Appropriate Genetic Approaches for Heat Tolerance and Maintaining Good Productivity in Tropical Poultry Production: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
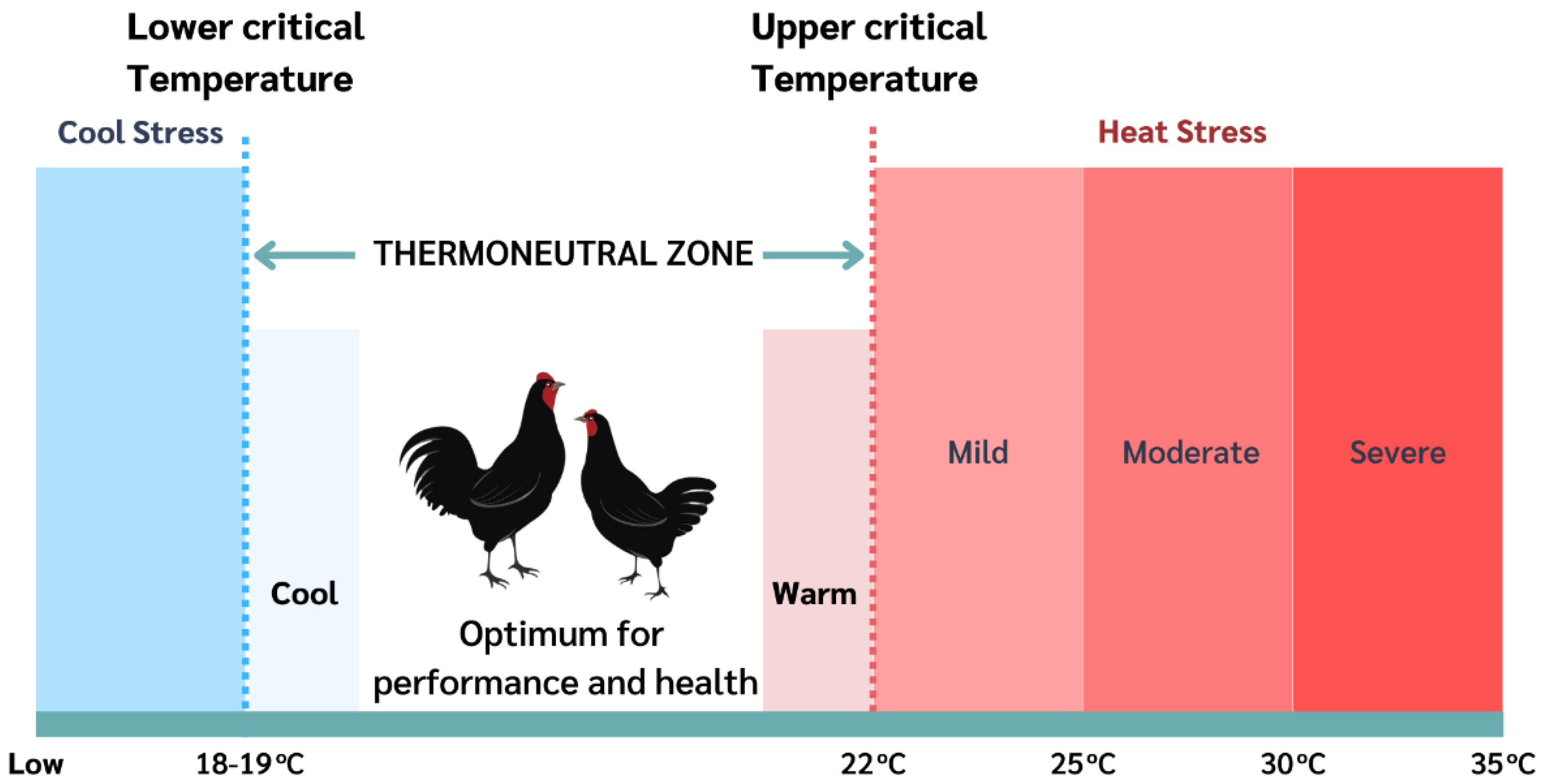
2. Heat Stress in Poultry
3. Mechanism of Thermoregulation in Poultry
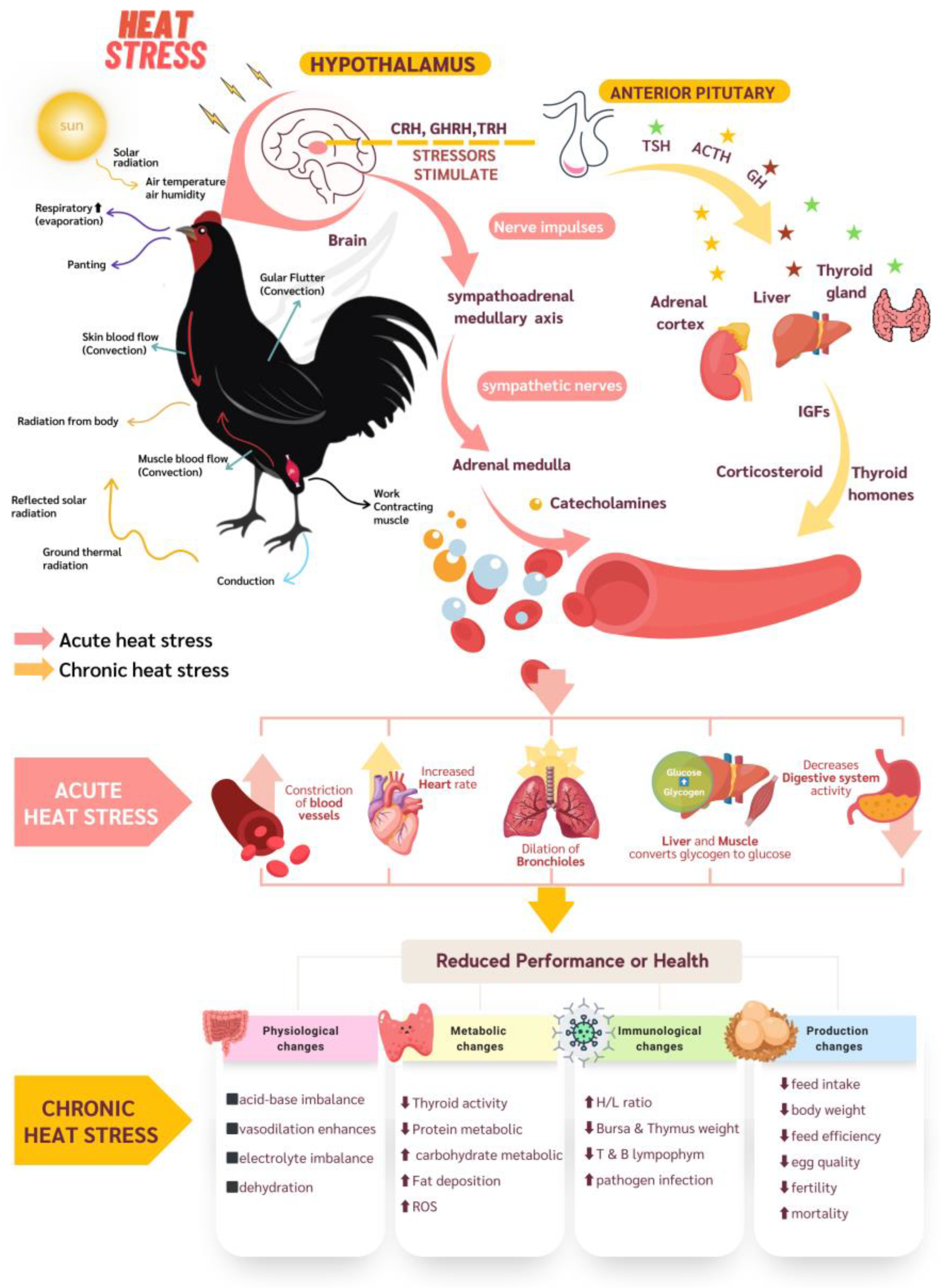
4. Effects of Heat Stress on Poultry
4.1. Physiological Changes
4.2. Metabolic Changes
4.3. Immunological Changes
4.4. Productivity Changes
| Parameters | Effects of Heat Stress | References |
|---|---|---|
| Physiological changes | ||
| Acid–base imbalance | respiratory alkalosis can occur when the body’s pH is shifted towards alkalinity due to a reduction in blood carbon dioxide (CO2) levels. | Popoola et al. [71] |
| Vasodilation | increases skin-surface blood vessel dilatation. this enhances radiative and convective heat loss from the core to the skin. | Chaiyabutr et al. [62]; Mota-Rojas et al. [74]; Hall et al. [75] |
| Electrolyte Imbalance | sweating and pant during heat stress, losing sodium chloride, potassium, and chloride. | Nawab et al. [76]; Wasti et al. [77] |
| Dehydration | rapid respiration risks dehydration and electrolyte imbalances due to higher water loss. | Khan et al. [78] |
| Metabolic changes | ||
| Thyroid activity declines | diminished thyroid hormone levels can diminish poultry metabolic rates, affecting growth and performance. | Del Vesco et al. [80] |
| Decreases Protein Metabolism | growth, reproduction, and immunity may be affected by decreased protein synthesis. | Zaboli et al. [82] |
| Increased Carbohydrate Metabolism | heat stress can elevate blood glucose levels through stress hormone release, potentially causing hyperglycemia. | Kikusato and Toyomizu [81] |
| The accumulation of fat increases | subcutaneous fat may decrease and abdominal fat rise. high temperatures reduce adipose tissue lipogenesis, altering meat quality and egg yolk composition. | Zaboli et al. [82] |
| Increased ROS | ROS from oxidative stress exceeds the bird’s antioxidant defenses. this damages tissues and cells. | Song et al. [84] Nanto-Hara et al. [85] |
| Immune changes | ||
| A higher H/L ratio. | heterophil to lymphocyte (h/l) ratios rise during heat stress, indicating immune system alterations. | Soleimani et al. [88]; Al-Murrani et al. [89] |
| Bursa and thymus weight decline. | prolonged heat stress can reduce bursa and thymus weights, affecting lymphoid organ growth and function. | Hirakawa et al. [85]; Kammon et al. [91] |
| Reduced T and B lymphocyte activity. | heat stress reduces t and b lymphocyte function, lowering the immune system’s ability to fight infections. | Honda et al. [92]; Mashaly et al. [73] |
| Pathogen susceptibility rises. | heat stress can decrease poultry immune systems, making them more susceptible to diseases. | Alhenaky et al. [93]; Quinteiro-Filho et al. [94]; Ahmad et al. [95] |
| Productivity changes | ||
| Reduced Feed Intake | lead to decreased appetite in poultry, resulting in lower feed consumption. | Rowland et al. [98]; Mazzoni et al. [99] |
| Reduced body weight | exposed to heat stress may experience slower growth rates and reduced body weight gain. | Awad et al. [100] |
| Feed efficiency reduction | The impairment of feed conversion efficiency results in elevated feed costs. | Sohao et al. [47] |
| Egg production decline | laying fewer eggs of reduced size and quality. | Yan et al. [40]; Loengbudnark et al. [51]; Rowland et al. [98] |
| Reducing fertility | impair the fertility of breeding poultry, leading to decreased hatchability. | Donoghue et al. [105]; Olusegun and Alabi [106] |
| Mortality rises | mortality rates can rise due to heat stress-induced physiological strain. | Aguanta et al. [109] |
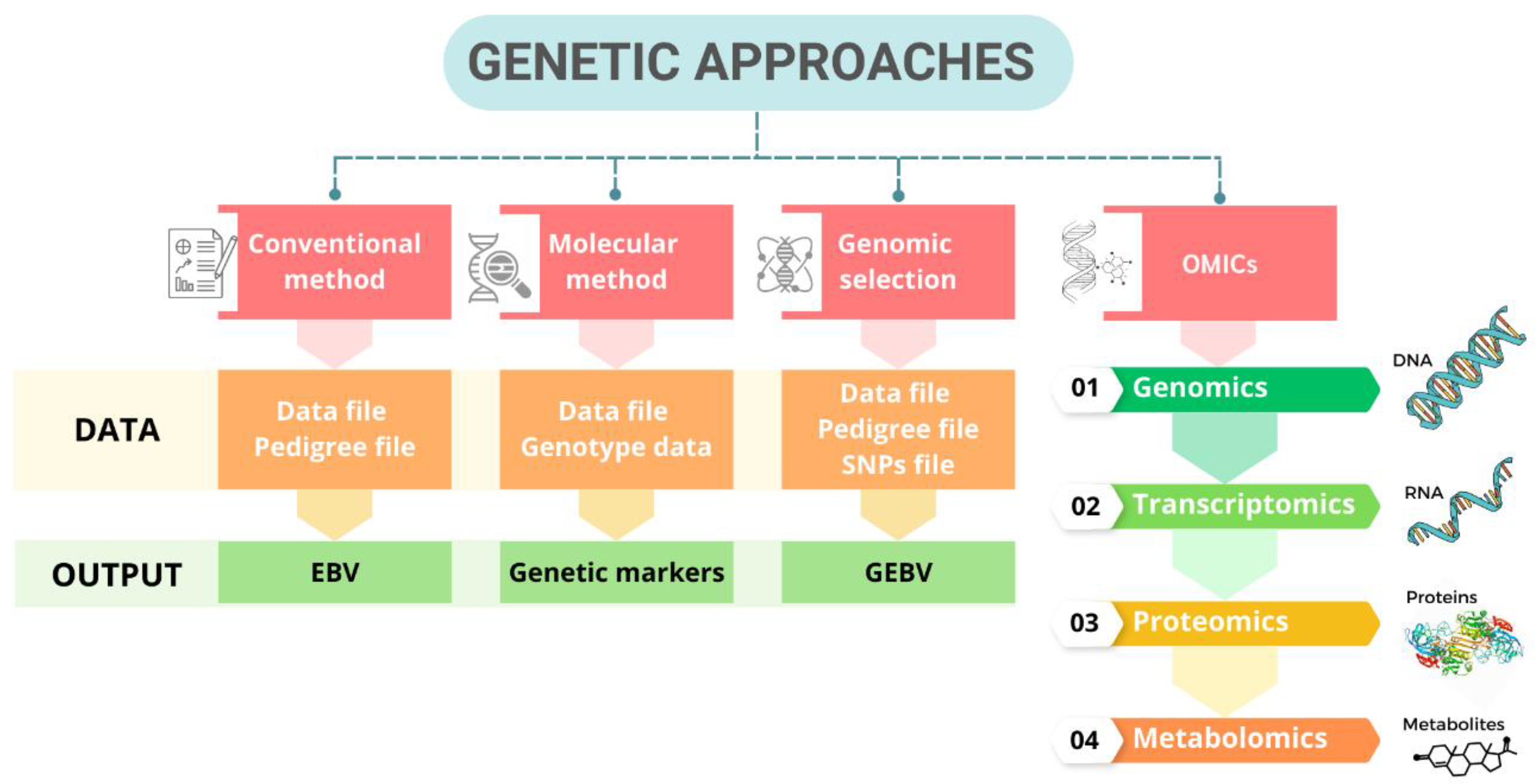
5. Genetic Approaches to Address Heat Stress in Poultry
5.1. Conventional Method
5.2. Molecular Method by Marker-Assisted Selection
5.3. Genomic Selection
5.4. OMICS Technology
6. Challenges of Improving Poultry Genetics in Tropical Areas
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Names of Genes and Their Full Names Given in Manuscript
| Genes | Full names of the genes |
| HSPA2 | Heat shock 70 kDa protein 2 |
| HSPH1 | Heat shock 105 kDa/110 kDa protein 1, |
| HSP25 | Heat shock protein 25 |
| RB1CC1 | RB1-inducible coiled-coil 1 |
| BAG3 | BCL2-associated athanogene 3 |
| CITED2 | Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 2 |
| CTSE | Cathepsin E |
| HSPD1 | Heat shock 60 kDa protein 1 |
| ID1 | Inhibitor Of DNA binding 1 |
| HSP90B1 | Heat shock protein 90 kDa beta member 1 |
| HSP60 | Heat shock protein 60 |
| PDIA2 | Protein disulfide isomerase family A member 2 |
| HSPA5 | Heat Shock Protein Family A (Hsp70) Member 5 |
| HSF1 | Heat shock factor protein 1 |
| HSF3 | Heat shock factor protein 3 |
| HSP70 | Heat shock protein 70 kDa |
| HSP90 | Heat shock protein 90 kDa |
| HSP40 | Heat shock protein 40 kDa |
| SERPINH1 | Serpin family H member 1 |
| HSP47 | Heat shock protein 47 |
| FABP2 | Fatty acid binding protein 2 [(human)] |
| RAMP3 | Receptor activity-modifying protein 3 |
| SUGCT | Succinyl-CoA:Glutarate-CoA Transferase |
| TSHR | thyroid stimulating hormone receptor |
| GLUT-2 | Glucose transporter 2 |
| FABP1 | Fatty acid binding protein 1 |
| CD36 | Cluster of differentiation 36 |
| TRMT1L | tRNA methyltransferase 1 |
| HS3ST5 | Heparan sulfate-glucosamine 3-sulfotransferase 5 |
| EOMES | Eomesodermin |
| NFAT5 | Nuclear factor of activated t-cells 5 |
| NF-κB | Nuclear factor kappa b |
| MRPL42 | Mitochondrial ribosomal protein L42 |
| EDN1 | Endothelin 1 |
| ACSF3 | Acyl-coA synthetase family member 3 |
| CYP4V2 | Cytochrome P450 4V2 |
| PLCB4 | Phospholipase C beta 4 |
| H1F0 | H1 histone family, member 0 |
| ACYP1 | Acylphosphatase 1 |
| JAK1 | Janus kinase 1 |
| JAK2 | Janus kinase 2 |
| TYK2 | Tyrosine kinase 2 |
| FGA | Fibrinogen alpha chain |
| LOXL2 | Lysyl oxidase like 2 |
| GINS1 | GINS Complex Subunit 1 |
| RRM2 | Ribonucleotide reductase regulatory subunit M2 |
| PDK | Pyruvate dehydrogenase kinase |
| PDK | Pyruvate dehydrogenase kinase |
| BVES | Blood Vessel Epicardial Substance |
| SMYD1 | SET And MYND Domain Containing 1 |
| IL18 | Interleukin 18 |
| PDGFRA | Platelet Derived Growth Factor Receptor Alpha |
| CORIN | Corin, Serine Peptidase |
| NRP1 | Neuropilin 1 |
| SIM2 | SIM BHLH Transcription Factor 2 |
| NALCN | Sodium Leak Channel, Non-Selective |
| CLPTM1L | CLPTM1 Like |
| APP | Amyloid Beta Precursor Protein |
| CRADD | CASP2 And RIPK1 Domain Containing Adaptor With Death Domain |
| PARK2 | Parkin RBR E3 Ubiquitin Protein Ligase 2 |
| AHR | Aryl Hydrocarbon Receptor |
| ESRRG | Estrogen Related Receptor Gamma |
| FAS | Fas Cell Surface Death Receptor |
| UBE4B | Ubiquitination Factor E4B |
| FABP1 | Fatty Acid Binding Protein 1 |
| MAP3K3 | Mitogen-Activated Protein Kinase Kinase Kinase 3 |
| SOCS2 | Suppressor Of Cytokine Signaling 2 |
| MAPKBP1 | Mitogen-Activated Protein Kinase Binding Protein 1 |
| SPON1 | Spondin 1 |
| HSP25 | Heat Shock Protein 25 |
| HSD17B1 | Hydroxysteroid 17-Beta Dehydrogenase 1 |
| APOB | Apolipoprotein B |
| PRDX4 | Peroxiredoxin 4 |
| SERPINH1 | Serpin Family H Member 1 |
| CIRBP | Cold Inducible RNA Binding Protein |
| CYP19A1 | Cytochrome P450 Family 19 Subfamily A Member 1 |
| SLC33A1 | Solute Carrier Family 33 Member 1 |
| TSHR | Thyroid Stimulating Hormone Receptor |
| NDUFS4 | NADH:Ubiquinone Oxidoreductase Subunit S4 |
| CAMK1d | Calcium/Calmodulin Dependent Protein Kinase ID |
| CCDC3 | Coiled-Coil Domain Containing 3 |
| TIRAP | TIR Domain Containing Adaptor Protein |
| ETS1 | ETS Proto-Oncogene 1, Transcription Factor |
| KIRREL3 | Kirre Like Nephrin Family Adhesion Molecule 3 |
| JAK1 | Janus Kinase 1 |
| JAK2 | Janus Kinase 2 |
| TYK2 | Tyrosine Kinase 2 |
| HSD17B7 | Hydroxysteroid 17-beta dehydrogenase 7 |
| STARD4 | StAR-related lipid transfer domain containing 4 |
| ACSBG2 | Acyl-CoA Synthetase Bubblegum Family Member 2 |
| SCD | Stearoyl-CoA Desaturase |
| INSIG1 | Insulin Induced Gene 1 |
| ATOX1 | Antioxidant 1 Copper Chaperone |
| SFTPA1 | Surfactant Protein A1 |
| ELK1 | ETS-like 1 |
| YY1 | Yin Yang 1 |
| ZFX | Zinc finger X-chromosomal protein |
| IRF3 | interferon regulatory factor 3 |
| MYLK2 | Myosin Light Chain Kinase 2 |
| BDKRB1 | Bradykinin Receptor B1 |
| FGG | Fibrinogen Gamma Chain |
| IL1R2 | Interleukin 1 Receptor Type 2 |
| IL13RA2 | Interleukin 13 Receptor Subunit Alpha 2 |
| BMP10 | Bone Morphogenetic Protein 10 |
| MYH7 | Myosin Heavy Chain 7 |
| PLK1 | Polo Like Kinase 1 |
| GADD45B | Growth Arrest And DNA Damage Inducible Beta |
| S100A8 | S100 Calcium Binding Protein A8 |
| FOS | Fos Proto-Oncogene, AP-1 Transcription Factor Subunit |
| CEBPD | CCAAT Enhancer Binding Protein Delta |
| CBFB | Core-Binding Factor Subunit Beta |
| SAT1 | Spermidine/Spermine N1-Acetyltransferase 1 |
| MPP1 | MAGUK P55 Scaffold Protein 1 |
| F8 | Coagulation Factor VIII |
| NMI | N-Myc And STAT Interactor |
| USP18 | Ubiquitin Specific Peptidase 18 |
| CMPK2 | Cytidine/Uridine Monophosphate Kinase 2 |
| IFI27L2 | Interferon Alpha Inducible Protein 27 Like 2 |
| DHX58 | DExH-Box Helicase 58 |
| IL-1β | Interleukin 1 Beta |
| IL-6 | Interleukin 6 |
| TNF-α | Tumor Necrosis Factor-Alpha |
| IFN-α | Interferon Alpha 1 |
| CTSD | Cathepsin D |
| CHMP1B | Charged Multivesicular Body Protein 1B |
| TNFAIP3 | TNF Alpha Induced Protein 3 |
| PARP3 | Poly(ADP-Ribose) Polymerase Family Member 3 |
| LUM | Lumican |
| PRKAA1 | Protein Kinase AMP-Activated Catalytic Subunit Alpha 1 |
| LYN | LYN Proto-Oncogene, Src Family Tyrosine Kinase |
| ABCA1 | ATP Binding Cassette Subfamily A Member 1 |
| CAT1 | Catalase 1 |
| DLD | Dihydrolipoamide Dehydrogenase |
| LDHB | Lactate Dehydrogenase B |
| ME1 | Malic Enzyme 1 |
| PCK1 | Phosphoenolpyruvate Carboxykinase 1 |
| PDHA1 | Pyruvate Dehydrogenase E1 Subunit Alpha 1 |
| COX5A | Cytochrome C Oxidase Subunit 5A |
| COX6C | Cytochrome C Oxidase Subunit 6C |
| NDUFS3 | NADH:Ubiquinone Oxidoreductase Core Subunit S3 |
| UQCRC1 | Ubiquinol-Cytochrome C Reductase Core Protein 1 |
| ACO2 | Aconitase 2 |
| ACAT1 | Acetyl-CoA Acetyltransferase 1 |
| CHGA | Chromogranin A |
| CHGB | Chromogranin B |
| HSPA5 | Heat shock 70 kDa protein 5 |
| HSPA8 | Heat shock 70 kDa protein 8 |
| HSP90AA1 | Heat shock protein 90 kDa alpha, class A member 1 |
| HSPA2 | Heat shock 70 kDa protein 2 |
| FKBP4 | FK506 binding protein 4 |
| HSP90α | Heat shock protein 90 kDa alpha |
| HSP70 | Heat shock 70 kDa |
| FABP7 | Fatty Acid Binding Protein 7 |
| FTH1 | Ferritin Heavy Chain 1 |
| GSTA1 | Glutathione S-Transferase Alpha 1 |
| ENO1 | Enolase 1 |
| TUBB | Tubulin Beta Class I |
References
- Attwood, S.; Hajat, C. How will the COVID-19 pandemic shape the future of meat consumption? Public Health Nutr. 2020, 23, 3116–3120. [Google Scholar] [CrossRef] [PubMed]
- Giller, K.E.; Hijbeek, R.; Andersson, J.A.; Sumberg, J. Regenerative agriculture: An agronomic perspective. Outlook Agric. 2021, 50, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. Economic effects of climate change on global agricultural production. Nat. Conserv. 2021, 44, 117–139. [Google Scholar] [CrossRef]
- FAO. The State of Food Security and Nutrition in the World 2022. Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Global Hunger Index Scores by 2021 GHI Rank. 2021. Available online: https://www.globalhungerindex.org/ranking.html (accessed on 4 July 2022).
- World Population Data Sheet. World Population Data Sheet Released. PRB. 2021. Available online: https://www.prb.org/news/2021-world-population-data-sheet-released (accessed on 4 July 2022).
- Whitton, C.; Bogueva, D.; Marinova, D.; Phillips, C.J.C. Are we approaching peak meat consumption? Analysis of meat consumption from 2000 to 2019 in 35 countries and its relationship to gross domestic product. Animals 2021, 11, 3466. [Google Scholar] [CrossRef] [PubMed]
- OECD-FAO. OECD-FAO Agricultural Outlook 2020–2029. 2021. Available online: https://www.oecd-ilibrary.org/sites/29248f46-en/index.html?itemId=/content/component/29248f46-en (accessed on 10 June 2022).
- Copernicus. Copernicus: Globally, the Seven Hottest Years on Record Were the Last Seven; Carbon Dioxide and Methane Concentrations Continue to Rise. 2022. Available online: https://climate.copernicus.eu/copernicus-globally-seven-hottest-years-record-were-last-seven (accessed on 25 March 2023).
- Kpomasse, C.C.; Oke, O.E.; Houndonougbo, F.M.; Tona, K. Broiler production challenges in the tropics: A review. Vet. Med. Sci. 2021, 7, 831–842. [Google Scholar] [CrossRef]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Pawar, S.S.; Basavaraj, S.; Dhansing, L.V.; Pandurang, K.N.; Sahebrao, K.A.; Vitthal, N.A.; Pandit, B.M.; Kumar, B.S. Assessing and mitigating the impact of heat stress in poultry. Adv. Anim. Vet. Sci. 2016, 4, 332–341. [Google Scholar] [CrossRef]
- Scanes, C.G.; Dridi, S. Sturkie’s Avian Physiology; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Vandana, G.D.; Sejian, V.; Lees, A.M.; Pragna, P.; Silpa, M.V.; Maloney, S.K. Heat stress and poultry production: Impact and amelioration. Int. J. Biometeorol. 2021, 65, 163–179. [Google Scholar] [CrossRef]
- Syafwan, S.; Kwakkel, R.P.; Verstegen, M.W.A. Heat stress and feeding strategies in meat-type chickens. Worlds Poult. Sci. J. 2011, 67, 653–674. [Google Scholar] [CrossRef]
- Farghly, M.F.A.; Abd El-Hack, M.E.; Alagawany, M.; Saadeldin, I.M.; Swelum, A.A. Wet feed and cold water as heat stress modulators in growing Muscovy ducklings. Poult. Sci. 2018, 97, 1588–1594. [Google Scholar] [CrossRef]
- Al-Sultan, S.I.; Abdel-Raheem, S.M.; Abd-Allah, S.M.S.; Edris, A.M. Alleviation of chronic heat stress in broilers by dietary supplementation of novel feed additive combinations. Slov. Vet. Res. 2019, 56, 269–279. [Google Scholar] [CrossRef]
- Gouda, A.; Amer, S.A.; Gabr, S.; Tolba, S.A. Effect of dietary supplemental ascorbic acid and folic acid on the growth performance, redox status, and immune status of broiler chickens under heat stress. Trop. Anim. Health Prod. 2020, 52, 2987–2996. [Google Scholar] [CrossRef] [PubMed]
- Lochi, G.M.; Shah, M.G.; Gandahi, J.A.; Gadahi, J.A.; Hadi, S.A.; Farooq, T.; Vistro, W.A.; Rahmani, M.M. Effect of selenium nanoparticles and chitosan on production performance and antioxidant integrity of heat-stressed broiler. Biol. Trace Elem. Res. 2023, 201, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Ogbuagu, N.E.; Ayo, J.O. Effects of l-serine administration on meat quality, characteristics and mineral content of tibia bone in heat-stressed broiler. Vet. Integr. Sci. 2023, 21, 131–144. [Google Scholar] [CrossRef]
- Du, M.; Cheng, Y.; Chen, Y.; Wang, S.; Zhao, H.; Wen, C.; Zhou, Y. Dietary supplementation with synbiotics improves growth performance, antioxidant status, immune function, and intestinal barrier function in broilers subjected to cyclic heat stress. Environ. Sci. Pollut. Res. 2023, 30, 18026–18038. [Google Scholar] [CrossRef] [PubMed]
- Abbass, G.; Abid, A.R. Effect of peppermint, fenugreek and their mixture on production traits of heat stressed broiler chickens. J. Surv. Fish. Sci. 2023, 10, 2704–2716. [Google Scholar]
- Laganá, C.; Ribeiro, A.M.L.; Kessler, A.M.; Kratz, L.R.; Pinheiro, C.C. Effect of the supplementation of vitamins and organic minerals on the performance of broilers under heat stress. Braz. J. Poult. Sci. 2007, 9, 39–43. [Google Scholar] [CrossRef]
- Ghazi Harsini, S.; Habibiyan, M.; Moeini, M.M.; Abdolmohammadi, A.R. Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol. Trace Elem. Res. 2012, 148, 322–330. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alagawany, M.; Noreldin, A.E. Managerial and nutritional trends to mitigate heat stress risks in poultry farms. In Sustainability of Agricultural Environment in Egypt: Part II. The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2018; Volume 77, pp. 325–338. [Google Scholar]
- Calik, A.; Emami, N.K.; White, M.B.; Walsh, M.C.; Romero, L.F.; Dalloul, R.A. Influence of dietary vitamin E and selenium supplementation on broilers subjected to heat stress, Part I: Growth performance, body composition and intestinal nutrient transporters. Poult. Sci. 2022, 101, 101857. [Google Scholar] [CrossRef]
- Awojobi, H.A.; Oluwole, B.O.; Adekunmisi, A.A.; Buraimo, R.A. Performance of finisher broilers fed wet mash with or without drinking water during wet season in the tropics. Int. J. Poult. Sci. 2009, 8, 592–594. [Google Scholar] [CrossRef]
- Saeed, M.; Abbas, G.; Alagawany, M.; Kamboh, A.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Chao, S. Heat stress management in poultry farms: A comprehensive overview. J. Therm. Biol. 2019, 84, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Polsky, L.; von Keyserlingk, M.A.G. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef] [PubMed]
- El-Kholy, M.S.; El-Hindawy, M.M.; Alagawany, M.; Abd El-Hack, M.E.; El-Sayed, S.A.A. Use of acetylsalicylic acid as an allostatic modulator in the diets of growing Japanese quails exposed to heat stress. J. Therm. Biol. 2018, 74, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.R.; Alagawany, M. Physiological alterations of poultry to the high environmental temperature. J. Therm. Biol. 2018, 76, 101–106. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef]
- Tokutake, Y.; Takanashi, R.; Kikusato, M.; Toyomizu, M.; Sato, K. Effect of dietary 4-phenylbuthyric acid supplementation on acute heat-stress-induced hyperthermia in broiler chickens. Animals 2022, 12, 2056. [Google Scholar] [CrossRef]
- Zaboli, G.-R.; Rahimi, S.; Shariatmadari, F.; Torshizi, M.A.K.; Baghbanzadeh, A.; Mehri, M. Thermal manipulation during pre and post-hatch on thermotolerance of male broiler chickens exposed to chronic heat stress. Poult. Sci. 2017, 96, 478–485. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J. Agric. Food Chem. 2017, 65, 11251–11258. [Google Scholar] [CrossRef]
- Meltzer, A.; Goodman, G.; Fistool, J. Thermoneutral zone and resting metabolic rate of growing White Leghorn-type chickens. Br. Poult. Sci. 1982, 23, 383–391. [Google Scholar] [CrossRef]
- Pace, N.; Rahlmann, D. Thermoneutral zone and scaling of metabolic rate on body mass in small mammals. Physiol. Suppl. 1983, 26, 19840041554. [Google Scholar]
- Silva, G.L.L.P.; Punyawardena, B.V.R.; Hettiarachchi, A.K.; Hulugalla, W.M.M.P.; Lokuge, G.M.S. Assessing thermal neutral zones in Sri Lanka for ten different dairy cattle breeds and crosses: An approach using temperature humidity index (THI). Int. J. Livest. Prod. 2021, 12, 112–121. [Google Scholar]
- Hadfield, J.; Hadfield, J.; Dallin, J.; Millward, L. Maintaining Pig Temperatures in the Summer and Winter Seasons; Utah State University Extension: Logan, UT, USA, 2023; pp. 1–4. [Google Scholar]
- Yan, L.; Hu, M.; Gu, L.; Lei, M.; Chen, Z.; Zhu, H.; Chen, R. Effect of heat stress on egg production, steroid hormone synthesis, and related gene expression in chicken preovulatory follicular granulosa cells. Animals 2022, 12, 1467. [Google Scholar] [CrossRef] [PubMed]
- Charles, D.R.; Walker, A.W. Poultry Environment Problems: A Guide to Solutions; University of Nottingham, Sutton Bonington Campus: Loughborough, UK, 2002; p. 88. [Google Scholar]
- Durmuş, İ.; Kamanli, S. Effects of cold and heat stress on egg quality traits of a newly developed native hybrid layer. Turk. J. Agric. Food. Sci. Technol. 2015, 3, 444–447. [Google Scholar] [CrossRef]
- Qaid, M.M.; Al-Garadi, M.A. Protein and amino acid metabolism in poultry during and after heat stress: A review. Animals 2021, 11, 1167. [Google Scholar] [CrossRef]
- Cândido, M.G.L.; Tinôco, I.F.F.; Herker, L.P.; Ireno, T.F.P.; Andrade, R.R.; Gates, R.S. Evaluation of a low cost thermographic camera for poultry temperature. In Proceedings of the 10th International Livestock Environment Symposium (ILES X), Omaha, NE, USA, 25–27 September 2018. [Google Scholar]
- Shakeri, M.; Oskoueian, E.; Le, H.H.; Shakeri, M. Strategies to combat heat stress in broiler chickens: Unveiling the roles of selenium, vitamin E and vitamin C. Vet. Sci. 2020, 7, 71. [Google Scholar] [CrossRef]
- Welay, K.; Amaha, N.; Demeke, S.; Debusho, L.K.; Girma, M. Growth performance and carcass characteristics of Koekoek chickens exposed to temperature variation with supplementary coriander seed powder. J. Therm. Biol. 2023, 116, 103674. [Google Scholar] [CrossRef]
- Sohail, M.U.; Hume, M.E.; Byrd, J.A.; Nisbet, D.J.; Ijaz, A.; Sohail, A.; Shabbir, M.Z.; Rehman, H. Effect of supplementation of prebiotic mannanoligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult. Sci. 2012, 91, 2235–2240. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Wang, X.; Zhao, F.; Wang, C.; Zhang, Q.; Chen, X.; Geng, Z.; Zhang, C. Resveratrol attenuates heat stress-induced impairment of meat quality in broilers by regulating the Nrf2 signaling pathway. Animals 2022, 12, 1889. [Google Scholar] [CrossRef]
- Attia, Y.A.; Abd El-Hamid, A.E.-H.E.; Abedalla, A.A.; Berika, M.A.; Al-Harthi, M.A.; Kucuk, O.; Sahin, K.; Abou-Shehema, B.M. Laying performance, digestibility and plasma hormones in laying hens exposed to chronic heat stress as affected by betaine, vitamin C, and/or vitamin E supplementation. SpringerPlus 2016, 5, 1619. [Google Scholar] [CrossRef]
- Boonkum, W.; Duangjinda, M.; Kananit, S.; Chankitisakul, V.; Kenchaiwong, W. Genetic effect and growth curve parameter estimation under heat stress in slow-growing Thai native chickens. Vet. Sci. 2021, 8, 297. [Google Scholar] [CrossRef]
- Loengbudnark, W.; Chankitisakul, V.; Boonkum, W. The genetic impact of heat stress on the egg production of Thai native chickens (Pradu Hang dum). PLoS ONE 2023, 18, e0281328. [Google Scholar] [CrossRef]
- Ribeiro, C.; Hennen-Bierwagen, T.A.; Myers, A.M.; Cline, K.; Settles, A.M. Engineering 6-phosphogluconate dehydrogenase improves grain yield in heat-stressed maize. Agric. Sci. 2020, 117, 33177–33185. [Google Scholar] [CrossRef] [PubMed]
- Strong, R.A.; Hester, P.Y.; Eicher, S.D.; Hu, J.; Cheng, H.-W. The effect of cooled perches on immunological parameters of caged White Leghorn hens during the hot summer months. PLoS ONE 2015, 10, e0141215. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Y.; Hester, P.Y.; Xiong, Y.; Gates, R.S.; Makagon, M.M.; Cheng, H.W. Effect of cooled perches on the efficacy of an induced molt in White Leghorn laying hens previously exposed to heat stress. Poult. Sci. 2019, 98, 4290–4300. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.M.U.; Ashraf, H.; Shahzad, K.; Sultan, M.; Miyazaki, T.; Usman, M.; Shamshiri, R.R.; Zhou, Y.; Ahmad, R. Investigating applicability of evaporative cooling systems for thermal comfort of poultry birds in Pakistan. Appl. Sci. 2020, 10, 4445. [Google Scholar] [CrossRef]
- Smit, B.; Zietsman, G.; Martin, R.O.; Cunningham, S.J.; McKechnie, A.E.; Hockey, P.A.R. Behavioural responses to heat in desert birds: Implications for predicting vulnerability to climate warming. Clim. Chang. Response 2016, 3, 9. [Google Scholar] [CrossRef]
- Ratnakaran, A.P.; Sejian, V.; Jose, V.S.; Vaswani, S.; Bagath, M.; Krishnan, G.; Beena, V.; Devi, P.I.; Varma, G.; Bhatta, R. Behavioral responses to livestock adaptation to heat stress challenges. Asian J. Anim. Sci. 2017, 11, 1–13. [Google Scholar] [CrossRef]
- Yang, L.; Tan, G.-Y.; Fu, Y.-Q.; Feng, J.-H.; Zhang, M.-H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 204–208. [Google Scholar] [CrossRef]
- Shahzad, K.; Sultan, M.; Bilal, M.; Ashraf, H.; Farooq, M.; Miyazaki, T.; Sajjad, U.; Ali, I.; Hussain, M.I. Experiments on energy-efficient evaporative cooling systems for poultry farm application in Multan (Pakistan). Sustainability 2021, 13, 2836. [Google Scholar] [CrossRef]
- Iyasere, O.S.; Edwards, S.A.; Bateson, M.; Mitchell, M.; Guy, J.H. Validation of an intramuscularly-implanted microchip and a surface infrared thermometer to estimate core body temperature in broiler chickens exposed to heat stress. Comput. Electron. Agric. 2017, 133, 1–8. [Google Scholar] [CrossRef]
- Wolf, B.O.; Walsberg, G.E. The role of the plumage in heat transfer processes of birds. Am. Zool. 2000, 40, 575–584. [Google Scholar] [CrossRef]
- Chaiyabutr, N. Physiological reactions of poultry to heat stress and methods to reduce its effects on poultry production. Thai J. Vet. Med. 2004, 34, 17–30. [Google Scholar] [CrossRef]
- Chen, S.; Yong, Y.; Ju, X. Effect of heat stress on growth and production performance of livestock and poultry: Mechanism to prevention. J. Therm. Biol. 2021, 99, 103019. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.H.; Amoah, K.; Leng, Q.Y.; Zheng, J.H.; Zhang, W.L.; Zhang, L. Poultry response to heat stress: Its physiological, metabolic, and genetic implications on meat production and quality including strategies to improve broiler production in a warming world. Front. Vet. Sci. 2021, 8, 699081. [Google Scholar] [CrossRef]
- Cockrem, J.F. Stress, corticosterone responses and avian personalities. J. Ornithol. 2007, 148, 169–178. [Google Scholar] [CrossRef]
- Kuo, T.; McQueen, A.; Chen, T.C.; Wang, J.C. Regulation of glucose homeostasis by glucocorticoids. Adv. Exp. Med. Biol. 2015, 872, 99–126. [Google Scholar]
- Akalestou, E.; Genser, L.; Rutter, G.A. Glucocorticoid metabolism in obesity and following weight loss. Front. Endocrinol. 2020, 11, 59. [Google Scholar] [CrossRef]
- Magomedova, L.; Cummins, C.L. Glucocorticoids and metabolic control. Metabolic Control. In Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2016; pp. 73–93. [Google Scholar]
- Riezman, H. Why do cells require heat shock proteins to survive heat stress? Cell Cycle 2004, 3, 60–62. [Google Scholar] [CrossRef]
- Ray, S.; Sharma, S.; Maheshwari, A.; Aneja, S.; Kumar, A. Heat stroke in an infant with hypohidrotic ectodermal dysplasia: Brain magnetic resonance imaging findings. J. Child Neurol. 2013, 28, 538–540. [Google Scholar] [CrossRef]
- Popoola, I.O.; Popoola, O.R.; Ojeniyi, M.O.; Olajide, O.O.; Iyayi, E.A. The roles of key electrolytes in balancing blood acid-base and nutrient in broiler chickens reared under tropical conditions. Nat. Sci. 2020, 12, 4–11. [Google Scholar] [CrossRef]
- Teeter, R.; Smith, M.; Owens, F.; Arp, S.; Sangiah, S.; Breazile, J. Chronic heat stress and respiratory alkalosis: Occurrence and treatment in broiler chicks. Poult. Sci. 1985, 64, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Mashaly, M.M.; Hendricks, G.L.; Kalama, M.A.; Gehad, A.E.; Abbas, A.O.; Patterson, P.H. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004, 83, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Titto, C.G.; de Mira Geraldo, A.; Martínez-Burnes, J.; Gómez, J.; Hernández-Ávalos, I.; Casas, A.; Domínguez, A.; José, N.; Bertoni, A.; et al. Efficacy and function of feathers, hair, and glabrous skin in the thermoregulation strategies of domestic animals. Animals 2021, 11, 3472. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.M.; Buettner, G.R.; Oberley, L.W.; Xu, L.; Matthes, R.D.; Gisolfi, C.V. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H509–H521. [Google Scholar] [CrossRef]
- Nawab, A.; Ibtisham, F.; Li, G.; Kieser, B.; Wu, J.; Liu, W.; Zhao, Y.; Nawab, Y.; Li, K.; Xiao, M.; et al. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018, 78, 131–139. [Google Scholar] [CrossRef]
- Wasti, S.; Sah, N.; Mishra, B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals 2020, 10, 1266. [Google Scholar] [CrossRef]
- Khan, R.U.; Naz, S.; Ullah, H.; Ullah, Q.; Laudadio, V.; Qudratullah; Bozzo, G.; Tufarelli, V. Physiological dynamics in broiler chickens under heat stress and possible mitigation strategies. Anim. Biotechnol. 2021, 34, 438–447. [Google Scholar] [CrossRef]
- Shakeri, M.; Le, H.H. Deleterious effects of heat stress on poultry production: Unveiling the benefits of betaine and polyphenols. Poultry 2022, 1, 147–156. [Google Scholar] [CrossRef]
- Del Vesco, A.; Gasparino, E.; Zancanela, V.; Grieser, D.; Stanquevis, C.; Pozza, P.; Oliveira Neto, A. Effects of selenium supplementation on the oxidative state of acute heat stress-exposed quails. J. Anim. Physiol. Anim. Nutr. 2017, 101, 170–179. [Google Scholar] [CrossRef]
- Kikusato, M.; Toyomizu, M. Mechanisms underlying the effects of heat stress on intestinal integrity, inflammation, and microbiota in chickens. Poult. Sci. J. 2023, 60, 2023021. [Google Scholar] [CrossRef]
- Zaboli, G.; Huang, X.; Feng, X.; Ahn, D.U. How can heat stress affect chicken meat quality?—A review. Poult. Sci. 2019, 98, 1551–1556. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.H.; Cheng, K.; Zheng, X.C.; Ahmad, H.; Zhang, L.L.; Wang, T. Effects of dietary supplementation with enzymatically treated artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018, 97, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Nanto-Hara, F.; Ohtsu, H.; Yamazaki, M.; Hirakawa, T.; Sato, K.; Murakami, H. Effects of dietary brown rice on the growth performance, systemic oxidative status, and splenic inflammatory responses of broiler chickens under chronic heat stress. J. Poult. Sci. 2021, 58, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.G.; Gomez-Raya, L.; Torres, O.; Cigarroa-Vazquez, F.A.; Davila, S.G.; Rauw, W.M. Heterophil/Lymphocyte response of local spanish breeds of laying hens to cold stress, heat stress, and water restriction. J. Therm. Biol. 2023, 113, 103542. [Google Scholar] [CrossRef]
- Videla, E.A.; Giayetto, O.; Fernández, M.E.; Chacana, P.A.; Marín, R.H.; Nazar, F.N. Immediate and transgenerational effects of thymol supplementation, inactivated salmonella and chronic heat stress on representative immune variables of Japanese quail. Sci. Rep. 2020, 10, 18152. [Google Scholar] [CrossRef]
- Soleimani, A.F.; Zulkifli, I.; Omar, A.R.; Raha, A.R. Physiological responses of 3 chicken breeds to acute heat stress. Poult. Sci. 2011, 90, 1435–1440. [Google Scholar] [CrossRef]
- Al-Murrani, W.; Kassab, A.; Al-Sam, H.; Al-Athari, A. Heterophil/Lymphocyte ratio as a selection criterion for heat resistance in domestic fowls. Br. Poult. Sci. 1997, 38, 159–163. [Google Scholar] [CrossRef]
- Hirakawa, R.; Nurjanah, S.; Furukawa, K.; Murai, A.; Kikusato, M.; Nochi, T.; Toyomizu, M. Heat stress causes immune abnormalities via massive damage to effect proliferation and differentiation of lymphocytes in broiler chickens. Front. Vet. Sci. 2020, 7, 46. [Google Scholar] [CrossRef]
- Kammon, A.; Alzentani, S.; Tarhuni, O.; Asheg, A. Effect of some organic acids on body weight, immunity and cecal bacterial count of chicken during heat stress. Int. J. Poult. Sci. 2019, 18, 293–300. [Google Scholar] [CrossRef]
- Honda, B.T.B.; Calefi, A.S.; Costola-de-Souza, C.; Quinteiro-Filho, W.M.; da Silva Fonseca, J.G.; de Paula, V.F.; Palermo-Neto, J. Effects of Heat stress on peripheral T and B Lymphocyte profiles and IgG and IgM serum levels in broiler chickens vaccinated for Newcastle disease virus. Poult. Sci. 2015, 94, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Alhenaky, A.; Abdelqader, A.; Abuajamieh, M.; Al-Fataftah, A.-R. The effect of heat stress on intestinal integrity and salmonella invasion in broiler birds. J. Therm. Biol. 2017, 70, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Quinteiro-Filho, W.M.; Gomes, A.; Pinheiro, M.L.; Ribeiro, A.; Ferraz-de-Paula, V.; Astolfi-Ferreira, C.S.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with salmonella enteritidis. Avian Pathol. 2012, 41, 421–427. [Google Scholar] [CrossRef]
- Ahmad, R.; Yu, Y.-H.; Hsiao, F.S.-H.; Su, C.-H.; Liu, H.-C.; Tobin, I.; Zhang, G.; Cheng, Y.-H. Influence of heat stress on poultry growth performance, intestinal inflammation, and immune function and potential mitigation by probiotics. Animals 2022, 12, 2297. [Google Scholar] [CrossRef]
- Ghulam Mohyuddin, S.; Khan, I.; Zada, A.; Qamar, A.; Arbab, A.A.I.; Ma, X.; Yu, Z.; Liu, X.-X.; Yong, Y.-H.; Ju, X.H.; et al. Influence of Heat Stress on Intestinal Epithelial Barrier Function, Tight Junction Protein, and Immune and Reproductive Physiology. Biomed. Res. Int. 2022, 2022, e8547379. [Google Scholar] [CrossRef] [PubMed]
- Calefi, A.S.; QUINTEIRO-FILHO, W.M.; Ferreira, A.J.P.; Palermo-Neto, J. Neuroimmunomodulation and heat stress in poultry. Worlds. Poult. Sci. J. 2017, 73, 493–504. [Google Scholar] [CrossRef]
- Rowland, K.; Ashwell, C.M.; Persia, M.E.; Rothschild, M.F.; Schmidt, C.; Lamont, S.J. Genetic Analysis of production, physiological, and egg quality traits in heat-challenged commercial white egg-laying hens using 600k snp array data. Genet. Sel. Evol. 2019, 51, 31. [Google Scholar] [CrossRef]
- Mazzoni, M.; Zampiga, M.; Clavenzani, P.; Lattanzio, G.; Tagliavia, C.; Sirri, F. Effect of chronic heat stress on gastrointestinal histology and expression of feed intake-regulatory hormones in broiler chickens. Animals 2022, 16, 100600. [Google Scholar] [CrossRef]
- Awad, W.A.; Ruhnau, D.; Hess, C.; Doupovec, B.; Schatzmayr, D.; Hess, M. Feeding of deoxynivalenol increases the intestinal paracellular permeability of broiler chickens. Arch. Toxicol. 2019, 93, 2057–2064. [Google Scholar] [CrossRef]
- Khan, R.U.; Naz, S.; Nikousefat, Z.; Tufarelli, V.; Javdani, M.; Rana, N.; Laudadio, V. Effect of vitamin E in heat-stressed poultry. Worlds Poult. Sci. J. 2011, 67, 469–478. [Google Scholar] [CrossRef]
- Sohail, M.U.; Ijaz, A.; Yousaf, M.S.; Ashraf, K.; Zaneb, H.; Aleem, M.; Rehman, H. Alleviation of cyclic heat stress in broilers by dietary supplementation of mannan-oligosaccharide and lactobacillus-based probiotic: Dynamics of cortisol, thyroid hormones, cholesterol, c-reactive protein, and humoral immunity. Poult. Sci. 2010, 89, 1934–1938. [Google Scholar] [CrossRef]
- Nanto-Hara, F.; Kikusato, M.; Ohwada, S.; Toyomizu, M. Heat stress directly affects intestinal integrity in broiler chickens. Jpn. Poult. Sci. 2020, 57, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Tabler, T.W.; Greene, E.S.; Orlowski, S.K.; Hiltz, J.Z.; Anthony, N.B.; Dridi, S. Intestinal barrier integrity in heat-stressed modern broilers and their ancestor wild jungle fowl. Front. Vet. Sci. 2020, 7, 249. [Google Scholar] [CrossRef]
- Donoghue, D.J.; Krueger, B.F.; Hargis, B.M.; Miller, A.M.; el Halawani, M. Thermal stress reduces serum luteinizing hormone and bioassayable hypothalamic content of luteinizing hormone-releasing hormone in hens. Biol. Reprod. 1989, 41, 419–424. [Google Scholar] [CrossRef]
- Olusegun, O.; Alabi, O. Influence of high environmental temperature on egg production and shell quality: A review. Worlds Poult. Sci. J. 2010, 66, 739–749. [Google Scholar]
- McDaniel, C.D.; Bramwell, R.K.; Wilson, J.L.; Howarth, J.B. Fertility of male and female broiler breeders following exposure to an elevated environmental temperature. Poult. Sci. 1995, 74, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Kamel, N.N.; Ahmed, A.M.H.; Mehaisen, G.M.K.; Mashaly, M.M.; Abass, A.O. Depression of leukocyte protein synthesis, immune function and growth performance induced by high environmental temperature in broiler chickens. Int. J. Biometeorol. 2017, 61, 1637–1645. [Google Scholar] [CrossRef]
- Aguanta, B.N.; Fuller, A.L.; Milfort, M.C.; Williams, S.M.; Rekaya, R.; Aggrey, S.E. Histologic effects of concurrent heat stress and coccidial infection on the lymphoid tissues of broiler chickens. Avian Dis. 2018, 62, 345–350. [Google Scholar] [CrossRef]
- Chomchuen, K.; Tuntiyasawasdikul, V.; Chankitisakul, V.; Boonkum, W. Comparative study of phenotypes and genetics related to the growth performance of crossbred Thai indigenous (KKU1 vs. KKU2) chickens under hot and humid conditions. Vet. Sci. 2022, 9, 263. [Google Scholar] [CrossRef]
- Chomchuen, K.; Tuntiyasawasdikul, V.; Chankitisakul, V.; Boonkum, W. Genetic evaluation of body weights and egg production traits using a multi-trait animal model and selection index in Thai native synthetic chickens (Kaimook e-san2). Animals 2022, 12, 335. [Google Scholar] [CrossRef]
- Rong, Y.; Zeng, M.; Guan, X.; Qu, K.; Liu, J.; Zhang, J.; Chen, H.; Huang, B.; Lei, C. Association of HSF1 genetic variation with heat tolerance in Chinese cattle. Animals 2019, 9, 1027. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, A.; Abdollahi-Arpanahi, R.; Aguilar, I.; Peñagaricano, F. Whole genome mapping reveals novel genes and pathways involved in milk production under heat stress in US Holstein cows. Front. Genet. 2019, 10, 928. [Google Scholar] [CrossRef] [PubMed]
- Carabaño, M.J.; Logar, B.; Bormann, J.; Minet, J.; Vanrobays, M.L.; Díaz, C.; Tychon, B.; Gengler, N.; Hammami, H. Modeling heat stress under different environmental conditions. J. Dairy Sci. 2016, 99, 3798–3814. [Google Scholar] [CrossRef] [PubMed]
- Pryce, J.E.; Nguyen, T.T.T.; Cheruiyot, E.K.; Marett, L.; Garner, J.B.; Haile-Mariam, M. Impact of hot weather on animal performance and genetic strategies to minimise the effect. Anim. Prod. Sci. 2022, 62, 726–735. [Google Scholar] [CrossRef]
- Wang, S.-H.; Cheng, C.-Y.; Tang, P.-C.; Chen, C.-F.; Chen, H.-H.; Lee, Y.-P.; Huang, S.-Y. Acute heat stress induces differential gene expressions in the testes of a broiler-type strain of Taiwan country chickens. PLoS ONE 2015, 10, e0125816. [Google Scholar] [CrossRef]
- Van Goor, A.; Bolek, K.J.; Ashwell, C.M.; Persia, M.E.; Rothschild, M.F.; Schmidt, C.J.; Lamont, S.J. Identification of quantitative trait loci for body temperature, body weight, breast yield, and digestibility in an advanced intercross line of chickens under heat stress. Genet. Sel. Evol. 2015, 47, 96. [Google Scholar] [CrossRef]
- Asadollahpour Nanaei, H.; Kharrati-Koopaee, H.; Esmailizadeh, A. Genetic diversity and signatures of selection for heat tolerance and immune response in Iranian native chickens. BMC Genom. 2022, 23, 224. [Google Scholar] [CrossRef]
- Dekkers, J.; Hospital, F. The use of molecular genetics in the improvement of agricultural populations. Nat. Rev. Genet. 2002, 3, 22–32. [Google Scholar] [CrossRef]
- Cedraz, H.; Gromboni, J.G.G.; Junior, A.A.P.G.; Filho, R.V.F.; Souza, T.M.; de Oliveira, E.R.; de Oliveira, E.B.; do Nascimento, C.S.; Meneghetti, C.; Wenceslau, A.A. Heat stress induces expression of HSP genes in genetically divergent chickens. PLoS ONE 2017, 12, e0186083. [Google Scholar] [CrossRef]
- Shehata, A.M.; Saadeldin, I.M.; Tukur, H.A.; Habashy, W.S. Modulation of heat-shock proteins mediates chicken cell survival against thermal stress. Animals 2020, 10, 2407. [Google Scholar] [CrossRef]
- Duangjinda, M.; Tunim, S.; Duangdaen, C.; Boonkum, W. Hsp70 genotypes and heat tolerance of commercial and native chickens reared in hot and humid conditions. Braz. J. Poult. Sci. 2017, 19, 07–18. [Google Scholar] [CrossRef]
- Luo, Q.B.; Song, X.Y.; Ji, C.L.; Zhang, X.Q.; Zhang, D.X. Exploring the molecular mechanism of acute heat stress exposure in broiler chickens using gene expression profiling. Gene 2014, 546, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, H.; Sheikhahmadi, A.; Wang, Y.; Jiao, H.; Lin, H.; Song, Z. Effects of heat stress on the gene expression of nutrient transporters in the jejunum of broiler chickens (Gallus gallus domesticus). Int. J. Biometeorol. 2015, 59, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Li, Z.; Shi, H.; Kang, Y.; Wang, J.; Huang, J.; Jiang, L. Effects of heat stress on respiratory burst, oxidative damage and SERPINH1 (HSP47) mRNA expression in rainbow trout Oncorhynchus mykiss. Fish Physiol. Biochem. 2016, 42, 701–710. [Google Scholar] [CrossRef]
- Tellechea, M.; Buxadé, M.; Tejedor, S.; Aramburu, J.; López-Rodríguez, C. NFAT5-regulated macrophage polarization supports the proinflammatory function of macrophages and T lymphocytes. J. Immunol. 2018, 200, 305–315. [Google Scholar] [CrossRef]
- Srikanth, K.; Kumar, H.; Park, W.; Byun, M.; Lim, D.; Kemp, S.; Te Pas, M.F.W.; Kim, J.M.; Park, J.E. Cardiac and skeletal muscle transcriptome response to heat stress in Kenyan chicken ecotypes adapted to low and high altitudes reveal differences in thermal tolerance and stress response. Front. Genet. 2019, 10, 993. [Google Scholar] [CrossRef]
- Kumar, M.; Ratwan, P.; Dahiya, S.P.; Nehra, A.K. Climate change and heat stress: Impact on production, reproduction and growth performance of poultry and its mitigation using genetic strategies. J. Therm. Biol. 2021, 97, 102867. [Google Scholar] [CrossRef]
- Asadollahi, H.; Torshizi, R.V.; Ehsani, A.; Masoudi, A.A. An association of CEP78, MEF2C, VPS13A and ARRDC3 genes with survivability to heat stress in an F2 chicken population. J. Anim. Breed. Genet. 2022, 139, 574–582. [Google Scholar] [CrossRef]
- De Maio, A.; Vazquez, D. Extracellular heat shock proteins: A new location, a new function. Shock 2013, 40, 239–246. [Google Scholar] [CrossRef]
- Dewe, J.M.; Fuller, B.L.; Lentini, J.M.; Kellner, S.M.; Fu, D. TRMT1-catalyzed tRNA modifications are required for redox homeostasis to ensure proper cellular proliferation and oxidative stress survival. Mol. Cell. Biol. 2017, 37, e00214-17. [Google Scholar] [CrossRef]
- Walugembe, M.; Bertolini, F.; Dematawewa, C.M.B.; Reis, M.P.; Elbeltagy, A.R.; Schmidt, C.J.; Lamont, S.J.; Rothschild, M.F. Detection of selection signatures among Brazilian, Sri Lankan, and Egyptian chicken populations under different environmental conditions. Front. Genet. 2019, 9, 737. [Google Scholar] [CrossRef]
- Szauter, K.M.; Jansen, M.K.; Okimoto, G.; Loomis, M.; Kimura, J.H.; Heller, M.; Ku, T.; Tiirikainen, M.; Boyd, C.D.; Csiszar, K.; et al. Persistent inflammatory pathways associated with early onset myocardial infarction in a medicated multiethnic Hawaiian cohort. Biochem. Insights 2011, 4, 13–27. [Google Scholar] [CrossRef]
- Zhang, J.; Marotel, M.; Fauteux-Daniel, S.; Mathieu, A.L.; Viel, S.; Marçais, A.; Walzer, T. T-bet and Eomes govern differentiation and function of mouse and human NK cells and ILC1. Eur. J. Immunol. 2018, 48, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zheng, H.; Yang, L.; Li, H.; Tian, Y.; Wang, Y.; Lyu, S.; Brockmann, G.A.; Kang, X.; Liu, X. Dynamic expression profile, regulatory mechanism and correlation with egg-laying performance of ACSF gene family in chicken (Gallus gallus). Sci. Rep. 2018, 8, 8457. [Google Scholar] [CrossRef] [PubMed]
- Claire D’Andre, H.; Paul, W.; Shen, X.; Jia, X.; Zhang, R.; Sun, L.; Zhang, X. Identification and characterization of genes that control fat deposition in chickens. J. Anim. Sci. Biotechnol. 2013, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Meuwissen, T.; Hayes, B.; Goddard, M. Genomic selection: A paradigm shift in animal breeding. Anim. Front. 2016, 6, 6–14. [Google Scholar] [CrossRef]
- Wang, Y.; Mette, M.F.; Miedaner, T.; Gottwald, M.; Wilde, P.; Reif, J.C.; Zhao, Y. The accuracy of prediction of genomic selection in elite hybrid rye populations surpasses the accuracy of marker-assisted selection and is equally augmented by multiple field evaluation locations and test years. BMC Genom. 2014, 15, 556. [Google Scholar] [CrossRef]
- Dekkers, J.C.M. Prediction of response to marker-assisted and genomic selection using selection index theory. J. Anim. Breed. Genet. 2007, 124, 331–341. [Google Scholar] [CrossRef]
- Ceballos, H.; Kawuki, R.S.; Gracen, V.E.; Yencho, G.C.; Hershey, C.H. Conventional breeding, marker-assisted selection, genomic selection and inbreeding in clonally propagated crops: A case study for cassava. Theor. Appl. Genet. 2015, 128, 1647–1667. [Google Scholar] [CrossRef]
- Misztal, I.; Legarra, A.; Aguilar, I. Computing procedures for genetic evaluation including phenotypic, full pedigree, and genomic information. J. Dairy. Sci. 2009, 92, 4648–4655. [Google Scholar] [CrossRef]
- Guo, Y.; Liao, J.-H.; Liang, Z.-L.; Balasubramanian, B.; Liu, W.-C. Hepatic lipid metabolomics in response to heat stress in local broiler chickens breed (Huaixiang chickens). Vet. Med. Sci. 2021, 7, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Bjorkquist, A.; Ashwell, C.; Persia, M.; Rothschild, M.F.; Schmidt, C.; Lamont, S.J. QTL for body composition traits during heat stress revealed in an advanced intercross line of chickens. In Proceedings of the 10th World Congress of Genetics Applied to Livestock Production, Vancouver, BC, Canada, 17–22 August 2014. [Google Scholar]
- Zhuang, Z.X.; Chen, S.-E.; Chen, C.-F.; Lin, E.-C.; Huang, S.-Y. Genomic regions and pathways associated with thermotolerance in layer-type strain Taiwan indigenous chickens. J. Therm. Biol. 2020, 88, 102486. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.-X.; Chen, S.-E.; Chen, C.-F.; Lin, E.-C.; Huang, S.-Y. Genome-wide association study on the body temperature changes of a broiler-type strain Taiwan country chickens under acute heat stress. J. Therm. Biol. 2019, 82, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Wolc, A.; Arango, J.; Settar, P.; Fulton, J.E.; O’Sullivan, N.P.; Dekkers, J.C.M. Genome wide association study for heat stress induced mortality in a white egg layer line. Poult. Sci. 2019, 98, 92–96. [Google Scholar] [CrossRef]
- Saelao, P.; Wang, Y.; Chanthavixay, G.; Gallardo, R.A.; Wolc, A.; Dekkers, J.C.M.; Lamont, S.J.; Kelly, T.; Zhou, H. Genetics and genomic regions affecting response to Newcastle disease virus infection under heat stress in layer chickens. Genes 2019, 10, 61. [Google Scholar] [CrossRef]
- Wells, K.L.; Hadad, Y.; Ben-Avraham, D.; Hillel, J.; Cahaner, A.; Headon, D.J. Genome-wide SNP scan of pooled DNA reveals nonsense mutation in FGF20 in the scaleless line of featherless chickens. BMC Genom. 2012, 13, 257. [Google Scholar] [CrossRef]
- Thudi, M.; Upadhyaya, H.D.; Rathore, A.; Gaur, P.M.; Krishnamurthy, L.; Roorkiwal, M.; Nayak, S.N.; Chaturvedi, S.K.; Basu, P.S.; Gangarao, N.V.P.R.; et al. Genetic dissection of drought and heat tolerance in chickpea through genome-wide and candidate gene-based association mapping approaches. PLoS ONE 2014, 9, e96758. [Google Scholar] [CrossRef]
- Lowe, E.K.; Cuomo, C.; Arnone, M.I. Omics approaches to study gene regulatory networks for development in echinoderms. Brief. Funct. Genom. 2017, 16, 299–308. [Google Scholar] [CrossRef]
- O’Connell, K.; Gannon, J.; Doran, P.; Ohlendieck, K. Proteomic profiling reveals a severely perturbed protein expression pattern in aged skeletal muscle. Int. J. Mol. Med. 2007, 20, 145–153. [Google Scholar] [CrossRef]
- Gu, J.; Liang, Q.; Liu, C.; Li, S. Genomic analyses reveal adaptation to hot arid and harsh environments in native chickens of China. Front. Genet. 2020, 11, 582355. [Google Scholar] [CrossRef]
- Asadollahi, H.; Torshizi, R.V.; Ehsani, A.; Masoudi, A. A genome-wide association study of survival to unexpected acute heat stress in a F2 chicken population. J. Agric. Sci. Technol. 2021, 23, 283–292. [Google Scholar]
- Cheng, C.Y.; Tu, W.L.; Chen, C.J.; Chan, H.J.; Chen, C.F.; Chen, H.H.; Tang, P.C.; Lee, Y.P.; Chen, S.E.; Huang, S.Y. Functional genomics study of acute heat stress response in the small yellow follicles of layer-type chickens. Sci. Rep. 2018, 8, 1320. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Shao, D.; Yang, L.; Liang, Q.; Han, W.; Xue, Q.; Qu, L.; Leng, L.; Li, Y.; Zhao, X.; et al. Whole genome analyses reveal novel genes associated with chicken adaptation to tropical and frigid environments. J. Adv. Res. 2023, 47, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, I.; de Koning, D.-J.; Hocking, P.M. Transcriptional profile of breast muscle in heat stressed layers is similar to that of broiler chickens at control temperature. Genet. Sel. Evol. 2017, 49, 69. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, X.; Hsieh, J.C.F.; Monson, M.S.; Zhang, J.; Shu, D.; Nie, Q.; Persia, M.E.; Rothschild, M.F.; Lamont, S.J. Transcriptome response of liver and muscle in heat-stressed laying hens. Genes 2021, 12, 255. [Google Scholar] [CrossRef]
- Saelao, P.; Wang, Y.; Chanthavixay, G.; Yu, V.; Gallardo, R.A.; Dekkers, J.C.M.; Lamont, S.J.; Kelly, T.; Zhou, H. Integrated proteomic and transcriptomic analysis of differential expression of chicken lung tissue in response to NDV infection during heat stress. Genes 2018, 9, 579. [Google Scholar] [CrossRef]
- Chanthavixay, G.; Kern, C.; Wang, Y.; Saelao, P.; Lamont, S.J.; Gallardo, R.A.; Rincon, G.; Zhou, H. Integrated transcriptome and histone modification analysis reveals NDV infection under heat stress affects bursa development and proliferation in susceptible chicken line. Front. Genet. 2020, 11, 567812. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Seong, P.; Arora, D.; Shin, D.; Park, W.; Park, J.-E. Transcriptomic response under heat stress in chickens revealed the regulation of genes and alteration of metabolism to maintain homeostasis. Animals 2021, 11, 2241. [Google Scholar] [CrossRef]
- Monson, M.S.; Van Goor, A.G.; Persia, M.E.; Rothschild, M.F.; Schmidt, C.J.; Lamont, S.J. Genetic lines respond uniquely within the chicken thymic transcriptome to acute heat stress and low dose lipopolysaccharide. Sci. Rep. 2019, 9, 13649. [Google Scholar] [CrossRef]
- Zhang, J.; Schmidt, C.J.; Lamont, S.J. Transcriptome analysis reveals potential mechanisms underlying differential heart development in fast- and slow-growing broilers under heat stress. BMC Genom. 2017, 18, 295. [Google Scholar] [CrossRef]
- Park, W.; Srikanth, K.; Lim, D.; Park, M.; Hur, T.; Kemp, S.; Dessie, T.; Kim, M.S.; Lee, S.-R.; te Pas, M.F.W.; et al. Comparative transcriptome analysis of Ethiopian indigenous chickens from low and high altitudes under heat stress condition reveals differential immune response. Anim. Genet. 2019, 50, 42–53. [Google Scholar] [CrossRef]
- Ma, D.; Liu, Q.; Zhang, M.; Feng, J.; Li, X.; Zhou, Y.; Wang, X. iTRAQ-based quantitative proteomics analysis of the spleen reveals innate immunity and cell death pathways associated with heat stress in broilers (Gallus gallus). J. Proteom. 2019, 196, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Meng, Q.; Gao, J.; Zhang, S.; Zhang, H.; Zhang, M. Label-free quantitative analysis of changes in broiler liver proteins under heat stress using SWATH-MS technology. Sci. Rep. 2015, 5, 15119. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Shim, K. Early heat exposure effects on proteomic changes of the broiler liver under acute heat stress. Animals 2021, 11, 1338. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-T.; Zhuang, Z.-X.; Chen, C.-J.; Liao, H.-Y.; Chen, H.-L.; Hsueh, H.-C.; Chen, C.-F.; Chen, S.-E.; Huang, S.-Y. Effects of acute heat stress on protein expression and histone modification in the adrenal gland of male layer-type country chickens. Sci. Rep. 2021, 11, 6499. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Cheng, C.-Y.; Chen, C.-J.; Chan, H.-L.; Chen, H.-H.; Tang, P.-C.; Chen, C.-F.; Lee, Y.-P.; Huang, S.-Y. Acute heat stress changes protein expression in the testes of a broiler-type strain of Taiwan country chickens. Anim. Biotechnol. 2019, 30, 129–145. [Google Scholar] [CrossRef]
- Park, J.S.; Kang, D.R.; Shim, K.S. Proteomic changes in broiler liver by body weight differences under chronic heat stress. Poult. Sci. 2022, 101, 101794. [Google Scholar]
- Goto, T.; Mori, H.; Shiota, S.; Tomonaga, S. Metabolomics approach reveals the effects of breed and feed on the composition of chicken eggs. Metabolites 2019, 9, 224. [Google Scholar] [CrossRef]
- Zampiga, M.; Laghi, L.; Zhu, C.; Cartoni Mancinelli, A.; Mattioli, S.; Sirri, F. Breast muscle and plasma metabolomics profile of broiler chickens exposed to chronic heat stress conditions. Animals 2021, 15, 100275. [Google Scholar]
- Lee, D.; Lee, H.J.; Jung, D.Y.; Kim, H.-J.; Jang, A.; Jo, C. Effect of an animal-friendly raising environment on the quality, storage stability, and metabolomic profiles of chicken thigh meat. Food. Res. Int. 2022, 155, 111046. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Serum metabolomics study of nutrient metabolic variations in chronic heat-stressed broilers. Br. J. Nutr. 2018, 119, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, Y.; Chowdhury, V.S.; Cockrem, J.F.; Bungo, T. Effects of thermal conditioning on changes in hepatic and muscular tissue associated with reduced heat production and body temperature in young chickens. Front. Vet. Sci. 2021, 7, 610319. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Xia, B.; Tang, S.; Cao, A.; Liu, L.; Zhong, R.; Chen, L.; Zhang, H. The effect of exogenous bile acids on antioxidant status and gut microbiota in heat-stressed broiler chickens. Front. Nutr. 2021, 8, 747136. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Dong, S.; Zhao, X.; Guo, K.J.; Gasco, L.; Zoccarato, I. Gene expressions and metabolomic research on the effects of polyphenols from the involucres of Castanea mollissima Blume on heat-stressed broilers chicks. Poult. Sci. 2016, 95, 1869–1880. [Google Scholar] [CrossRef]
- Brown, C.L.J.; Zaytsoff, S.J.M.; Montina, T.; Inglis, G.D. Corticosterone-mediated physiological stress alters liver, kidney, and breast muscle metabolomic profiles in chickens. Animals 2021, 11, 3056. [Google Scholar] [CrossRef]
| Genes | Expression | Heat Control Functions | References |
|---|---|---|---|
| HSPA2, HSPH1, HSP25 | Increase | provide cellular protection and healing. | Wang et al. [116] |
| RB1CC1, BAG3, CITED2 | Increase | negative regulation of apoptosis and programmed cell death. | Wang et al. [116]; Luo et al. [123] |
| ID1 | Decrease | It plays a role in embryonic development, tissue regeneration, and the control of cell proliferation. | Luo et al. [123] |
| HSP90B1, HSPD1, PDIA2, HSPA5 | Increase | stabilize and refold denatured proteins in the endoplasmic reticulum and mitochondrial. | De Maio and Vazquez [130] |
| HSF1, HSF3 | Increase | protects cells from heat damage. | Cedraz et al. [120]; De Maio and Vazquez [130] |
| HSP70, HSP90, HSP40 | Increase | stabilize and refold denatured proteins, which is crucial for heat-stress cell survival. | |
| SERPINH1 | Increase | facilitate protein folding, reduce aggregation, and recover misfolded proteins. | Wang et al. [125]; De Maio and Vazquez [130] |
| GLUT-2, FABP1, CD36 | Decrease | decrease feed intake and intestinal damage. | Sun et al. [124] |
| TRMT1L | Increase | require for redox homeostasis to ensure proper cellular proliferation and oxidative stress survival. | Dewe et al. [131]; Walugembe et al. [132] |
| HS3ST5 | Unknown | involve immunity and defense molecular functions. | Walugembe et al. [132]; Szauter et al. [133] |
| EOMES | Increase | stimulate immunity and control homeostasis. | Walugembe et al. [132]; Zhang et al. [134] |
| NFAT5, NF-κB | Increase | stimulate the expression of various proinflammatory cytokines. | Tellechea et al. [126]; Zhang et al. [134] |
| MRPL42 | Increase | disrupt of DNA synthesis, transcription, RNA processing, and translation. | Van Goor et al. [117] |
| EDN1 | Unknown | augment apoptosis in cancer cells induced by mild hyperthermia. | Wang et al. [116] |
| ACSF | Unknown | alter in energy metabolism during heat stress. | Tian et al. [135] |
| CYP4V2 | Increase | increase fat deposition. | Claire De’Andre et al. [136] |
| PLCB4 | Increase | assist in the regulation of metabolic energy | Nanaei et al. [118] |
| H1F0, ACYP | Increase | reduce heat-induced apoptosis and repair DNA damage. | Srikanth et al. [127] |
| PDK | Increase | maintain glucose and reduce heat from combustion. | Luo et al. [123]; Kumar et al. [128] |
| Number of SNPs | The Number of the Genotype | Breeds | Traits | References |
|---|---|---|---|---|
| 23,098 SNPs | 192 | Taiwan indigenous chickens | Pathways associated with thermotolerance | Zhuang et al. [144] |
| 580,954 SNPs | 200 | Taiwan country chickens | Body temperature change | Zhuang et al. [145] |
| 113,344 SNPs | 118 | White Leghorn layer line. | Mortality in a white egg layer line | Wolc et al. [146] |
| 304,500 SNPs | 526 | Hy-Line Brown | Controlling traits related to NDV infection during heat stress | Saelao et al. [147] |
| 56,702 SNPs | 206 | Scaleless chickens | Feather development | Wells et al. [148] |
| 210,117 SNPs | 458 | broiler × Fayoumi | Body temperature, body weight, breast yield, and digestibility | Van Goor et al. [117] |
| 261,509 SNPs | 374 | White Leghorns | Production traits, feed intake, body weight, digestibility, egg quality | Rowland et al. [98] |
| Techniques | Chicken Breeds | Analyzed | Genes | Functions | References |
|---|---|---|---|---|---|
| Genomics | Native Chickens | Blood and Muscle | BVES, SMYD1, IL18, PDGFRA, NRP1, CORIN | The circulatory system and blood vessel development | Gu et al. [152] |
| SIM2, NALCN | Central nervous system development | ||||
| CLPTM1L, APP, CRADD, PARK2 | Related to apoptosis | ||||
| AHR, ESRRG, FAS, UBE4B | Responded to stimuli | ||||
| FABP1 | Fatty acid metabolism | ||||
| Fayoumis | Blood | MAP3K3, SOCS2 | Cellular response to stress suppressing cytokine signaling. | Van Goor et al. [117] | |
| Blood | MAPKBP1, SPON1 | Response to heat stress | Asadollahi et al. [153] | ||
| Taiwan country chickens | Blood | CTL, H4R0, H4R2, H4R6 | Response to acute heat stress | Cheng et al. [154] | |
| Native Chickens | Blood | SLC33A1, TSHR, NDUFS4 | Biomarkers to assess the adaptation to extreme environments. | Shi et al. [155] | |
| Hy-Line Brown | Blood | CAMK1d, CCDC3 TIRAP, ETS1, KIRREL3 | Associated with response to NDV during heat stress | Saelao et al. [147] | |
| Transcriptomics | Ross 308, White Leghorn | Muscle and meat quality | JAK1, 2JAK2, TYK2 | Wound healing and tissue regeneration | Zahoor et al. [156] |
| Hy-Line | Liver and Muscle | HSD17B7, STARD4, ACSBG2, SCD, INSIG1, | Response to changes in energy metabolism | Wang et al. [157] | |
| Leghorns, Fayoumis | Lung Tissue | IL17REL | Cytokine-mediated signaling | Saelao et al. [158] | |
| NOX4, PRDX1, RAB7B | The phagosome maturation pathway. | ||||
| Leghorns, Fayoumis | Bursa tissue | H3K27ac, H3K4me1 | Associated with cell cycle and receptor signaling of lymphocytes. | Chanthavixay et al. [159] | |
| Ross 308 | Blood | MYLK2, BDKRB1 | Calcium signaling pathway, Response to inflammation and tissue damage | Kim et al. [160] | |
| Fayoumi, broilers | Thymus | FGG, IL18, IL1R2, IL13RA2 | The immune response. | Monson et al. [161] | |
| Ross 708, Illinois | Heart | BMP10, MYH7, ANGPT2 | Related to cardiovascular function | Zhang et al. [162] | |
| Ethiopian chickens | Heart, breast muscle, spleen | IFI27L2, F8, USP18, CEBPD | Immune response | Park et al. [163] | |
| Proteomics | Broilers | Spleen | IL-1β, IL-6, TNF-α, IFN-α | Reveals innate immunity | Ma et al. [164] |
| CTSD, PARP3, IAP3 | Related to apoptosis | ||||
| CHMP1B, TNFAIP3, PARP3, IAP3 | Related to necroptosis | ||||
| Arbor Acres | Liver | HSP90AA1, LUM, PRKAA1, LYN, ABCA1 | Regulate the phagocytic ability of macrophages | Tang et al. [165] | |
| Ross chicks | Liver | CAT1, DLD, LDHB, ME1, PCK1, PDHA1 | Carbohydrate metabolism | Kang and Shim [166] | |
| COX5A, COX6C, NDUFS3, UQCRC1 | Energy metabolism | ||||
| ACO2, ACAT1 | Lipid metabolism | ||||
| Taiwan country chickens | Adrenal gland | H3K27me3 | Body temperature homeostasis | Zheng et al. [167] | |
| Taiwan country chickens | Testis | HSP90α, HSPA5, HSPA8 | Attenuate the testicular injury | Wang et al. [168] | |
| Ross-308 | Liver | MRP-126, FABP7, AGMAT, FTH1, GSTA1, TUBB, ENO1, HSP60 | Response to oxidative stress | Park et al. [169] | |
| Metabolomics | Rhode Island Red and Australorp | Egg yolk and albumen | Investigated breed and feed effects on 10 egg traits | Goto et al. [170] | |
| Ross 308 | Breast muscle and plasma | Body energy homeostasis, growth performance, and meat quality traits | Zampiga et al. [171] | ||
| Cobb chicks | Thigh meat | Comparing the physicochemical properties, storage stability, and metabolomic profile of thigh meat from broilers | Lee et al. [172] | ||
| Broiler chickens | Serum | Nutrient metabolic variations | Lu et al. [173] | ||
| Young Chickens (Chunky) | Hepatic and muscular tissue | Study was to clarify the effect of thermal conditioning at young ages on heat production and heat dissipation in chickens | Ouchi et al. [174] | ||
| Huaixiang chickens | Serum | Lipid metabolism | Guo et al. [142] | ||
| Arbor Acres | Bile acids | Investigating whether HS alters the composition of the bile acids pool and whether exogenous bile acids can alleviate heat stress by its characteristics described above. | Yin et al. [175] | ||
| Arbor Acres | Serum and jejunum mucosa | Analyze some growth and antioxidative related gene expressions of jejunum mucosa | Xiong et al. [176] | ||
| White leghorn | Kidney, liver, and breast muscle | Effects of CORT, on the metabolome of chicken kidney, liver, and breast muscle | Brown et al. [177] |
| Methods/Criteria | Minium Data Records | Budget (US Dollars) | Analysis Accuracy | Analysis Time | Suitability of the Area to Use the Technique | Traits | Application |
|---|---|---|---|---|---|---|---|
| Conventional | ≥1000 records | ≥1000 | 45–70% | 1–2 days | Underdeveloped and developing countries | Any traits | Easy to farm animals of all sizes. |
| Molecular | ≥50 sample/gene | ≥5000 | 45–70% | 1 week | Underdeveloped and developing countries | Any traits | Easy to farm animals of all sizes. |
| Genomic selection | ≥300 genotyped animal records | ≥100,000 | >70% | At least 1 month | Developing and developed countries | Emphasis on yield and fertility traits as well as cost-reduce traits | Use in case GP and GGP farm |
| OMICS technology | ≥5 samples | ≥100,000 | >90% | At least 1 month | Developed countries | Functional traits Longevity traits | Use in case GGP farm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juiputta, J.; Chankitisakul, V.; Boonkum, W. Appropriate Genetic Approaches for Heat Tolerance and Maintaining Good Productivity in Tropical Poultry Production: A Review. Vet. Sci. 2023, 10, 591. https://doi.org/10.3390/vetsci10100591
Juiputta J, Chankitisakul V, Boonkum W. Appropriate Genetic Approaches for Heat Tolerance and Maintaining Good Productivity in Tropical Poultry Production: A Review. Veterinary Sciences. 2023; 10(10):591. https://doi.org/10.3390/vetsci10100591
Chicago/Turabian StyleJuiputta, Jiraporn, Vibuntita Chankitisakul, and Wuttigrai Boonkum. 2023. "Appropriate Genetic Approaches for Heat Tolerance and Maintaining Good Productivity in Tropical Poultry Production: A Review" Veterinary Sciences 10, no. 10: 591. https://doi.org/10.3390/vetsci10100591
APA StyleJuiputta, J., Chankitisakul, V., & Boonkum, W. (2023). Appropriate Genetic Approaches for Heat Tolerance and Maintaining Good Productivity in Tropical Poultry Production: A Review. Veterinary Sciences, 10(10), 591. https://doi.org/10.3390/vetsci10100591






