol. 2016, 7, 259–262. [Google Scholar] [CrossRef]
- Hassan, M.I.; Gabr, H.S.; Abdel-Shafy, S.; Hammad, K.M.; Mokhtar, M.M. Prevalence of tick-vectors of Theileria annulata infesting the one-humped camels in Giza, Egypt. J. Egypt. Soc. Parasitol. 2017, 47, 425–432. [Google Scholar] [CrossRef]
- Alanazi, A.; Abdullah, S.; Helps, C.; Wall, R.; Puschendorf, R.; ALHarbi, S.; Abdel-Shafy, S.; Shaapan, R. Tick-borne pathogens in ticks and blood samples collected from camels in Riyadh Province, Saudi Arabia. Int. J. Zool. Res. 2018, 14, 30–36. [Google Scholar]
- Perveen, N.; Muzaffar, S.B.; Al-Deeb, M.A. Four tick-borne microorganisms and their prevalence in Hyalomma ticks collected from livestock in United Arab Emirates. Pathogens 2021, 10, 1005. [Google Scholar] [CrossRef]
- Amira, A.-H.; Răileanu, C.; Tauchmann, O.; Fischer, S.; Nijhof, A.M.; Silaghi, C. Tick species identification and molecular detection of tick-borne pathogens in blood and ticks collected from cattle in Egypt. Ticks Tick-Borne Dis. 2021, 12, 101676. [Google Scholar]
- Raza, N.; Durrani, A.Z.; Saleem, M.H.; Sheikh, A.A.; Usman, M.; Mujahid, Q.; Iqbal, M.Z.; Rizwan, M. Seroprevalence of Borrelia burgdorferi sensu lato in Camel (Camelus dromedarius) in Punjab, Pakistan. Pakistan J. Zool. 2021, 64, 1–4. [Google Scholar] [CrossRef]
- Said, M.B.; Belkahia, H.; Alberti, A.; Abdi, K.; Zhioua, M.; Daaloul-Jedidi, M.; Messadi, L. First molecular evidence of Borrelia burgdorferi sensu lato in goats, sheep, cattle and camels in Tunisia. Ann. Agric. Environ. Med. 2016, 23, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Sazmand, A.; Harl, J.; Eigner, B.; Hodžić, A.; Beck, R.; Hekmatimoghaddam, S.; Mirzaei, M.; Fuehrer, H.-P.; Joachim, A. Vector-borne bacteria in blood of camels in Iran: New data and literature review. Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Knoll, S.; Springer, A.; Hauck, D.; Schunack, B.; Pachnicke, S.; Fingerle, V.; Strube, C. Distribution of Borrelia burgdorferi sl and Borrelia miyamotoi in Ixodes tick populations in Northern Germany, co-infections with Rickettsiales and assessment of potential influencing factors. Med. Vet. Entomol. 2021, 35, 595–606. [Google Scholar] [CrossRef]
- Bankole, A.A.; Kumsa, B.; Mamo, G.; Ogo, N.I.; Elelu, N.; Morgan, W.; Cutler, S.J. Comparative Analysis of Tick-Borne Relapsing Fever Spirochaetes from Ethiopia and Nigeria. Pathogens 2023, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Adham, F.K.; El-Samie-Abd, E.M.; Gabre, R.M.; El Hussein, H. Detection of tick blood parasites in Egypt using PCR assay II-Borrelia burgdorferi sensu lato. J. Egypt. Soc. Parasitol 2010, 40, 553–564. [Google Scholar] [PubMed]
- Elhelw, R.A.; El-Enbaawy, M.I.; Samir, A. Lyme borreliosis: A neglected zoonosis in Egypt. Acta Trop. 2014, 140, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, S.-H.; Shin, S.; Kwak, D. Molecular Identification of Borrelia spp. from Ticks in Pastures Nearby Livestock Farms in Korea. Insects 2021, 12, 1011. [Google Scholar] [CrossRef]
- Răileanu, C.; Tauchmann, O.; Vasić, A.; Wöhnke, E.; Silaghi, C. Borrelia miyamotoi and Borrelia burgdorferi (sensu lato) identification and survey of tick-borne encephalitis virus in ticks from north-eastern Germany. Parasites Vectors 2020, 13, 106. [Google Scholar] [CrossRef]
- Kurtenbach, K.; De Michelis, S.; Etti, S.; Schäfer, S.M.; Sewell, H.-S.; Brade, V.; Kraiczy, P. Host association of Borrelia burgdorferi sensu lato–the key role of host complement. Trends Microbiol. 2002, 10, 74–79. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Wang, Z.; Zhang, L.; Cai, Y.; Liu, Q. Prevalence and identification of Borrelia burgdorferi sensu lato genospecies in ticks from Northeastern China. Vector-Borne Zoonotic Dis. 2019, 19, 309–315. [Google Scholar] [CrossRef]
- Parola, P.; Diatta, G.; Socolovschi, C.; Mediannikov, O.; Tall, A.; Bassene, H.; Trape, J.F.; Raoult, D. Tick-borne relapsing fever borreliosis, rural Senegal. Emerg. Infect. Dis. 2011, 17, 883. [Google Scholar] [CrossRef]
- Ndiaye, E.H.I.; Diouf, F.S.; Ndiaye, M.; Bassene, H.; Raoult, D.; Sokhna, C.; Parola, P.; Diatta, G. Tick-borne relapsing fever Borreliosis, a major public health problem overlooked in Senegal. PLoS Negl. Trop. Dis. 2021, 15, e0009184. [Google Scholar] [CrossRef]
- Reed, K.D.; Meece, J.K.; Henkel, J.S.; Shukla, S.K. Birds, migration and emerging zoonoses: West Nile virus, Lyme disease, influenza A and enteropathogens. Clin. Med. Res. 2003, 1, 5–12. [Google Scholar] [CrossRef]
- James, C.A. The Epidemiology of Ixodes scapularis and Borrelia burgdorferi Collected from Pet Dogs in an Emerging Lyme Disease Risk Area of Southeastern Ontario, Canada. Doctoral Dissertation, University of Guelph, Guelph, ON, Canada, 2017. [Google Scholar]
- Boulanger, N.; Boyer, P.; Talagrand-Reboul, E.; Hansmann, Y. Ticks and tick-borne diseases. Med. Et Mal. Infect. 2019, 49, 87–97. [Google Scholar] [CrossRef]
- Platonov, A.E.; Karan, L.S.; Kolyasnikova, N.M.; Makhneva, N.A.; Toporkova, M.G.; Maleev, V.V.; Fish, D.; Krause, P.J. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis. 2011, 17, 1816. [Google Scholar] [CrossRef]
- Heglasová, I.; Rudenko, N.; Golovchenko, M.; Zubriková, D.; Miklisová, D.; Stanko, M. Ticks, fleas and rodent-hosts analyzed for the presence of Borrelia miyamotoi in Slovakia: The first record of Borrelia miyamotoi in a Haemaphysalis inermis tick. Ticks Tick-Borne Dis. 2020, 11, 101456. [Google Scholar] [CrossRef]
- de la Fuente, J.; Contreras, M.; Estrada-Peña, A.; Cabezas-Cruz, A. Targeting a global health problem: Vaccine design and challenges for the control of tick-borne diseases. Vaccine 2017, 35, 5089–5094. [Google Scholar] [CrossRef]


| Pathogen & Tick | Target Gene (bp) | Primer | Sequence (5′-3′) | Thermal Cycles | References |
|---|---|---|---|---|---|
| Borrelia spp. | 16S-23S rRNA (1007 bp) | Bospp-IGS-F | GTATGTTTAGTGAGGGGGGTG | 95 °C, 7 min; 35 cycles (95 °C 45 s; 55.5 °C, 1 min; 72 °C, 45 s), 72 °C, 7 min | [35,36] |
| Bospp-IGS-R | GGATCATAGCTCAGGTGGTTAG | ||||
| 16S-23S rRNA (300–600 bp) | Bospp-IGS-Fi | AGGGGGGTGAAGTCGTAACAAG | |||
| Bospp-IGS-Ri | GTCTGATAAACCTGAGGTCGGA | ||||
| B.burgdorferi | 16S rRNA (577 bp) | BbF | GGGATGTAGCAATACATTC | 94 °C, 1 min; 35 cycles (95 °C 1 min; 50 °C, 1 min; 72 °C, 1.5 min), 72 °C, 10 min | [39] |
| BbR | ATATAGTTTCCAACATAGG | ||||
| B. miyamotoi | glpQ (424 bp) | Q1 | CACCATTGATCATAGCTCACAG | 95 °C, 5 min; 35 cycles (95 °C 30 s; 50 °C,45 s; 72 °C, 45 s), 72 °C, 7 min | [35,36] |
| Q2 | CTGTTGGTGCTTCATTCCAGTC | ||||
| Q3 | GCTAGTGGGTATCTTCCAGAAC | Annealing: 52 °C | |||
| Q4 | CTTGTTGTTTATGCCAGAAGGGT | ||||
| Tick identification | cox1 (732–820 bp) | CO1-F | GGAACAATATATTTAATTTTTGG | 94 °C, 5 min; 30 cycles (94 °C 1 min; 45 °C, 1 min; 72 °C, 1 min), 72 °C, 10 min | [38] |
| CO1-R | ATCTATCCCTACTGTAAATATATG |
| Tick Species | No. (%) |
|---|---|
| H. dromedarii | 880 (59.4) |
| H. marginatum | 297 (20.1) |
| H. excavatum | 115 (7.2) |
| H. anatolicum | 66 (4.4) |
| Total/genera | 1358 (85.1%) |
| R. pulchellus | 8 (0.5) |
| R. annulatus | 23 (1.6) |
| Total/genera | 31 (1.9%) |
| Am. hebraeum | 165 (11.1) |
| Am. testudinarium | 12 (0.8) |
| Am. lepidum | 11 (0.7) |
| Am. variegatum | 10 (0.7) |
| Am. cohaerens | 6 (0.4) |
| Am. gemma | 3 (0.2) |
| Total/genera | 207 (13.0%) |
| Total No collected | 1596 |
| Borrelia spp. | Positive Samples | |
|---|---|---|
| Camels Blood | Ticks | |
| B. burgdorferi | 1 (9.1) | 3 (14.3) |
| B. afzelii | 0 (0.0) | 2 (9.5) |
| B. crocidurae | 1 (9.1) | 0 (0.0) |
| B. miyamotoi | 9 (81.8) | 16 (76.2) |
| Total positive | 11 (8.3) | 21 (1.3) |
| Tick Species | Total No. Ticks Examined | Total Borrelia spp. /One Tick spp. | B. afzelii (IGS) | B. burgdorferi (16S rRNA) | B. miyamotoi (glpQ) |
|---|---|---|---|---|---|
| H. dromedarii | 880 | 11 (1.3) | 1 (0.1) | 2 (0.2) | 8 (0.9) |
| H. marginatum | 297 | 1 (0.3) | 1 (0.3) | - | - |
| H. excavatum | 115 | - | - | - | - |
| H. anatolicum | 66 | - | - | - | - |
| Am. hebraeum | 165 | 1 (0.6) | - | - | 1 (0.6) |
| Am. testudinarium | 12 | 1 (8.3) | - | 1 (8.3) | - |
| Am. lepidum | 11 | 2 (18.1) | - | - | 2 (18.1) |
| Am. variegatum | 10 | 2 (20.0) | - | - | 2 (20.0) |
| Am. cohaerens | 6 | 1 (16.6) | - | - | 1 (16.7) |
| Am. gemma | 3 | - | - | - | - |
| R. annulatus | 23 | 2 (8.7) | - | - | 2 (8.7) |
| R. pulchellus | 8 | - | - | - | - |
| Total No. | 1596 | 21 (1.3) | 2 (0.1) | 3 (0.2) | 16 (1.0) |
| Borrelia spp. | Accession Number | Isolation Source | Percent Identity | Target Gene |
|---|---|---|---|---|
| B. burgdorferi | OL437191 | Am. testudinarium | AF139510 (99.68%) | IGS (16S-23S) |
| B. afzelii | OL437192 | H. dromedarii | OL840591 (99.14%) | |
| OL840591 | H. marginatum | MK945805 (87.90%) | ||
| B. miyamotoi | OL405578 | Am. lepidum | LC540659 (98.93%) | |
| OL405574 | Am. variegatum | CP046389 (97.35%) | ||
| B. crocidurae | OM022095 | Blood | KF176330 (100.00%) | |
| B.burgdorferi | OM057683 | KM269456 (99.74%) | ||
| B.burgdorferi | OM812932 | Blood | OM033638 (100.00%) | 16SRNA |
| OM812925 | Am. testudinarium | |||
| B. miyamotoi | OL347931 OL439926 | H. dromedarii | KJ003841.2 100.00% | glpQ gene |
| OL439928 | Am. lipidium | |||
| OM103428 | Am. cohaerens | |||
| OM103426 | Am. hebraeum | |||
| OM144474 | R. annulatus | |||
| OL439927 | Blood | |||
| Tick Species | Accession number | Isolation source | Percent identity | Target gene |
| H. dromedarii | OK484562 | Hard ticks | KU323789 (100.00%) | cox1 |
| H. marginatum | OQ154976 | KX000644 (99.87%) | ||
| H. excavatum | OL818342 | MZ505538 (100.00%) | ||
| H. anatolicum | OK340836 | KF583576 (100.00%) | ||
| Am. hebraeum | OL908962 | MT549816 (100.00%) | ||
| Am. testudinarium | OQ154978 | KX712284 (99.83%) | ||
| Am. lepidum | OQ154975 | KT307492 (100.00%) | ||
| Am. variegatum | OQ154974 | MN150169 (100.00%) | ||
| Am. cohaerens | OQ154972 | MN150171 (100.00%) | ||
| Rh. annulatus | OQ154973 | KX228542 (100.00%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashour, R.; Hamza, D.; Kadry, M.; Sabry, M.A. The Surveillance of Borrelia Species in Camelus dromedarius and Associated Ticks: The First Detection of Borrelia miyamotoi in Egypt. Vet. Sci. 2023, 10, 141. https://doi.org/10.3390/vetsci10020141
Ashour R, Hamza D, Kadry M, Sabry MA. The Surveillance of Borrelia Species in Camelus dromedarius and Associated Ticks: The First Detection of Borrelia miyamotoi in Egypt. Veterinary Sciences. 2023; 10(2):141. https://doi.org/10.3390/vetsci10020141
Chicago/Turabian StyleAshour, Radwa, Dalia Hamza, Mona Kadry, and Maha A. Sabry. 2023. "The Surveillance of Borrelia Species in Camelus dromedarius and Associated Ticks: The First Detection of Borrelia miyamotoi in Egypt" Veterinary Sciences 10, no. 2: 141. https://doi.org/10.3390/vetsci10020141
APA StyleAshour, R., Hamza, D., Kadry, M., & Sabry, M. A. (2023). The Surveillance of Borrelia Species in Camelus dromedarius and Associated Ticks: The First Detection of Borrelia miyamotoi in Egypt. Veterinary Sciences, 10(2), 141. https://doi.org/10.3390/vetsci10020141





