Urolithiasis Problems in Finishing Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General Description of the Farms and Anamnesis
2.2. Drinking Water Intake
2.3. Drinking Water Quality
2.4. Feed Analysis
2.5. Blood Parameters
2.6. Examination of Bladders and Urine in Slaughtered Pigs from Farm B
2.7. Analysis of Urine Samples Taken at the Farm
2.8. Analysis of the Mineral Composition of Uroliths
3. Results
3.1. Drinking Water Intake
3.2. Drinking Water Analysis
3.3. Feed Analysis
3.4. Blood Parameters
3.5. Urinalysis of Samples Taken at the Farm
3.6. Mineral Composition of Uroliths
4. Discussion
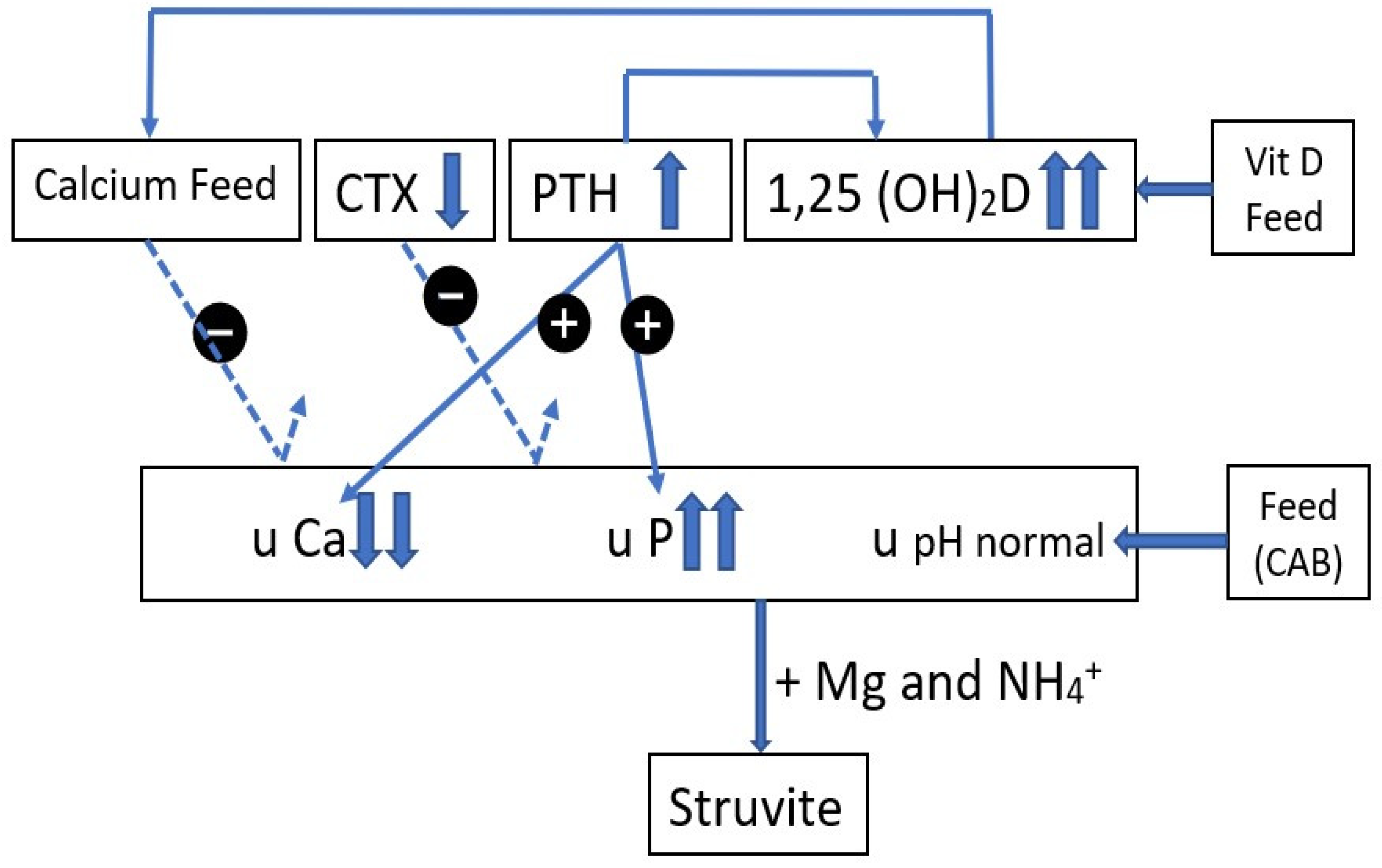
4.1. Differences in Crystal and Stone Composition between the Farms
4.2. Differences in Urinary Potassium and Citrate Excretion between the Farms
4.3. Differences in Urinary Magnesium Excretion between the Farms
4.4. Differences in Urinary P Excretion between the Farms
4.5. Differences in Urinary Calcium Excretion on Both Farms
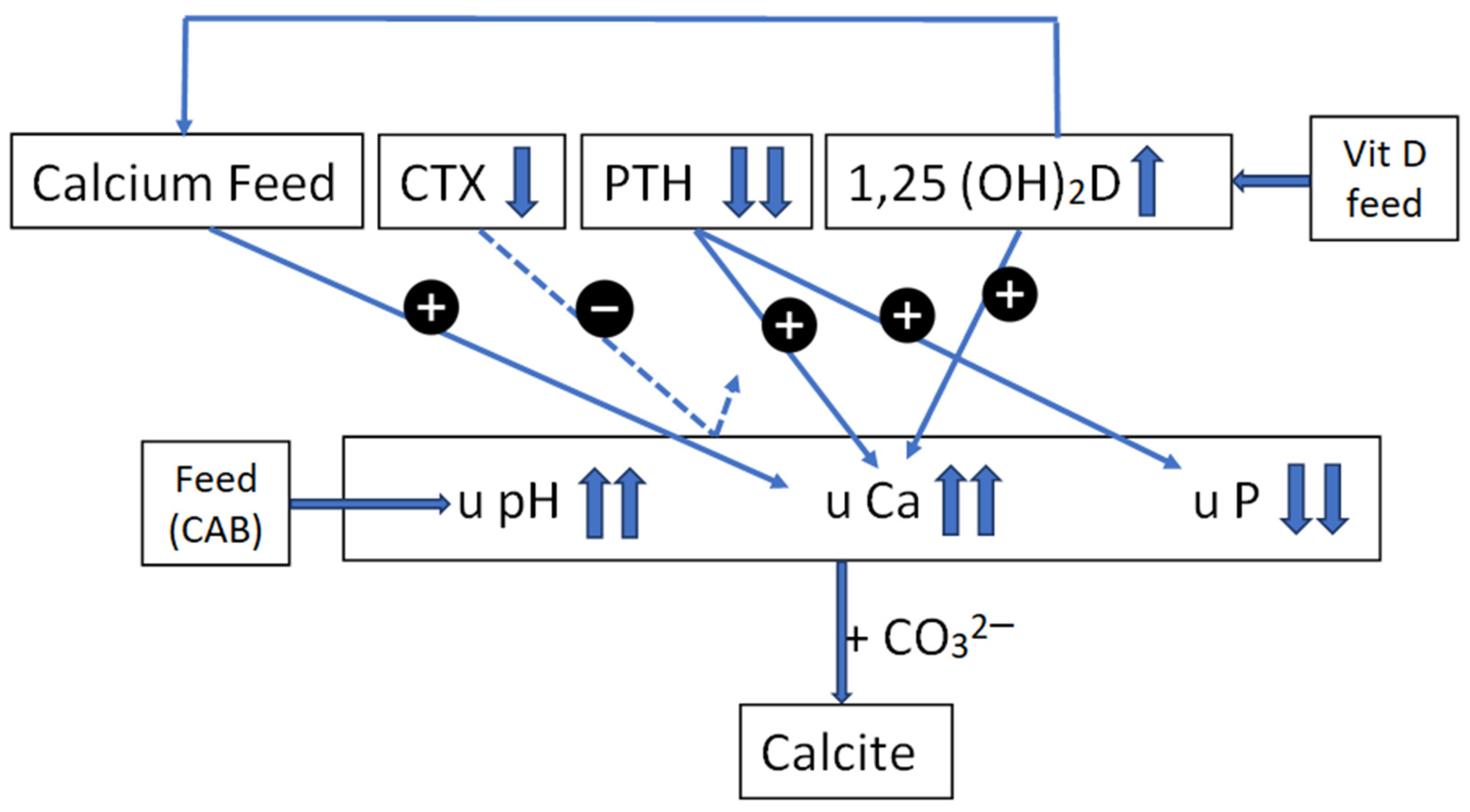
4.6. Calcium Metabolism on Farm A
4.7. Factors Influencing the pH of the Urine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maes, D.; Vrielinck, J.; Millet, S.; Janssens, G.P.; Deprez, P. Urolithiasis in finishing pigs. Vet. J. 2004, 168, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Lorenzett, M.P.; Cruz, R.A.S.; Cecco, B.S.; Schwertz, C.I.; Hammerschmitt, M.E.; Schu, D.T.; Driemeier, D.; Pavarini, S.P. Obstructive urolithiasis in growing-finishing pigs. Pesqui. Veterinária Bras. 2019, 39, 382–387. [Google Scholar] [CrossRef]
- Moe, O.W. Kidney stones: Pathophysiology and medical management. Lancet 2006, 367, 333–344. [Google Scholar] [CrossRef]
- Baumann, J.M.; Affolter, B. From crystalluria to kidney stones, some physicochemical aspects of calcium nephrolithiasis. World J. Nephrol. 2014, 4, 256. [Google Scholar] [CrossRef] [PubMed]
- Dogliotti, E.; Vezzoli, G.; Nouvenne, A.; Meschi, T.; Terranegra, A.; Mingione, A.; Brasacchino, C.; Raspini, B.; Cusi, D.; Soldati, L. Nutrition in calcium nephrolithiasis. J. Transl. Med. 2013, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Kienzle, E. Ernährung und Urolithiasis bei Haussäugetieren. Uebers Tierernaehrg 1991, 19, 157–200. [Google Scholar]
- Ratkaldar, V.N.; Kleinmann, J.G. Mechanisms of stone formation. Clin. Rev. Bone Min. Metab 2011, 9, 187–197. [Google Scholar] [CrossRef]
- Khan, A. Prevalence, pathophysiological mechanisms and factors affecting urolithiasis. Int. Urol. Nephrol. 2018, 50, 799–806. [Google Scholar] [CrossRef]
- Nwaokorie, E.E.; Osborne, C.A.; Lulich, J.P.; Fletcher, T.F.; Ulrich, L.K.; Koehler, L.A.; Buettner, M.T. Risk factors for calcium carbonate urolithiasis in goats. J. Am. Vet. Med. Assoc. 2015, 247, 293–299. [Google Scholar] [CrossRef]
- Miano, R.; Germani, S.S.; Vespasiani, G. Stones and urinary tract infections. Urol. Int. 2007, 79, 32–36. [Google Scholar] [CrossRef]
- Carr, J.; Walton, J.R.; Done, S. Cystitis and ascending pyelonephritis in the sow. Practice 1995, 17, 71–79. [Google Scholar] [CrossRef]
- Carr, J.; Walton, J.R. Characteristics of plasma and urine from adult swine and changes found in sows with either asymptomatic bacteriuria or cystitis and pyelonephritis. Proc. Congr. Int. Pig Vet. Soc. 1992, 12, 263. [Google Scholar]
- Samal, L.; Pattanaik, A.K.; Mishra, C.; Maharana, B.R.; Sarangi, N.S.; Baithalu, R.K. Nutritional strategies to prevent Urolithiasis in Animals. Veterinay World 2011, 4, 142–144. [Google Scholar] [CrossRef]
- Vrielinck, J.; Janssens, G.P.J.; Chantziaras, I.; Cools, A.; Maes, D. Effect of Feed Supplementation with Tripotassium Citrate or Sodium Chloride on the Development of Urinary Calcium Oxalate Crystals in Fattening Pigs. Vet. Sci. 2022, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Beker, S.; Kienzle, E.; Dobenecker, B. Untersuchungen zur Einstellung des Harn-pH-Wertes bei Sauen. Lohmann Inf. 1999, ausgabe 1. [Google Scholar]
- Lindermayer, H.; Propstmeier, G. Practical experiences with feed mixtures used for the acidification of the urine of breeding sows. Lohmann Inf. 1999, ausgabe 1. [Google Scholar]
- Kienzle, E.; Wilms-Eilers, S. Struvite diet in cats: Effect of ammonium chloride and carbonates on acid base balance of cats. J. Nutr. 1994, 124, 2652S–2659S. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D status: Measurement, interpretation, and clinical application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef]
- Groth, E.M.; Lulich, J.P.; Chew, D.J.; Parker, V.J.; Furrow, E. Vitamin D metabolism in dogs with and without hypercalciuric calcium oxalate urolithiasis. J. Vet. Intern. Med. 2019, 33, 758–763. [Google Scholar] [CrossRef]
- Geudeke, T.; Groenland, G.; Greijdanus, S.; Kroeske, K.; Counotte, G. Use of bone markers osteocalcin an C-telopeptide to diagnose metabolic bone disorders in pigs. In Proceedings of the 11th European Symposium of Porcine Health Management (ESPHM), Utrecht, The Netherlands, 22–24 May 2019. [Google Scholar]
- Loynachan, A.T. Cardiovascular and hematopoietic systems. In Diseases of Swine, 10th ed.; Zimmerman, J.J., Karriker, L.A., Raminez, A., Schwartz, K.J., Stevenson, G.W., Eds.; 2019; Volume 14, pp. 189–198. [Google Scholar]
- Madson, D.M.; Ensley, S.M.; Gauger, P.C.; Schwartz, K.J.; Stevenson, G.W.; Cooper, V.L.; Janke, B.H.; Burrough, E.R.; Goff, L.P.; Horst, R.L. Rickets: Case series and diagnostic review of hypovitaminosis D in swine. J. Vet. Diagn. Investig. 2012, 24, 1137–1144. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Vrielinck, J.; Sarrazin, S.; Schoos, A.; Janssens, G.P.J.; Maes, D. Prevalence and chemical composition of uroliths in fattening pigs in Belgium. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1828–1836. [Google Scholar] [CrossRef]
- Massey, L.K.; Whiting, S.J. Dietary salt, urinary calcium, and kidney stone risk. Nutr. Rev. 1995, 53, 131–134. [Google Scholar] [CrossRef]
- Muldowney, F.P.; Freany, R.; Moloney, M.F. Importance of dietary sodium in the hypercalciuria syndrome. Kidney Int. 1982, 22, 292–296. [Google Scholar] [CrossRef]
- Parks, J.H.; Coe, F.L. A urinary calcium-citrate index for the evaluation of nephrolithiasis. Kidney Int. 1986, 30, 85–90. [Google Scholar] [CrossRef]
- Rimer, J.D.; Sakhaee, K.; Maalouf, N.M. Citrate therapy for calcium phosphate stones. Curr. Opin. Nephrol. Hypertens. 2019, 28, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Caudarella, R.; Vescini, F.; Buffa, A.; Stefoni, S. Urinary citrate and renal stone disease: The preventive role of alkali citrate treatment. Arch. Ital. Urol. Androl. 2009, 81, 1084–1106. [Google Scholar]
- Hess, B. Acid–base metabolism: Implications for kidney stone formation. Urol. Res. 2006, 34, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Hamm, L.L.; Hering-Smith, K.S. Pathophysiology of hypocitraturic nephrolithiasis. Endocrinol. Metab. Clin. 2002, 31, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Quamme, G.A. Renal magnesium handling: New insights in understanding old problems. Kidney Int. 1997, 52, 1180–1195. [Google Scholar] [CrossRef] [PubMed]
- Blaine, J.; Choncol, M.; Levi, M. Renal control of calcium, phosphate and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef]
- Belgian Feed Association (BFA). Available online: https://bfa.be/ (accessed on 1 January 2021).
- Crenshaw, T.D.; Rortvedt, L.A.; Hassen, Z. Triennial Growth Symposium: A novel pathway for vitamin D-mediated phosphate homeostasis: Implications for skeleton growth and mineralization. J. Anim. Sci. 2011, 89, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Moor, M.B.; Bonny, O. Ways of calcium reabsorption in the kidney. Am. J. Physiol.-Ren. Physiol. 2016, 310, F1337–F1350. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.K.; Kelly, A.K.; Rajauria, G.; Jakobsen, J.; Clarke, L.C.; Monahan, F.J.; Dowling, K.G.; Hull, G.; Galvin, K.; Cashman, K.D.; et al. The use of synthetic and natural vitamin D sources in pig diets to improve meat quality and vitamin D content. Meat Sci. 2018, 143, 60–68. [Google Scholar] [CrossRef]
- Ackerman, A.L.; Chai, T.C. The bladder is not sterile: An update on the urinary microbiome. Curr. Bladder Dysfunct. Rep. 2019, 14, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Dornbier, R.A.; Bajic, P.; Van Kuiken, M.; Jardaneh, A.; Lin, H.; Gao, X.; Knudsen, B.; Dong, Q.; Wolfe, A.J.; Schwaderer, A.L. The microbiome of calcium-based urinary stones. Urolithiasis 2020, 48, 191–199. [Google Scholar] [CrossRef]
- Zhao, E.; Zhang, W.; Geng, B.; You, W.; Li, X. Intestinal dysbacteriosis leads to kidney stone disease. Mol. Med. Rep. 2021, 23, 180. [Google Scholar] [CrossRef]
- Yuan, T.; Xia, Y.X.; Yu, W.; Ye, Z.; Yan, X.; Song, B.; Li, L.; Lin, F.; Cheng, F. Gut microbiota in patients with kidney stones: A systematic review and meta-analysis. BMC Microbiol. 2023, 23, 143. [Google Scholar] [CrossRef]
- Whittamore, J.M.; Hatch, M. The role of intestinal oxalate transport in hyperoxaluria and formation of kidney stones in animals and man. Urolithiasis 2017, 45, 89–108. [Google Scholar] [CrossRef]
| Farm A | Farm B | |
|---|---|---|
| Type of farm | Farrow-to-finish (20% of piglets are sold) | Fattening farm |
| Number of fattening pigs | 4000 | 8000 |
| Breed of fattening pigs | Topigs × Piétrain | PIC × Piétrain |
| Male pigs | Surgically castrated | Vaccinated against boar taint (Improvac®) at 12 and 20 weeks of age |
| Housing of males and females | Mixed in the same pen | In separate pens |
| Pen size (m2) | 10 | 10 |
| Stocking density (m2/pig) | 0.85 | 0.72 |
| Number of feeding places per pen | 2 | 2 |
| Number of drinking places per pen | 2 | 1 |
| Flow of drinking nipple | 1 Lit /min | >0.5–1.0 Lit/min |
| Source of water | Deep pit (50 m) | 80% phreatic water (3–5 m depth)/20% rainwater |
| Type of floor | Concrete, fully slatted | Concrete, fully slatted |
| Feeding | ad libitum | ad libitum |
| Type of feed | Mash | Pellets |
| Number of feeds during the fattening period | 2 | 2 |
| Mortality rate (%) during the fattening period | 3 | 2–3 |
| Farm A | Farm B | Reference Values a | ||
|---|---|---|---|---|
| Biochemical Analysis | Minimum | Maximum | ||
| pH | 8.1 | 7.8 | 4 | 9 |
| Nitrate (mg/L) | 7.3 | 18 | 200 | |
| Nitrite (mg/L) | <0.03 | 0.38 | 0.5 | |
| Bicarbonates (mg/L) | 369 | 414 | ||
| Carbonates (mg/L) | 0 | 0 | ||
| Chlorides (mg/L) | 40 | 80 | 250 | |
| Sulfates (mg/L) | 83 | 137 | 250 | |
| Total hardness (F°) | 17.87 | 49.39 | 35.3 | |
| Ca (mg/L) | 33 | 170 | 270 | |
| Mg (mg/L) | 23.1 | 17 | 50 | |
| Na (mg/L) | 116 | 46 | 400 | |
| K (mg/L) | 10.8 | 4.4 | ||
| P (mg/L) | <0.05 | <0.05 | ||
| B (mg/L) | 0.34 | <0.2 | ||
| Fe (mg/L) | <0.052 | 0.48 | ||
| Mn (mg/L) | <0.02 | 0.27 | 1 | |
| Cu (mg/L) | <0.02 | <0.02 | ||
| Zn (mg/L) | <0.08 | <0.08 | ||
| Microbiological analysis | ||||
| Bacterial count at 22 °C (CFU/mL) b | 39 | 1937 | 100,000 | |
| Bacterial count at 37 °C (CFU/mL) | 8 | 297 | 100,000 | |
| Coliform bacteria (CFU/100 mL) | 0 | 72 | 100 | |
| Escherichia coli (CFU/100 mL) | 0 | 0 | 10,000 | |
| Enterococci (CFU/100 mL) | 0 | 7 | 0 | |
| Sulfite-reducing clostridia | 1 | 24 | 0 | |
| Farm A | Farm B | Control Farm | |
|---|---|---|---|
| Macronutrients (%) | |||
| Dry matter | 89.17 | 90.2 | |
| Crude protein | 16.11 | 15.77 | |
| Ether extract | 3.98 | 2.88 | |
| Crude fiber | 4.33 | 4.55 | |
| Crude ash | 4.74 | 4.80 | |
| Nitrogen-free extract | 60.01 | 62.2 | |
| Macrominerals (g/kg) | |||
| Ca | 6.75 | 7.31 | 8.65 |
| P | 3.81 | 3.77 | 3.98 |
| Na | 1.74 | 3.83 | 2.21 |
| Mg | 2.11 | 1.52 | 1.76 |
| K | 7.68 | 7.64 | 6.36 |
| Cl | 3.08 | 3.75 | 4.04 |
| S | 0.64 | 0.83 | 0.56 |
| Microminerals | |||
| Cu, mg/kg | 20 | 17 | 13 |
| Fe, mg/kg | 209 | 237 | 231 |
| Mn, mg/kg | 71 | 71 | 83 |
| Zn, mg/kg | 94 | 96 | 97 |
| Derived parameters | |||
| Na+K-Cl, meq/kg | 185 | 256 | 145 |
| Extended cation-anion balance; meq/kg | 376 | 411 | 399 |
| Ca:P ratio, g/g | 1.77 | 1.93 | 2.17 |
| Parameter | Unit | Farm | ||||||
|---|---|---|---|---|---|---|---|---|
| A (n = 5) | B (n = 10) | Control (n = 30) | ||||||
| Mean | Median | Mean | Median | Mean | Median | Reference Interval | ||
| CTX | ng/mL | 0.116 | 0.110 | 0.125 | 0.120 | 0.170 | 0.150 | 0.3 a |
| PTH | ng/L | <1.20 | 1.20 | 4.20 | 3.1 | 5.43 | 2.87 | |
| Ca | mmol/L | 2.66 | 2.65 | 2.65 | 2.65 | 2.3 | 2.42 | 2.5–3.1 b |
| K | 5.56 | 5.55 | 5.58 | 5.51 | 6.57 | 6.17 | 4.8–7.8 b | |
| P | 2.68 | 2.68 | 2.82 | 2.80 | 3.40 | 2.84 | 2.8–4.3 b | |
| Na | 144.6 | 144.6 | 141.9 | 141.7 | - | - | 143–156 b | |
| 25(OH)D | ng/mL | 25.80 | 26.98 | 27.44 | 26.72 | 30.15 | 29.31 | 18–30 c |
| 1,25(OH)2D | pg/mL | 95.27 | 91.36 | 110.49 | 112.75 | 78.54 | 74.22 | |
| 24,25(OH)2D | ng/mL | 19.38 | 18.20 | 15.20 | 14.62 | 19.36 | 19.49 | |
| 25(OH)D:24,25(OH)2D | ng/ng | 1.33 | 1.48 | 1.80 | 1.82 | 1.55 | 1.50 | |
| 1,25(OH)2D:25(OH)D | pg/ng | 3.69 | 3.69 | 4.02 | 4.21 | 2.60 | 2.53 | |
| 1,25(OH)2D:24,25(OH)2D | pg/ng | 4.91 | 5.01 | 7.26 | 7.71 | 4.05 | 3.80 | |
| . | Farm A | Farm B | |||||
|---|---|---|---|---|---|---|---|
| S | pH | SG (g/mL) | Microscopic Findings of Sediment (Score b) | S | pH | SG (g/mL) | Microscopic Findings of Sediment (Score b) |
| 1 | 8.5 | 1.020 | Calcite (5), COD (2) | 1 | 7.5 | 1.015 | Struvite (5), calcite (2) |
| 2 | 8.5 | 1.021 | Calcite (5), | 2 | 7.5 | 1.016 | Struvite (5), calcite (5) |
| 3 | 8.5 | 1.022 | Calcite (5) | 3 | 7.0 | 1.025 | Struvite (5), calcite (1) |
| 4 | 8.5 | 1.014 | Calcite (5), COD (2) | 4 | 7.5 | 1.018 | Struvite.(5), calcite(1), amorph. (5) |
| 5 | 8.5 | 1.019 | Calcite (5 | 5 | 7.5 | 1.016 | Struvite (5), calcite (2) |
| 6 | 8.5 | 1.019 | Calcite (5) | 6 | 8.0 | 1.017 | Struvite (5), amorphous (5) |
| 7 | 7.0 | 1.021 | Calcite (1), COD (1) | 7 | 7.5 | 1.017 | Struvite (5), calcite (5), amorph.(5) |
| Parameter | Unit | Farm | |||||
|---|---|---|---|---|---|---|---|
| A (n = 5) | B (n = 7) | Control (n = 15) | |||||
| Mean | Median | Mean | Median | Mean | Median | ||
| Ca | mmol/g creatinine | 6.34 | 6.80 | 0.48 | 0.44 | 1.80 | 1.68 |
| P | 0.83 | 0.87 | 4.32 | 4.33 | 1.19 | 0.41 | |
| Mg | 9.19 | 9.23 | 1.27 | 1.41 | 3.19 | 2.61 | |
| Na | 26.85 | 23.07 | 39.99 | 35.76 | 18.01 | 13.14 | |
| Cl | 29.84 | 26.21 | 27.45 | 30.74 | 29.21 | 22.62 | |
| K | 78.52 | 81.39 | 28.44 | 27.38 | 35.0 | 32.9 | |
| Citrate | 2.29 | 2.61 | 0.11 | 0.12 | 0.18 | 0.16 | |
| Citrate | mmol/L | 3.71 | 4.16 | 0.25 | 0.23 | 0.74 | 0.70 |
| Creatinine | g/L | 1.77 | 1.62 | 2.25 | 2.21 | 4.18 | 4.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrielinck, J.; Janssens, G.P.J.; Chantziaras, I.; Cools, A.; Maes, D. Urolithiasis Problems in Finishing Pigs. Vet. Sci. 2023, 10, 688. https://doi.org/10.3390/vetsci10120688
Vrielinck J, Janssens GPJ, Chantziaras I, Cools A, Maes D. Urolithiasis Problems in Finishing Pigs. Veterinary Sciences. 2023; 10(12):688. https://doi.org/10.3390/vetsci10120688
Chicago/Turabian StyleVrielinck, Joris, Geert P. J. Janssens, Ilias Chantziaras, An Cools, and Dominiek Maes. 2023. "Urolithiasis Problems in Finishing Pigs" Veterinary Sciences 10, no. 12: 688. https://doi.org/10.3390/vetsci10120688
APA StyleVrielinck, J., Janssens, G. P. J., Chantziaras, I., Cools, A., & Maes, D. (2023). Urolithiasis Problems in Finishing Pigs. Veterinary Sciences, 10(12), 688. https://doi.org/10.3390/vetsci10120688







