Comparison of the Trachea in Normocephalic versus Brachycephalic Cats on the Basis of CT-Derived Measurements
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Inclusion Criteria
2.2. Tracheal Measurements
2.3. Statistical Analyses
3. Results
3.1. Cohort Characteristics
3.2. Trachea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haynes, A.M.; Seibert, R.; Sura, P.A. Chapter 102: Trachea and Bronchi. In Veterinary Surgery: Small Animal Expert Consult, 2nd ed.; Johnston, S.M., Tobias, K.M., Eds.; Elsevier Health Sciences: St. Louis, MO, USA, 2018; Volume 2, pp. 1963–1983. [Google Scholar]
- Schultheiss, P.C.; Gardner, S.A.; Owens, J.M.; Wenger, D.A.; Thrall, M.A. Mucopolysaccharidosis VII in a cat. Vet. Pathol. 2000, 37, 502–505. [Google Scholar] [CrossRef]
- Hendricks, J.C.; O’Brien, J.A. Tracheal collapse in two cats. J. Am. Vet. Med. Assoc. 1985, 187, 418–419. [Google Scholar]
- Viñeta, C.; López, M.C.; Verdés, J.; Roura, X. Medical management of primary tracheal collapse in a cat. Vet. Rec. Case Rep. 2023, 11, e523. [Google Scholar] [CrossRef]
- Fujita, M.; Miura, H.; Yasuda, D.; Hasegawa, D.; Orima, H. Tracheal narrowing secondary to airway obstruction in two cats. J. Small Anim. Pract. 2004, 45, 29–31. [Google Scholar] [CrossRef]
- Bell, R.; Philbey, A.W.; Martineau, H.; Nielsen, L.; Pawson, P.; Dukes-McEwan, J. Dynamic tracheal collapse associated with disseminated histiocytic sarcoma in a cat. J. Small Anim. Pract. 2006, 47, 461–464. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, J.R.; Taylor, H.W.; Lee, Y.-S. Primary extranodal lymphosarcoma of the trachea in a cat. J. Vet. Med. Sci. 1996, 58, 703–706. [Google Scholar] [CrossRef]
- Vincenti, S.; Betting, A.; Durand, A.; Campos, M.; Scanziani, E.; Martin, S.S. Total laryngectomy in a cat with a laryngeal peripheral nerve sheath tumor. Vet. Surg. 2021, 50, 1533–1541. [Google Scholar] [CrossRef]
- Jelinek, F.; Vozkova, D. Carcinoma in the trachea in a cat. J. Comp. Pathol. 2012, 147, 177–180. [Google Scholar] [CrossRef]
- Howard, J.; Fisher, J.; Tolbert, M.K. Invasive tracheal neoplasia in eight cats: Descriptive cases and review of the current literature. J. Feline Med. Surg. Open Rep. 2017, 3, 2055116917690074. [Google Scholar] [CrossRef]
- Coyne, B.E.; Fingland, R.B. Hypoplasia of the trachea in dogs: 103 cases (1974–1990). J. Am. Vet. Med. Assoc. 1992, 201, 768–772. [Google Scholar]
- Rieks, T.W.; Birchard, S.J.; Stephens, J.A. Surgical correction of brachycephalic syndrome in dogs: 62 cases (1991–2004). J. Am. Vet. Med. Assoc. 2007, 230, 1324–1328. [Google Scholar] [CrossRef]
- Stordalen, M.B.; Silveira, F.; Fenner, J.V.H.; Demetriou, J.L. Outcome of temporary tracheostomy tube-placement following surgery for brachycephalic obstructive airway syndrome in 42 dogs. J. Small Anim. Pract. 2020, 61, 292–299. [Google Scholar] [CrossRef]
- Gobbetti, M.; Romussi, S.; Buracco, P.; Bronzo, V.; Gatti, S.; Cantatore, M. Long-term outcome of permanent tracheostomy in 15 dogs with severe laryngeal collapse secondary to brachycephalic airway obstructive syndrome. Vet. Surg. 2018, 47, 648–653. [Google Scholar] [CrossRef]
- Harvey, C.E.; Fink, E.A. Tracheal diameter: Analysis of radiographic measurements in brachycephalic and nonbrachycephalic dogs. J. Am. Anim. Hosp. Assoc. 1982, 18, 570–576. [Google Scholar]
- Nicholson, I.; Baines, S. Complications associated with temporary tracheostomy tubes in 42 dogs (1998–2007). J. Small Anim. Pract. 2021, 53, 108–114. [Google Scholar] [CrossRef]
- Occhipinti, L.L.; Hauptman, J.G. Long-term outcome of permanent tracheostomies in dogs: 21 cases (2000–2012). Can. Vet. J. 2014, 55, 357–360. [Google Scholar]
- Harvey, C.E.; O’Brien, J.A. Tracheotomy in the dog and cat: Analysis of 89 episodes in 79 animals. J. Am. Anim. Hosp. Assoc. 1982, 18, 563–566. [Google Scholar]
- Hedlund, C.S.; Tagner, C.H.; Montgomery, D.L.; Hobson, H.P. A procedure for permanent tracheostomy and its effects on tracheal mucosa. Vet. Surg. 1982, 11, 13–17. [Google Scholar] [CrossRef]
- Hedlund, C.S.; Tagner, C.H.; Waldron, D.R.; Hobson, H.P. Permanent tracheostomy: Perioperative and long-term data from 34 cases. J. Am. Anim. Hosp. Assoc. 1988, 24, 585–591. [Google Scholar]
- Colley, P.; Huber, M.; Henderson, R. Tracheostomy techniques and management. Compend. Contin. Educ. Pract. Vet. 1999, 21, 44–53. [Google Scholar]
- Stepnik, M.W.; Mehl, M.L.; Hardie, E.M.; Kass, P.H.; Reimer, S.B.; Campbell, B.G.; Mison, M.B.; Schmiedt, C.W.; Gregory, C.R.; Hobson, H.P. Outcome of permanent tracheostomy for treatment of upper airway obstruction in cats: 21 cases (1990–2007). J. Am. Vet. Med. Assoc. 2009, 234, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Guenther-Yenke, C.L.; Rozanski, E.A. Tracheostomy in cats: 23 cases (1998–2006). J. Feline Med. Surg. 2007, 9, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Hardie, E. Trachea and bronchus. In Feline Soft Tissue and General Surgery, 1st ed.; Langley-Hobbs, S.J., Demetriou, J.L., Ladlow, J.F., Eds.; Elsevier Health Sciences: St. Louis, MO, USA, 2014; pp. 531–540. [Google Scholar]
- Kara, M.E.; Turan, E.; Dabanoglu, I.; Ocal, M.K. Computed tomographic assessment of the trachea in the German shepherd dog. Ann. Anat. 2004, 186, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Jeong, J.; Heng, H.G.; Sung, S.; Choi, Y.; Oh, H.; Kim, K.; Jung, Y.; Lee, K. Computed tomographic features of tracheal shapes and dimensions in awake dogs. Vet. Med. 2018, 63, 131–136. [Google Scholar] [CrossRef]
- Hammond, G.; Geary, M.; Coleman, E.; Gunn-Moore, D. Radiographic measurements of the trachea in domestic shorthair and Persian cats. J. Feline Med. Surg. 2011, 13, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Brunner, A.; Underberg, J.; Vincenti, S. CT measurements of tracheal diameter and length in normocephalic cats. J. Feline Med. Surg. 2023, 25, 1098612X231158578. [Google Scholar] [CrossRef]
- Dabanoglu, I.; Ocal, M.; Kara, M.E. A Quantitative Study on the Trachea of the Dog. Anat. Histol. Embryol. 2001, 30, 57–59. [Google Scholar] [CrossRef]
- Mims, H.L.; Hancock, R.B.; Leib, M.S.; Waldron, D.R. Primary Tracheal Collapse in a Cat. J. Am. Vet. Med. Assoc. 2008, 44, 149–153. [Google Scholar] [CrossRef] [PubMed]
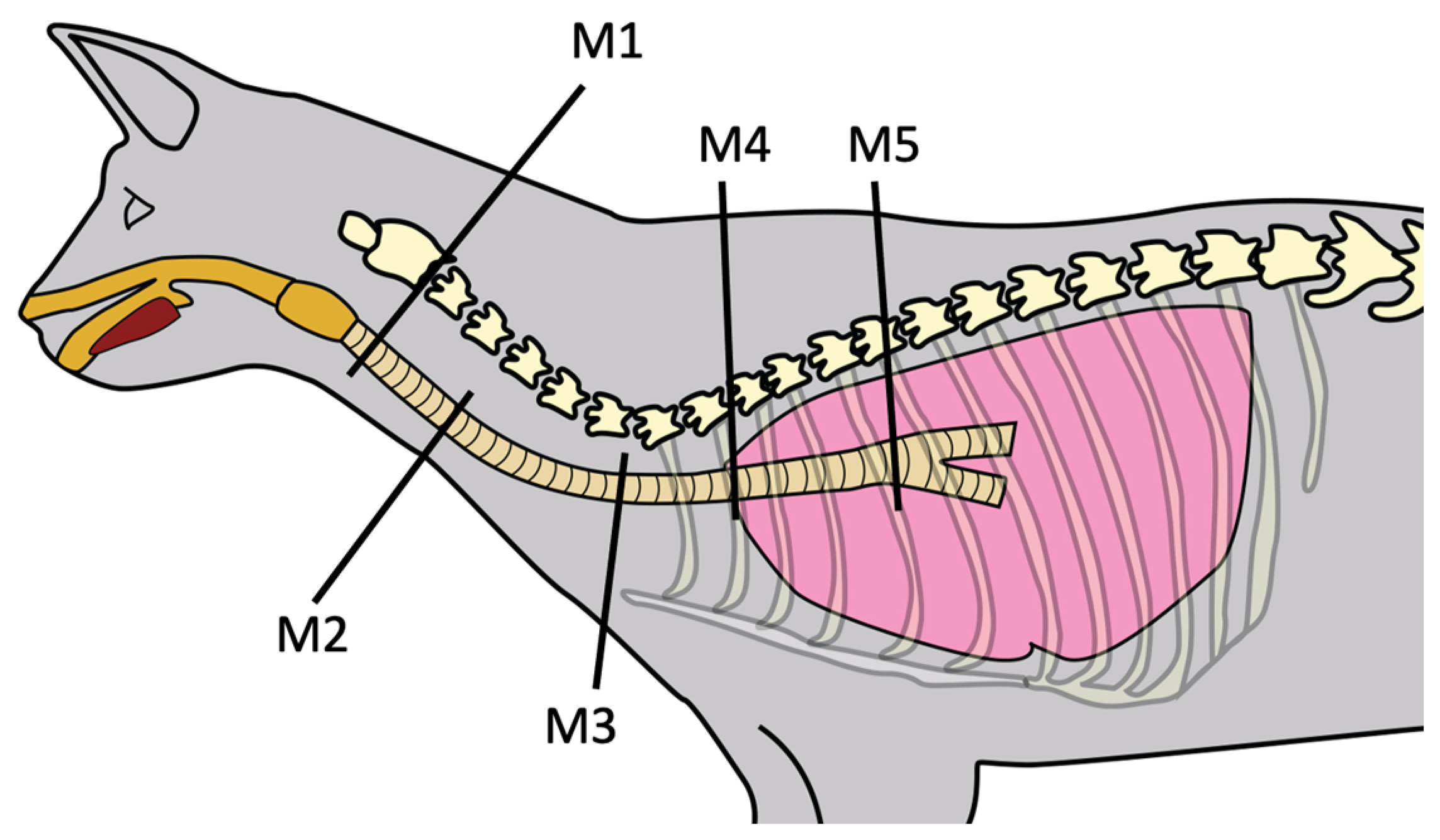
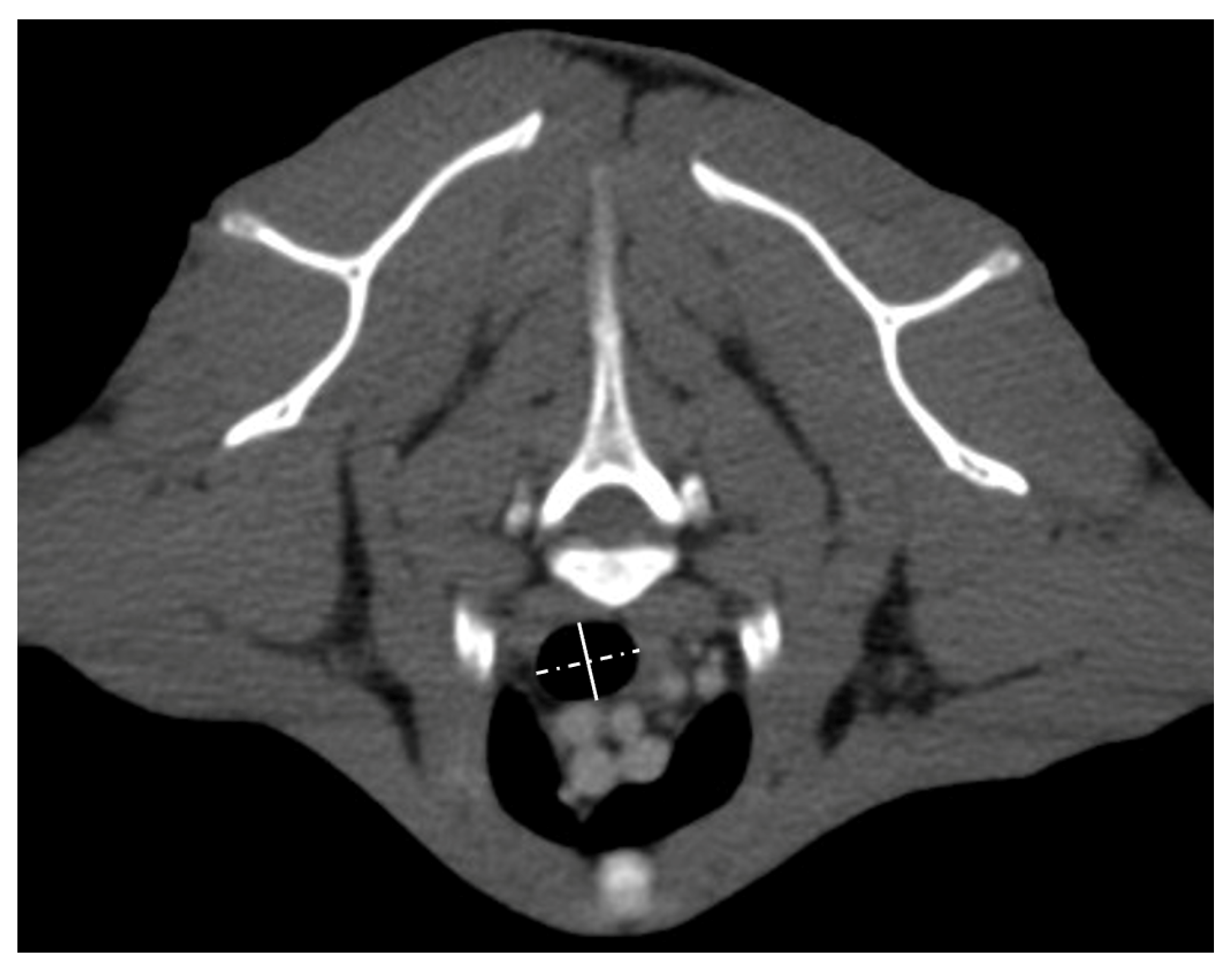
| Variable | Normocephalic Cats (n = 15) | Brachycephalic Cats (n = 14) | p-Value | |
|---|---|---|---|---|
| Length (mm) | 135.747 (+/−12.785 mm) | 115.085 (+/−8.224) | 0.0001 | |
| M1 | ML (mm) | 7.425 (5.725–11.575) | 7.500 (6.475–9.000) | 0.827 |
| DV (mm) | 7.575 (6.525–11.475) | 7.375 (6.050–8.000) | 0.183 | |
| Ratio ML/DV | 1.000 (0.814–1.116) | 1.062 (0.863–1.210) | 0.052 | |
| M2 | ML (mm) | 8.200 (5.425–9.650) | 8.000 (6.300–9.700) | 0.984 |
| DV (mm) | 7.175 (6.475–9.750) | 7.550 (5.100–8.550) | 0.570 | |
| Ratio ML/DV | 1.096 (0.746–1.306) | 1.087 (0.965–1.382) | 0.704 | |
| M3 | ML (mm) | 8.550 (6.150–9.625) | 8.000 (5.975–9.775) | 0.153 |
| DV (mm) | 7.650 (5.400–9.500) | 7.487 (5.250–8.625) | 0.325 | |
| Ratio ML/DV | 1.120 (0.992–1.339) | 1.145 (1.020–1.306) | 0.654 | |
| M4 | ML (mm) | 8.200 (5.975–9.000) | 7.213 (5.600–10.070) | 0.079 |
| DV (mm) | 7.200 (5.150–8.475) | 6.512 (5.075–8.800) | 0.381 | |
| Ratio ML/DV | 1.124 (1.026–1.432) | 1.141 (1.038–1.336) | 0.513 | |
| M5 | ML (mm) | 7.500 (4.875–8.175) | 6.700 (5.475–8.600) | 0.085 |
| DV (mm) | 7.425 (5.575–8.550) | 6.987 (5.350–8.475) | 0.507 | |
| Ratio ML/DV | 1.023 (0.874–1.218) | 1.003 (0.859–1.086) | 0.425 | |
| Measurement Point | ML | DV | Ratio ML/DV | p-Value |
|---|---|---|---|---|
| M1 | 7.425 (5.725–11.157) | 7.575 (6.525–11.475) | 1.000 (0.814–1.116) | 0.885 |
| M2 | 8.200 (5.425–9.650) | 7.175 (6.475–9.750) | 1.096 (0.746–1.306) | 0.144 |
| M3 | 8.550 (6.150–9.625) | 7.650 (5.400–9.500) | 1.120 (0.992–1.339) | 0.035 |
| M4 | 8.200 (5.975–9.000) | 7.200 (5.150–8.475) | 1.124 (1.026–1.432) | 0.005 |
| M5 | 7.500 (4.875–8.175) | 7.425(5.575–8.550) | 1.023 (0.874–1.218) | 0.724 |
| Measurement Point | ML | DV | Ratio ML/DV | p-Value |
|---|---|---|---|---|
| M1 | 7.500 (6.475–9.000) | 7.375 (6.050–8.000) | 1.062 (0.863–1.210) | 0.136 |
| M2 | 8.000 (6.300–9.700) | 7.550 (5.100–8.550) | 1.087 (0.965–1.382) | 0.113 |
| M3 | 8.100 (6.325–9.775) | 7.487 (5.250–8.625) | 1.145 (1.020–1.306) | 0.031 |
| M4 | 7.350 (5.600–10.075) | 6.512 (5.075–8.675) | 1.141 (1.038–1.336) | 0.035 |
| M5 | 6.700 (5.475–8.600) | 6.987 (5.350–8.475) | 1.003 (0.859–1.086) | 0.725 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunner, A.; Underberg, J.; Zimmermann, J.; Vincenti, S. Comparison of the Trachea in Normocephalic versus Brachycephalic Cats on the Basis of CT-Derived Measurements. Vet. Sci. 2023, 10, 602. https://doi.org/10.3390/vetsci10100602
Brunner A, Underberg J, Zimmermann J, Vincenti S. Comparison of the Trachea in Normocephalic versus Brachycephalic Cats on the Basis of CT-Derived Measurements. Veterinary Sciences. 2023; 10(10):602. https://doi.org/10.3390/vetsci10100602
Chicago/Turabian StyleBrunner, Anna, Julius Underberg, Jeannette Zimmermann, and Simona Vincenti. 2023. "Comparison of the Trachea in Normocephalic versus Brachycephalic Cats on the Basis of CT-Derived Measurements" Veterinary Sciences 10, no. 10: 602. https://doi.org/10.3390/vetsci10100602
APA StyleBrunner, A., Underberg, J., Zimmermann, J., & Vincenti, S. (2023). Comparison of the Trachea in Normocephalic versus Brachycephalic Cats on the Basis of CT-Derived Measurements. Veterinary Sciences, 10(10), 602. https://doi.org/10.3390/vetsci10100602






