Biological Variation in Biochemistry Analytes in Laboratory Guinea Pigs (Cavia porcellus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sampling
2.3. Analytical Methods
2.4. Statistical Analysis
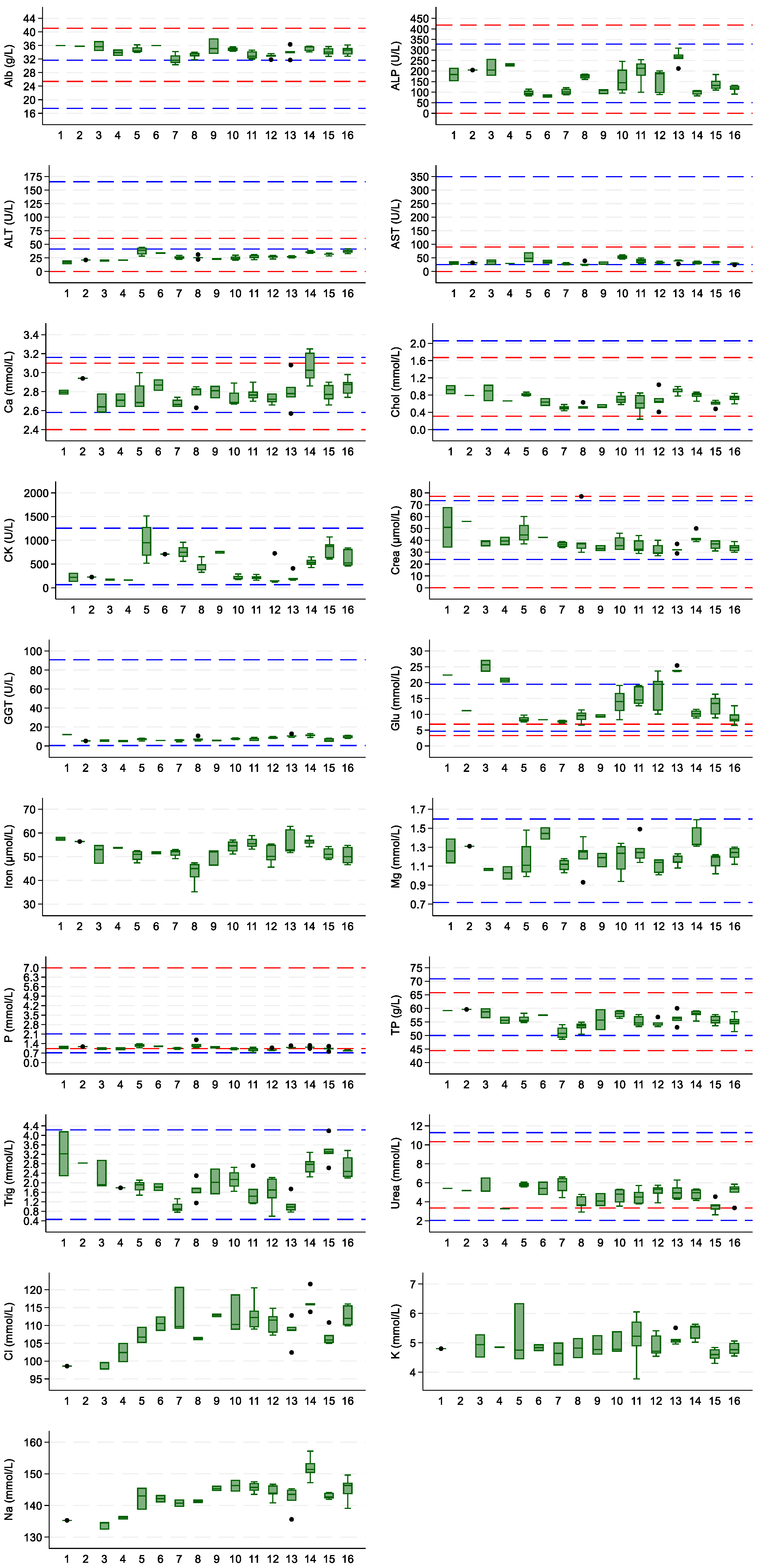
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birck, M.M.; Tveden-Nyborg, P.; Lindblad, M.M.; Lykkesfeldt, J. Non-terminal blood sampling techniques in guinea pigs. J. Vis. Exp. 2014, 92, e51982. [Google Scholar] [CrossRef]
- Padilla-Carlin, D.J.; McMurray, D.N.; Hickey, A.J. The guinea pig as a model of infectious diseases. Comp. Med. 2008, 58, 324–340. [Google Scholar]
- Struillou, X.; Boutigny, H.; Soueidan, A.; Layrolle, P. Experimental animal models in periodontology: A review. Open Dent. J. 2010, 4, 37–47. [Google Scholar] [CrossRef]
- Riggs, S.M. GUINEA PIGS. In Manual of Exotic Pet Practice; Mitchell, M.A., Tully, T.N., Eds.; W.B. Saunders: St. Louis, MO, USA, 2009; pp. 456–473. [Google Scholar]
- Fraser, C.G. Reference change values. Clin. Chem. Lab. Med. 2011, 50, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Campora, C.; Freeman, K.P.; Baral, R. Clinical application of biological variation data to facilitate interpretation of canine and feline laboratory results. J. Small Anim. Pract. 2018, 59, 3–9. [Google Scholar] [CrossRef]
- Flatland, B.; Baral, R.M.; Freeman, K.P. Current and emerging concepts in biological and analytical variation applied in clinical practice. J. Vet. Intern. Med. 2020, 34, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Walton, R.M. Subject-based reference values: Biological variation, individuality, and reference change values. Vet. Clin. Pathol. 2012, 41, 175–181. [Google Scholar] [CrossRef]
- Freeman, K.P.; Baral, R.M.; Dhand, N.K.; Nielsen, S.S.; Jensen, A.L. Recommendations for designing and conducting veterinary clinical pathology biologic variation studies. Vet. Clin. Pathol. 2017, 46, 211–220. [Google Scholar] [CrossRef]
- Genzer, S.C.; Huynh, T.; Coleman-Mccray, J.D.; Harmon, J.R.; Welch, S.R.; Spengler, J.R. Hematology and Clinical Chemistry Reference Intervals for Inbred Strain 13/n Guinea Pigs (Cavia porcellus). J. Am. Assoc. Lab. Anim. Sci. 2019, 58, 293–303. [Google Scholar] [CrossRef]
- Spittler, A.P.; Afzali, M.F.; Bork, S.B.; Burton, L.H.; Radakovich, L.B.; Seebart, C.A.; Moore, A.R.; Santangelo, K.S. Age- and sex-associated differences in hematology and biochemistry parameters of Dunkin Hartley guinea pigs (Cavia porcellus). PLoS ONE 2021, 16, e0253794. [Google Scholar] [CrossRef]
- Waner, T.; Avidar, Y.; Peh, H.C.; Zass, R.; Bogin, E. Hematology and clinical chemistry values of normal and euthymic hairless adult male Dunkin-Hartley guinea pigs (Cavia porcellus). Vet. Clin. Pathol. 1996, 25, 61–64. [Google Scholar] [CrossRef]
- Kitagaki, M.; Yamaguchi, M.; Nakamura, M.; Sakurada, K.; Suwa, T.; Sasa, H. Age-related changes in haematology and serum chemistry of Weiser-Maples guineapigs (Cavia porcellus). Lab. Anim. 2005, 39, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Carney, P.C.; Prieto, J.M.; Miller, M.L.; Randolph, J.F.; Farace, G.; Peterson, S.; Bilbrough, G.; Peterson, M.E. Biological variation of biochemical analytes determined at 8-week intervals for 1 year in clinically healthy cats. Vet. Clin. Pathol. 2023, 52, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Baral, R.M.; Freeman, K.P.; Flatland, B. Analytical quality performance goals for symmetric dimethylarginine in cats. Vet. Clin. Pathol. 2021, 50, 57–61. [Google Scholar] [CrossRef]
- Baral, R.M.; Dhand, N.K.; Freeman, K.P.; Krockenberger, M.B.; Govendir, M. Biological variation and reference change values of feline plasma biochemistry analytes. J. Feline Med. Surg. 2014, 16, 317–325. [Google Scholar] [CrossRef]
- Colburn, M.E.; Schnelle, A.N.; Wong, Y.K.; Whitmore, E.M.; Reilly, J.D.; Adamovicz, L.A.; Keller, K.A.; Allender, M.C. SHORT-TERM BIOLOGICAL VARIABILITY OF HEMATOLOGY PARAMETERS IN THE BEARDED DRAGON (POGONA VITTICEPS). J. Zoo Wildl. Med. 2022, 53, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Perrin, K.L.; Kristensen, A.T.; Gray, C.; Nielsen, S.S.; Bertelsen, M.F.; Kjelgaard-Hansen, M. BIOLOGICAL VARIATION OF HEMATOLOGY AND BIOCHEMISTRY PARAMETERS FOR THE ASIAN ELEPHANT (ELEPHAS MAXIMUS), AND APPLICABILITY OF POPULATION-DERIVED REFERENCE INTERVALS. J. Zoo Wildl. Med. 2020, 51, 643–651. [Google Scholar] [CrossRef]
- Ruaux, C.G.; Carney, P.C.; Suchodolski, J.S.; Steiner, J.M. Estimates of biological variation in routinely measured biochemical analytes in clinically healthy dogs. Vet. Clin. Pathol. 2012, 41, 541–547. [Google Scholar] [CrossRef] [PubMed]
- VetBiologicalVariation. Available online: http://www.vetbiologicalvariation.org/ (accessed on 27 September 2023).
- Flatland, B.; Freeman, K.P.; Friedrichs, K.R.; Vap, L.M.; Getzy, K.M.; Evans, E.W.; Harr, K.E. ASVCP quality assurance guidelines: Control of general analytical factors in veterinary laboratories. Vet. Clin. Pathol. 2010, 39, 264–277. [Google Scholar] [CrossRef]
- Vangel, M.G. Confidence Intervals for a Normal Coefficient of Variation. Am. Stat. 1996, 50, 21–26. [Google Scholar] [CrossRef]
- Flatland, B.; Camus, M.S.; Baral, R.M. Analytical quality goals-a review. Vet. Clin. Pathol. 2018, 47, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Baldrey, V.; Ashpole, I. Interpreting blood profiles in non-domestic small mammals. Vet. Times 2012, 1–9. [Google Scholar]
- Rabe, H. Reference ranges for biochemical parameters in guinea pigs for the Vettest®8008 blood analyzer. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2011, 39, 170–175. [Google Scholar]
- Pritt, S. Taxonomy and History. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Suckow, M.A., Stevens, K.A., Wilson, R.P., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2012; pp. 563–574. [Google Scholar]
- Canning, B.J.; Chou, Y. Using guinea pigs in studies relevant to asthma and COPD. Pulm. Pharmacol. Ther. 2008, 21, 702–720. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.; Nijkamp, F.; De Rose, V.; Folkerts, G. The guinea pig as an animal model for asthma. Curr. Drug Targets 2008, 9, 452–465. [Google Scholar] [CrossRef]
- Adner, M.; Canning, B.J.; Meurs, H.; Ford, W.; Ramos Ramírez, P.; van den Berg, M.P.; Birrell, M.A.; Stoffels, E.; Lundblad, L.K.; Nilsson, G.P.; et al. Back to the future: Re-establishing guinea pig in vivo asthma models. Clin. Sci. 2020, 134, 1219–1242. [Google Scholar] [CrossRef]
- Aamir, R.; Safadi, G.S.; Mandelik, J.; Cornish, K.; Melton, A.L.; Pien, L.C.; Wagner, W.O.; Battisto, J.R. A guinea pig model of hypersensitivity to allergenic fractions of natural rubber latex. Int. Arch. Allergy Immunol. 1996, 110, 187–194. [Google Scholar] [CrossRef]
- Merayo-Lloves, J.; Calonge, M.; Foster, C.S. Experimental model of allergic conjunctivitis to ragweed in guinea pig. Curr. Eye Res. 1995, 14, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Krause, K.K.; Azouz, F.; Nakano, E.; Nerurkar, V.R. A guinea pig model of Zika virus infection. Virol. J. 2017, 14, 75. [Google Scholar] [CrossRef]
- Hensel, M.E.; Arenas-Gamboa, A.M. A Neglected Animal Model for a Neglected Disease: Guinea Pigs and the Search for an Improved Animal Model for Human Brucellosis. Front. Microbiol. 2018, 9, 2593. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.S.; Wehner, N.G. The guinea pig as an animal model for developmental and reproductive toxicology studies. Birth Defects Res. B Dev. Reprod. Toxicol. 2009, 86, 92–97. [Google Scholar] [CrossRef]
- Canizo, J.; Zhao, C.; Vandal, K.; Biondic, S.; Petropoulos, S. O-234 The guinea pig embryo: A potential new model for human development. Hum. Reprod. 2022, 37, deac106.016. [Google Scholar] [CrossRef]
- Taylor, D.K.; Lee, V.K. Chapter 25—Guinea Pigs as Experimental Models. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Suckow, M.A., Stevens, K.A., Wilson, R.P., Eds.; Academic Press: Boston, MA, USA, 2012; pp. 705–744. [Google Scholar]
- Musci, R.V.; Walsh, M.A.; Konopka, A.R.; Wolff, C.A.; Peelor, F.F.; Reiser, R.F.; Santangelo, K.S.; Hamilton, K.L. The Dunkin Hartley Guinea Pig Is a Model of Primary Osteoarthritis That Also Exhibits Early Onset Myofiber Remodeling That Resembles Human Musculoskeletal Aging. Front. Physiol. 2020, 11, 571372. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Salamanna, F.; Martini, L.; Fini, M. Naturally Occurring Osteoarthritis Features and Treatments: Systematic Review on the Aged Guinea Pig Model. Int. J. Mol. Sci. 2022, 23, 7309. [Google Scholar] [CrossRef]
- Lund, F.; Hyltoft Petersen, P.; Fraser, C.G. A dynamic reference change value model applied to ongoing assessment of the steady state of a biomarker using more than two serial results. Ann. Clin. Biochem. 2019, 56, 283–294. [Google Scholar] [CrossRef] [PubMed]
- López-Olvera, J.R.; Montané, J.; Marco, I.; Martínez-Silvestre, A.; Soler, J.; Lavín, S. Effect of venipuncture site on hematologic and serum biochemical parameters in marginated tortoise (Testudo marginata). J. Wildl. Dis. 2003, 39, 830–836. [Google Scholar] [CrossRef]
- Fernández, I.; Peña, A.; Del Teso, N.; Pérez, V.; Rodríguez-Cuesta, J. Clinical biochemistry parameters in C57BL/6J mice after blood collection from the submandibular vein and retroorbital plexus. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 202–206. [Google Scholar]
- Abatan, O.I.; Welch, K.B.; Nemzek, J.A. Evaluation of saphenous venipuncture and modified tail-clip blood collection in mice. J. Am. Assoc. Lab. Anim. Sci. 2008, 47, 8–15. [Google Scholar]
- Kachhawa, K.; Kachhawa, P.; Varma, M.; Behera, R.; Agrawal, D.; Kumar, S. Study of the Stability of Various Biochemical Analytes in Samples Stored at Different Predefined Storage Conditions at an Accredited Laboratory of India. J. Lab. Physicians 2017, 9, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Cray, C.; Rodriguez, M.; Zaias, J.; Altman, N.H. Effects of storage temperature and time on clinical biochemical parameters from rat serum. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 202–204. [Google Scholar]
- Thoresen, S.I.; Tverdal, A.; Havre, G.; Morberg, H. Effects of storage time and freezing temperature on clinical chemical parameters from canine serum and heparinized plasma. Vet. Clin. Pathol. 1995, 24, 129–133. [Google Scholar] [CrossRef] [PubMed]
| Measurand | Method | Measuring Range | Model (ME or FE) | Correlation Structure (AR1 or IND) |
|---|---|---|---|---|
| Albumin (Alb) | Bromocresol green | 2–60 g/L | ME | IND |
| Alkaline phosphatase (ALP) | Colorimetric IFCC | 3–1200 U/L | ME | IND |
| Alanine transaminase (ALT) | Colorimetric IFCC w/o P5P | 2–700 U/L | ME | AR1 |
| Aspartate aminotransferase (AST) | Colorimetric IFCC w/o P5P | 2–700 U/L | ME | IND |
| Calcium (Ca) | Colorimetric NM-BAPTA | 0.2–5.0 mmol/L | ME | IND |
| Cholesterol (Chol) | Colorimetric | 0.1–20.7 mmol/L | ME | IND |
| Creatine kinase (CK) | UV-test | 7–2000 U/L | ME | IND |
| Creatinine (Crea) | Jaffé | 18–1300 μmol/L | FE | N/A |
| Gamma-glutamyl transferase (GGT) | Enzymatic colorimetric IFCC | 3–1200 U/L | ME | AR1 |
| Glucose (Glu) | Enzymatic with hexokinase | 0.24–40 mmol/L | ME | IND |
| Iron | FerroZine | 0.9–179 μmol/L | ME | AR1 |
| Magnesium (Mg) | Colorimetric Chlorophosphonazo III | 0.15–2.5 mmol/L | ME | IND |
| Phosphorus (P) | Endpoint method with sample blanking | 0.1–6.46 mmol/L | ME | IND |
| Total protein (TP) | Colorimetric biuret | 2–120 g/L | ME | IND |
| Triglycerides (Trig) | Enzymatic colorimetric | 0.1–10 mmol/L | ME | IND |
| Urea | Kinetic test with urease and glutamate dehydrogenase | 0.5–40 mmol/L | ME | IND |
| Electrolytes (Sodium, Na; Chloride, Cl; Potassium, K) | Ion-selective electrodes, using undiluted samples (ISE) | K: FE Na, Cl: ME | K: N/A Na, Cl: IND |
| ID Sex | 1 F | 2 F | 3 F | 4 F | 5 M | 6 M | 7 M | 8 M | 9 M | 10 F | 11 F | 12 F | 13 F | 14 M | 15 M | 16 M |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W1 | X | X | X | X | X | X | ||||||||||
| W2 | X | X | X | X | X | X | X | X | ||||||||
| W3 | X | X | X | X | X | X | ||||||||||
| W4 | X | X | X | X | ||||||||||||
| W5 | X | X | X | X | ||||||||||||
| W6 | X | X | X | X |
| Formulae | |
|---|---|
| Index of individuality | |
| Reference change value | where Z = 1.65 for a 95% confidence interval if only increasing or decreasing concentration is of clinical concerns (e.g., enzymes); Z = 1.95 for a 95% confidence interval if both high and low concentrations are clinically important. |
| CV Opt | Recommended optimal analytical variation (imprecision) based on CVA < 0.25CVI |
| CV Des | Recommended desirable analytical variation (imprecision) based on CVA < 0.5CVI |
| CV Min | Recommended minimally acceptable analytical variation (imprecision) based on CVA < 0.75CVI |
| Bias Opt | Recommended optimal bias (inaccuracy) based on <0.125( + )1/2 |
| Bias Des | Recommended desirable bias (inaccuracy) based on <0.25( + )1/2 |
| Bias Min | Recommended minimally acceptable bias (inaccuracy) based on <0.375( + )1/2 |
| TEa Opt | Recommended optimal allowable total error based on <1.65(0.25CVI) + 0.125( + )1/2 |
| TEa Des | Recommended desirable allowable total error based on <1.65(0.5CVI) + 0.25( + )1/2 |
| TEa Min | Recommended minimally acceptable allowable total error based on <1.65(0.75CVI) + 0.375( + )1/2 |
| Alt TEa Opt | Alternative recommended optimal allowable total error based on <1.65(CV Opt) |
| Alt TEa Des | Alternative recommended desirable allowable total error based on <1.65(CV Des) |
| Alt TEa Min | Alternative recommended minimally acceptable allowable total error based on <1.65(CV Min) |
| Biological Variation | Traditional Quality Specifications Based on Biological Variation | Alternative TEa Based on Biologic Variation | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | CVI ± SD (%) (95% CI) | CVG ± SD (%) (95% CI) | CVA ± SD (%) | CVA:CVI | IoI | RCV (%) | CV Opt | CV Des | CV Min | Bias Opt | Bias Des | Bias Min | TEa Opt | TEa Des | TEa Min | Alt TEa Opt | Alt TEa Des | Alt TEa Min | |
| Alb | 34.4 | 3.4 ± 1.2 (2.9–4.2) | 3.0 ± 1.0 (2.2–3.6) | 0.9 ± 1.6 | 0.3 | 1.2 | 9.84 | 0.86 | 1.71 | 2.57 | 0.57 | 1.14 | 1.71 | 2.0 | 4.0 | 6.0 | 1.4 | 2.8 | 4.2 |
| ALP | 156.8 | 22.3 ± 35.0 (18.9–27.4) | 32.7 ± 51.3 (23.0–40.6) | 0.4 ± 0.4 | 0.0 | 0.7 | 52.16 | 5.59 | 11.17 | 16.76 | 4.95 | 9.90 | 14.85 | 14.2 | 28.3 | 42.5 | 9.2 | 18.4 | 27.7 |
| ALT | 27.1 | 16.6 ± 4.5 (14.1–20.3) | 20.4 ± 5.5 (14.6–24.9) | 1.6 ± 1.2 | 0.1 | 0.8 | 38.93 | 4.15 | 8.30 | 12.45 | 3.29 | 6.58 | 9.86 | 10.1 | 20.3 | 30.4 | 6.8 | 13.7 | 20.5 |
| AST | 34.9 | 19.4 ± 6.8 (16.4–23.8) | 19.2 ± 6.7 (13.7–23.6) | 0.7 ± 1.2 | 0.0 | 1.0 | 45.30 | 4.85 | 9.70 | 14.55 | 3.41 | 6.82 | 10.24 | 11.4 | 22.8 | 34.2 | 8.0 | 16.0 | 24.0 |
| Ca | 2.8 | 3.9 ± 0.1 (3.3–4.7) | 2.9 ± 0.1 (2.1–3.6) | 0.5 ± 0.6 | 0.1 | 1.3 | 10.93 | 0.98 | 1.95 | 2.93 | 0.61 | 1.22 | 1.84 | 2.2 | 4.4 | 6.7 | 1.6 | 3.2 | 4.8 |
| Chol | 0.7 | 18.1 ± 0.1 (15.3–22.1) | 16.2 ± 0.1 (11.6–19.8) | 0.6 ± 0.7 | 0.0 | 1.1 | 50.16 | 4.52 | 9.04 | 13.56 | 3.04 | 6.07 | 9.11 | 10.5 | 21.0 | 31.5 | 7.5 | 14.9 | 22.4 |
| CK | 458.8 | 37.6 ± 172.5 (31.3–47.2) | 56.8 ± 260.7 (37.6–74.6) | 0.7 ± 1.1 | 0.0 | 0.7 | 104.24 | 9.40 | 18.80 | 28.20 | 8.51 | 17.03 | 25.54 | 24.0 | 48.0 | 72.1 | 15.5 | 31.0 | 46.5 |
| GGT | 7.6 | 22.1 ± 1.7 (18.7–27.1) | 25.7 ± 1.9 (18.3–31.6) | 3.2 ± 3.1 | 0.1 | 0.9 | 52.12 | 5.53 | 11.05 | 16.58 | 4.24 | 8.48 | 12.72 | 13.4 | 26.7 | 40.1 | 9.1 | 18.2 | 27.3 |
| Glu | 14.1 | 19.1 ± 2.7 (16.1–23.3) | 41.8 ± 5.9 (28.9–52.7) | 0.3 ± 0.2 | 0.0 | 0.5 | 52.81 | 4.76 | 9.53 | 14.29 | 5.74 | 11.48 | 17.23 | 13.6 | 27.2 | 40.8 | 7.9 | 15.7 | 23.6 |
| Iron | 52.4 | 7.1 ± 3.7 (6.0–8.6) | 6.2 ± 3.3 (4.5–7.5) | 0.7 ± 0.5 | 0.1 | 1.1 | 19.77 | 1.78 | 3.55 | 5.33 | 1.18 | 2.36 | 3.54 | 4.1 | 8.2 | 12.3 | 2.9 | 5.9 | 8.8 |
| Mg | 1.2 | 9.9 ± 0.1 (8.4–12.1) | 6.9 ± 0.1 (5.0–8.4) | 0.9 ± 0.6 | 0.1 | 1.4 | 27.56 | 2.48 | 4.95 | 7.43 | 1.51 | 3.01 | 4.52 | 5.6 | 11.2 | 16.8 | 4.1 | 8.2 | 12.3 |
| P | 1.1 | 10.5 ± 0.1 (8.9–12.7) | 9.5 ± 0.1 (6.9–11.5) | 0.6 ± 0.7 | 0.1 | 1.1 | 29.15 | 2.62 | 5.25 | 7.87 | 1.77 | 3.54 | 5.31 | 6.1 | 12.2 | 18.3 | 4.3 | 8.7 | 13.0 |
| TP | 56.0 | 3.5 ± 2.0 (3.0–4.2) | 3.3 ± 1.8 (2.4–4.0) | 0.5 ± 0.4 | 0.1 | 1.1 | 9.80 | 0.87 | 1.75 | 2.62 | 0.60 | 1.20 | 1.80 | 2.0 | 4.1 | 6.1 | 1.4 | 2.9 | 4.3 |
| Trig | 2.1 | 23.9 ± 0.5 (20.2–29.3) | 30.9 ± 0.6 (21.9–38.3) | 0.5 ± 0.4 | 0.0 | 0.8 | 66.24 | 5.97 | 11.95 | 17.92 | 4.89 | 9.77 | 14.66 | 14.7 | 29.5 | 44.2 | 9.9 | 19.7 | 29.6 |
| Urea | 4.8 | 15.4 ± 0.7 (13.0–18.7) | 13.5 ± 0.6 (9.7–16.4) | 1.3 ± 1.0 | 0.1 | 1.1 | 42.76 | 3.84 | 7.68 | 11.53 | 2.55 | 5.11 | 7.66 | 8.9 | 17.8 | 26.7 | 6.3 | 12.7 | 19.0 |
| Cl | 108.9 | 3.1 ± 3.4 (2.6–3.8) | 4.4 ± 4.8 (3.1–5.5) | 1.2 ± 1.0 | 0.4 | 0.8 | 9.24 | 0.78 | 1.56 | 2.34 | 0.68 | 1.36 | 2.03 | 2.0 | 3.9 | 5.9 | 1.3 | 2.6 | 3.9 |
| Na | 142.6 | 1.8 ± 2.6 (1.6–2.3) | 3.0 ± 4.3 (2.1–3.7) | 1.1 ± 1.2 | 0.6 | 0.7 | 5.86 | 0.45 | 0.90 | 1.35 | 0.44 | 0.87 | 1.31 | 1.2 | 2.4 | 3.5 | 0.7 | 1.5 | 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, G.; Liu, K.-F.; Kershaw, H.; Riddell, D.; Hyndman, T.H.; Monks, D.; Musk, G.C. Biological Variation in Biochemistry Analytes in Laboratory Guinea Pigs (Cavia porcellus). Vet. Sci. 2023, 10, 621. https://doi.org/10.3390/vetsci10100621
Rossi G, Liu K-F, Kershaw H, Riddell D, Hyndman TH, Monks D, Musk GC. Biological Variation in Biochemistry Analytes in Laboratory Guinea Pigs (Cavia porcellus). Veterinary Sciences. 2023; 10(10):621. https://doi.org/10.3390/vetsci10100621
Chicago/Turabian StyleRossi, Gabriele, Kwei-Farn Liu, Helen Kershaw, Dayna Riddell, Timothy H. Hyndman, Deborah Monks, and Gabrielle C. Musk. 2023. "Biological Variation in Biochemistry Analytes in Laboratory Guinea Pigs (Cavia porcellus)" Veterinary Sciences 10, no. 10: 621. https://doi.org/10.3390/vetsci10100621
APA StyleRossi, G., Liu, K.-F., Kershaw, H., Riddell, D., Hyndman, T. H., Monks, D., & Musk, G. C. (2023). Biological Variation in Biochemistry Analytes in Laboratory Guinea Pigs (Cavia porcellus). Veterinary Sciences, 10(10), 621. https://doi.org/10.3390/vetsci10100621






