Long-Term Changes in Pain Sensitivity in an Animal Model of Social Anxiety
Abstract
:1. Introduction
2. Experimental Section

3. Results and Discussion
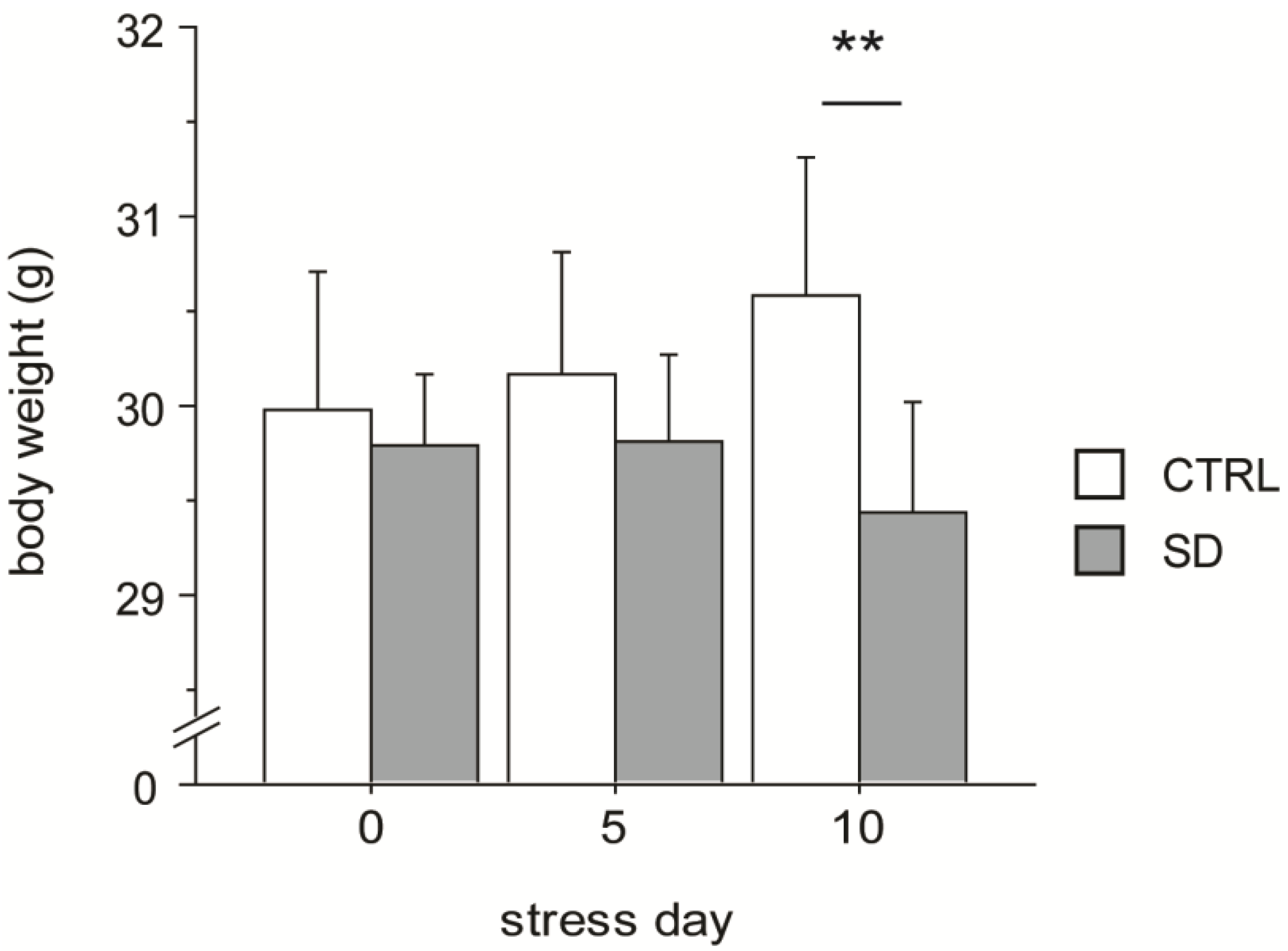
3.1. Body Weight
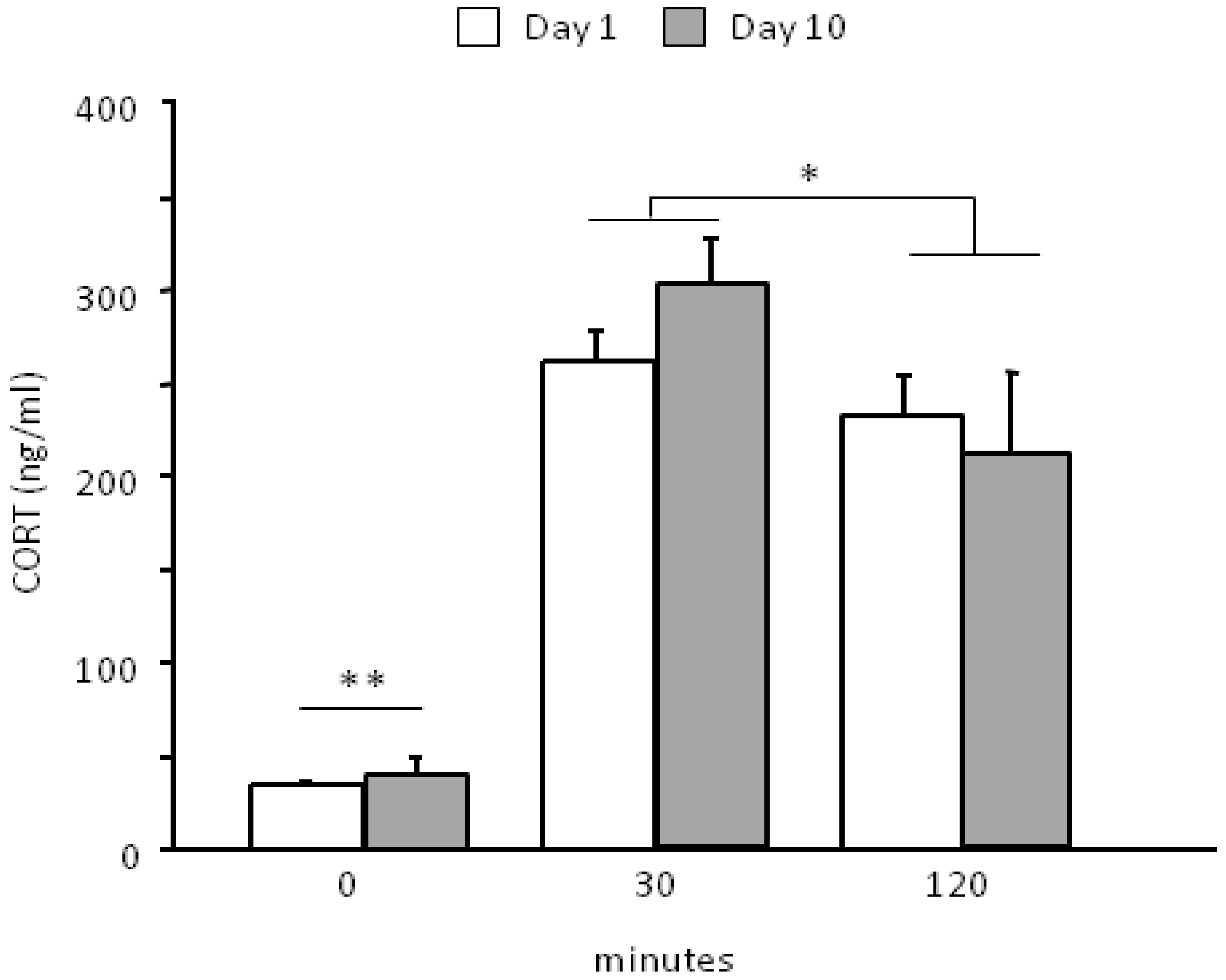
3.2. Neuroendocrine Activation
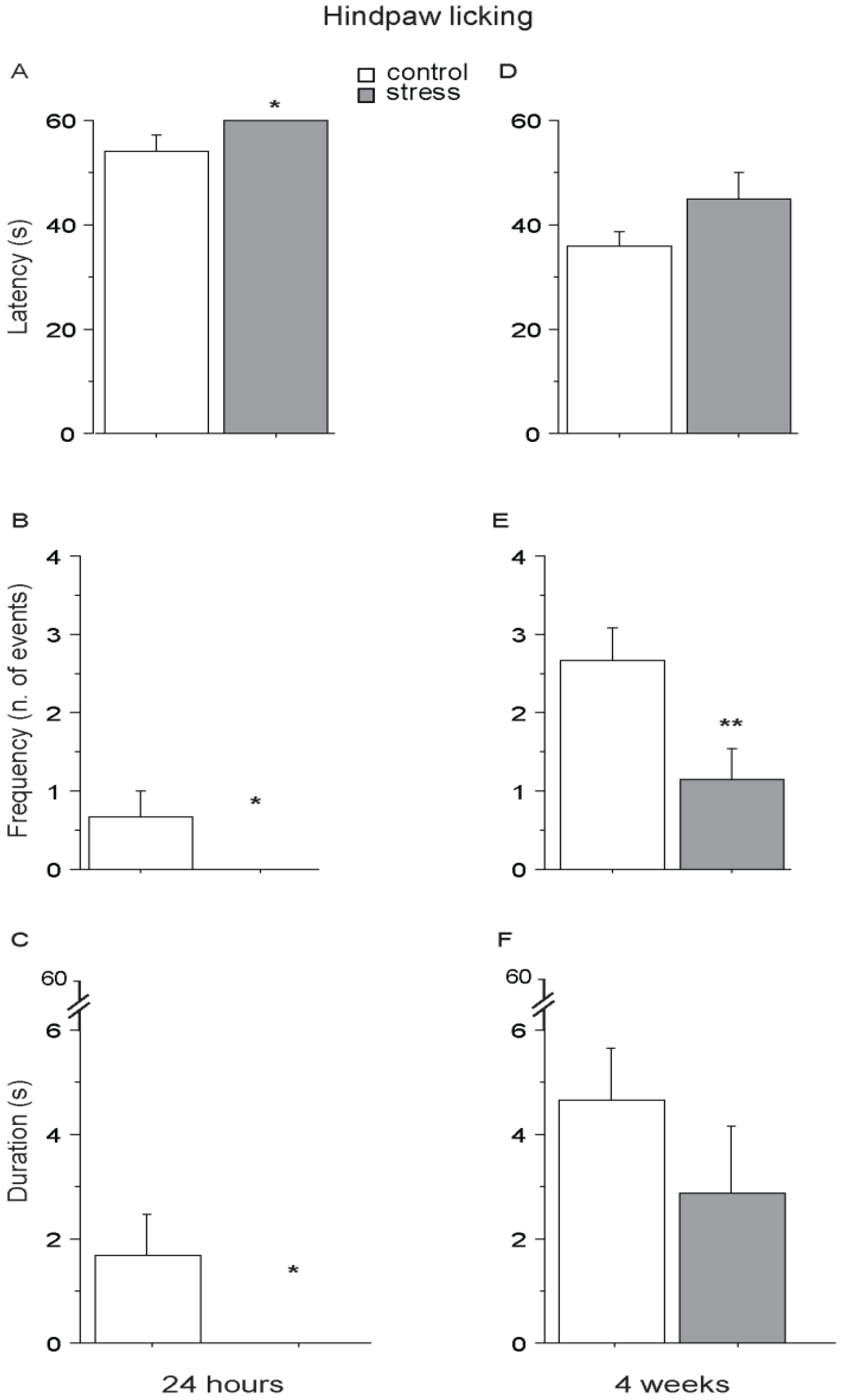
3.3. Social Avoidance Test—SAT
3.3.1. Short-Term Effects (24 h)

3.3.2. Long-Term Effects (4 Weeks)
3.4. Hot Plate (HP) Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anisman, H.; Merali, Z.; Stead, J.D. Experiential and genetic contributions to depressive- and anxiety-like disorders: Clinical and experimental studies. Neurosci. Biobehav. Rev. 2008, 32, 1185–1206. [Google Scholar] [CrossRef]
- Cirulli, F.; Alleva, E. The NGF saga: From animal models of psychosocial stress to stress-related psychopathology. Front Neuroendocrinol. 2009, 30, 379–395. [Google Scholar]
- Cryan, J.F.; Holmes, A. The ascent of mouse: Advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005, 4, 775–790. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; de Boer, S.F.; Buwalda, B.; van Reenen, K. Individual variation in coping with stress: A multidimensional approach of ultimate and proximate mechanisms. Brain Behav. Evol. 2007, 70, 218–226. [Google Scholar] [CrossRef]
- Miczek, K.A.; de Wit, H. Challenges for translational psychopharmacology research—Some basic principles. Psychopharmacology (Berl.) 2008, 199, 291–301. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; de Boer, S.F.; de Rutter, A.J.; Meerlo, P.; Sgoifo, A. Social stress in rats and mice. Acta Physiol. Scand Suppl. 1997, 640, 69–72. [Google Scholar]
- Tamashiro, K.L.; Nguyen, M.M.; Sakai, R.R. Social stress: From rodents to primates. Front Neuroendocrinol. 2005, 26, 27–40. [Google Scholar] [CrossRef]
- Willner, P. The validity of animal models of depression. Psychopharmacology (Berl.) 1984, 83, 1–16. [Google Scholar] [CrossRef]
- Berry, A.; Bellisario, V.; Capoccia, S.; Tirassa, P.; Calza, A.; Alleva, E.; Cirulli, F. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology 2012, 37, 762–772. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Bakshtanovskaya, I.V.; Koryakina, L.A. Social model of depression in mice of C57BL/6J strain. Pharmacol. Biochem. Behav. 1991, 38, 315–320. [Google Scholar] [CrossRef]
- Sheridan, J.F.; Stark, J.L.; Avitsur, R.; Padgett, D.A. Social disruption, immunity, and susceptibility to viral infection. Role of glucocorticoid insensitivity and NGF. Ann. N. Y. Acad. Sci. 2000, 917, 894–905. [Google Scholar]
- Vialou, V.; Robison, A.J.; Laplant, Q.C.; Covington, H.E., 3rd.; Dietz, D.M.; Ohnishi, Y.N.; Mouzon, E.; Rush, A.J., 3rd.; Watts, E.L.; Wallace, D.L.; et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat. Neurosci. 2010, 13, 745–752. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Palanza, P.; Sacerdote, P.; Panerai, A.E.; Sgoifo, A.; Dantzer, R.; Parmigiani, S. Social factors and individual vulnerability to chronic stress exposure. Neurosci. Biobehav. Rev. 2005, 29, 67–81. [Google Scholar] [CrossRef]
- Beitia, G.; Garmendia, L.; Azpiroz, A.; Vegas, O.; Brain, P.F.; Arregi, A. Time-dependent behavioral, neurochemical, and immune consequences of repeated experiences of social defeat stress in male mice and the ameliorative effects of fluoxetine. Brain Behav. Immun. 2005, 19, 530–539. [Google Scholar] [CrossRef]
- Fuchs, E. Social stress in tree shrews as an animal model of depression: an example of a behavioral model of a CNS disorder. CNS Spectr. 2005, 10, 182–190. [Google Scholar]
- Berton, O.; McClung, C.A.; Dileone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef]
- Brown, G.W. Social roles, context and evolution in the origins of depression. J. Health Soc. Behav. 2002, 43, 255–276. [Google Scholar] [CrossRef]
- Tidey, J.W.; Miczek, K.A. Acquisition of cocaine self-administration after social stress: Role of accumbens dopamine. Psychopharmacology (Berl.) 1997, 130, 203–212. [Google Scholar]
- Avgustinovich, D.F.; Kovalenko, I.L.; Kudryavtseva, N.N. A model of anxious depression: Persistence of behavioral pathology. Neurosci. Behav. Physiol. 2005, 35, 917–924. [Google Scholar] [CrossRef]
- Huhman, K.L.; Solomon, M.B.; Janicki, M.; Harmon, A.C.; Lin, S.M.; Israel, J.E.; Jasnow, A.M. Conditioned defeat in male and female Syrian hamsters. Horm. Behav. 2003, 44, 293–299. [Google Scholar] [CrossRef]
- Siegfried, B.; Frischknecht, H.R.; Waser, P.G. Defeat, learned submissiveness, and analgesia in mice: Effect of genotype. Behav. Neural. Biol. 1984, 42, 91–97. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Meerlo, P.; de Boer, S.F.; Strubbe, J.H.; Bohus, B. The temporal dynamics of the stress response. Neurosci. Biobehav. Rev. 1997, 21, 775–782. [Google Scholar] [CrossRef]
- Meerlo, P.; de Boer, S.F.; Koolhaas, J.M.; Daan, S.; van den Hoofdakker, R.H. Changes in daily rhythms of body temperature and activity after a single social defeat in rats. Physiol. Behav. 1996, 59, 735–739. [Google Scholar] [CrossRef]
- Ruis, M.A.; te Brake, J.H.; Buwalda, B.; de Boer, S.F.; Meerlo, P.; Korte, S.M.; Blokhuis, H.J.; Koolhaas, J.M. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology 1999, 24, 285–300. [Google Scholar] [CrossRef]
- Van der Kolk, B.A.; Greenberg, M.S.; Orr, S.P.; Pitman, R.K. Endogenous opioids, stress induced analgesia, and posttraumatic stress disorder. Psychopharmacol. Bull. 1989, 25, 417–421. [Google Scholar]
- Pitman, R.K.; van der Kolk, B.A.; Orr, S.P.; Greenberg, M.S. Naloxone-reversible analgesic response to combat-related stimuli in posttraumatic stress disorder. A pilot study. Arch. Gen. Psychiatry 1990, 47, 541–544. [Google Scholar] [CrossRef]
- Ludascher, P.; Valerius, G.; Stiglmayr, C.; Mauchnik, J.; Lanius, R.A.; Bohus, M.; Schmahl, C. Pain sensitivity and neural processing during dissociative states in patients with borderline personality disorder with and without comorbid posttraumatic stress disorder: A pilot study. J. Psychiatry Neurosci. 2010, 35, 177–184. [Google Scholar] [CrossRef]
- Amir, S.; Brown, Z.W.; Amit, Z. The role of endorphins in stress: Evidence and speculations. Neurosci. Biobehav. Rev. 1980, 4, 77–86. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Kelly, D.D.; Brutus, M.; Glusman, M. Stress-induced analgesia: Neural and hormonal determinants. Neurosci. Biobehav. Rev. 1980, 4, 87–100. [Google Scholar] [CrossRef]
- Chance, W.T. Autoanalgesia: Opiate and non-opiate mechanisms. Neurosci. Biobehav. Rev. 1980, 4, 55–67. [Google Scholar] [CrossRef]
- Lewis, J.W.; Cannon, J.T.; Liebeskind, J.C. Opioid and nonopioid mechanisms of stress analgesia. Science 1980, 208, 623–625. [Google Scholar]
- Watkins, L.R.; Mayer, D.J. Organization of endogenous opiate and nonopiate pain control systems. Science 1982, 216, 1185–1192. [Google Scholar]
- Butler, R.K.; Finn, D.P. Stress-induced analgesia. Prog. Neurobiol. 2009, 88, 184–202. [Google Scholar] [CrossRef]
- Bolles, R.C.; Fanselow, M.S. A perceptual-defensive-recuperative model of fear and pain. Behav. Brain Sci. 1980, 3, 291–301. [Google Scholar] [CrossRef]
- Miczek, K.A.; Thompson, M.L.; Shuster, L. Opioid-like analgesia in defeated mice. Science 1982, 215, 1520–1522. [Google Scholar]
- Rodgers, R.J.; Hendrie, C.A. Social conflict activates status-dependent endogenous analgesic or hyperalgesic mechanisms in male mice: Effects of naloxone on nociception and behaviour. Physiol. Behav. 1983, 30, 775–780. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Hendrie, C.A.; Waters, A.J. Naloxone partially antagonizes post-encounter analgesia and enhances defensive responding in male rats exposed to attack from lactating conspecifics. Physiol. Behav. 1983, 30, 781–786. [Google Scholar] [CrossRef]
- Teskey, G.C.; Kavaliers, M.; Hirst, M. Social conflict activates opioid analgesic and ingestive behaviors in male mice. Life Sci. 1984, 35, 303–315. [Google Scholar] [CrossRef]
- Jackson, R.L.; Maier, S.F.; Coon, D.J. Long-term analgesic effects of inescapable shock and learned helplessness. Science 1979, 206, 91–93. [Google Scholar]
- Bartlang, M.S.; Neumann, I.D.; Slattery, D.A.; Uschold-Schmidt, N.; Kraus, D.; Helfrich-Forster, C.; Reber, S.O. Time matters: Pathological effects of repeated psychosocial stress during the active, but not inactive, phase of male mice. J. Endocrinol. 2012, 215, 425–437. [Google Scholar] [CrossRef]
- Fluttert, M.; Dalm, S.; Oitzl, M.S. A refined method for sequential blood sampling by tail incision in rats. Lab. Anim. 2000, 34, 372–378. [Google Scholar] [CrossRef]
- Sadler, A.M.; Bailey, S.J. Validation of a refined technique for taking repeated blood samples from juvenile and adult mice. Lab. Anim. 2013, 47, 316–319. [Google Scholar] [CrossRef]
- Berry, A.; Capone, F.; Giorgio, M.; Pelicci, P.G.; de Kloet, E.R.; Alleva, E.; Minghetti, L.; Cirulli, F. Deletion of the life span determinant p66Shc prevents age-dependent increases in emotionality and pain sensitivity in mice. Exp. Gerontol. 2007, 42, 37–45. [Google Scholar] [CrossRef]
- Cirulli, F.; Berry, A.; Alleva, E. Intracerebroventricular administration of brain-derived neurotrophic factor in adult rats affects analgesia and spontaneous behaviour but not memory retention in a Morris Water Maze task. Neurosci. Lett. 2000, 287, 207–210. [Google Scholar] [CrossRef]
- Savignac, H.M.; Finger, B.C.; Pizzo, R.C.; O’Leary, O.F.; Dinan, T.G.; Cryan, J.F. Increased sensitivity to the effects of chronic social defeat stress in an innately anxious mouse strain. Neuroscience 2011, 192, 524–536. [Google Scholar] [CrossRef]
- Reber, S.O.; Obermeier, F.; Straub, R.H.; Falk, W.; Neumann, I.D. Chronic intermittent psychosocial stress (social defeat/overcrowding) in mice increases the severity of an acute DSS-induced colitis and impairs regeneration. Endocrinology 2006, 147, 4968–4976. [Google Scholar] [CrossRef]
- Savignac, H.M.; Hyland, N.P.; Dinan, T.G.; Cryan, J.F. The effects of repeated social interaction stress on behavioural and physiological parameters in a stress-sensitive mouse strain. Behav. Brain Res. 2011, 216, 576–584. [Google Scholar] [CrossRef]
- Krishnan, V.; Han, M.H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; Laplant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef]
- Razzoli, M.; Carboni, L.; Andreoli, M.; Ballottari, A.; Arban, R. Different susceptibility to social defeat stress of BalbC and C57BL6/J mice. Behav. Brain Res. 2010, 216, 100–108. [Google Scholar]
- Razzoli, M.; Carboni, L.; Andreoli, M.; Michielin, F.; Ballottari, A.; Arban, R. Strain-specific outcomes of repeated social defeat and chronic fluoxetine treatment in the mouse. Pharmacol. Biochem. Behav. 2010, 97, 566–576. [Google Scholar]
- Golden, S.A.; Covington, H.E., 3rd.; Berton, O.; Russo, S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef]
- Hammamieh, R.; Chakraborty, N.; De Lima, T.C.; Meyerhoff, J.; Gautam, A.; Muhie, S.; D’Arpa, P.; Lumley, L.; Carroll, E.; Jett, M. Murine model of repeated exposures to conspecific trained aggressors simulates features of post-traumatic stress disorder. Behav. Brain Res. 2012, 235, 55–66. [Google Scholar] [CrossRef]
- Litvin, Y.; Murakami, G.; Pfaff, D.W. Effects of chronic social defeat on behavioral and neural correlates of sociality: Vasopressin, oxytocin and the vasopressinergic V1b receptor. Physiol. Behav. 2011, 103, 393–403. [Google Scholar] [CrossRef]
- Girardi, C.E.; Tiba, P.A.; Llobet, G.B.; Levin, R.; Abilio, V.C.; Suchecki, D. Contextual exploration previous to an aversive event predicts long-term emotional consequences of severe stress. Front. Behav. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Zhang, Y.; Gandhi, P.R.; Standifer, K.M. Increased nociceptive sensitivity and nociceptin/orphanin FQ levels in a rat model of PTSD. Mol. Pain 2012, 8. [Google Scholar] [CrossRef]
- Ludascher, P.; Bohus, M.; Lieb, K.; Philipsen, A.; Jochims, A.; Schmahl, C. Elevated pain thresholds correlate with dissociation and aversive arousal in patients with borderline personality disorder. Psychiatry Res. 2007, 149, 291–296. [Google Scholar] [CrossRef]
- Beckham, J.C.; Crawford, A.L.; Feldman, M.E.; Kirby, A.C.; Hertzberg, M.A.; Davidson, J.R.; Moore, S.D. Chronic posttraumatic stress disorder and chronic pain in Vietnam combat veterans. J. Psychosom. Res. 1997, 43, 379–389. [Google Scholar] [CrossRef]
- Shipherd, J.C.; Keyes, M.; Jovanovic, T.; Ready, D.J.; Baltzell, D.; Worley, V.; Gordon-Brown, V.; Hayslett, C.; Duncan, E. Veterans seeking treatment for posttraumatic stress disorder: What about comorbid chronic pain? J. Rehabil. Res. Dev. 2007, 44, 153–166. [Google Scholar] [CrossRef]
- Asmundson, G.J.; Katz, J. Understanding the co-occurrence of anxiety disorders and chronic pain: State-of-the-art. Depress Anxiety 2009, 26, 888–901. [Google Scholar] [CrossRef]
- Rubinstein, M.; Mogil, J.S.; Japon, M.; Chan, E.C.; Allen, R.G.; Low, M.J. Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc. Natl. Acad. Sci. USA 1996, 93, 3995–4000. [Google Scholar] [CrossRef]
- Yamada, K.; Nabeshima, T. Stress-induced behavioral responses and multiple opioid systems in the brain. Behav. Brain Res. 1995, 67, 133–145. [Google Scholar] [CrossRef]
- Pike, J.L.; Smith, T.L.; Hauger, R.L.; Nicassio, P.M.; Patterson, T.L.; McClintick, J.; Costlow, C.; Irwin, M.R. Chronic life stress alters sympathetic, neuroendocrine, and immune responsivity to an acute psychological stressor in humans. Psychosom. Med. 1997, 59, 447–457. [Google Scholar] [CrossRef]
- Birrell, A.M.; Heffernan, S.J.; Ansselin, A.D.; McLennan, S.; Church, D.K.; Gillin, A.G.; Yue, D.K. Functional and structural abnormalities in the nerves of type I diabetic baboons: Aminoguanidine treatment does not improve nerve function. Diabetologia 2000, 43, 110–116. [Google Scholar] [CrossRef]
- Van Goozen, S.H.; van den Ban, E.; Matthys, W.; Cohen-Kettenis, P.T.; Thijssen, J.H.; van Engeland, H. Increased adrenal androgen functioning in children with oppositional defiant disorder: A comparison with psychiatric and normal controls. J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 1446–1451. [Google Scholar] [CrossRef]
- Bremner, J.D. Long-term effects of childhood abuse on brain and neurobiology. Child Adolesc. Psychiatr. Clin. North Am. 2003, 12, 271–292. [Google Scholar] [CrossRef]
- Claes, L.; Vandereycken, W.; Vertommen, H. Pain experience related to self-injury in eating disorder patients. Eat. Behav. 2006, 7, 204–213. [Google Scholar] [CrossRef]
- Zanarini, M.C.; Yong, L.; Frankenburg, F.R.; Hennen, J.; Reich, D.B.; Marino, M.F.; Vujanovic, A.A. Severity of reported childhood sexual abuse and its relationship to severity of borderline psychopathology and psychosocial impairment among borderline inpatients. J. Nerv. Ment. Dis. 2002, 190, 381–387. [Google Scholar] [CrossRef]
- Gratz, K.L.; Hepworth, C.; Tull, M.T.; Paulson, A.; Clarke, S.; Remington, B.; Lejuez, C.W. An experimental investigation of emotional willingness and physical pain tolerance in deliberate self-harm: The moderating role of interpersonal distress. Compr. Psychiatry 2011, 52, 63–74. [Google Scholar] [CrossRef]
- Joyce, P.R.; McKenzie, J.M.; Mulder, R.T.; Luty, S.E.; Sullivan, P.F.; Miller, A.L.; Kennedy, M.A. Genetic, developmental and personality correlates of self-mutilation in depressed patients. Aust. N. Z. J. Psychiatry 2006, 40, 225–229. [Google Scholar] [CrossRef]
- Eisenberger, N.I. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat. Rev. Neurosci. 2012, 13, 421–434. [Google Scholar] [CrossRef]
- Moeller-Bertram, T.; Keltner, J.; Strigo, I.A. Pain and post traumatic stress disorder—Review of clinical and experimental evidence. Neuropharmacology 2012, 62, 586–597. [Google Scholar] [CrossRef]
- Hache, G.; Guiard, B.P.; le Dantec, Y.; Orvoen, S.; David, D.J.; Gardier, A.M.; Coudore, F. Antinociceptive effects of fluoxetine in a mouse model of anxiety/depression. Neuroreport 2012, 23, 525–529. [Google Scholar] [CrossRef]
- Moll, J.; Krueger, F.; Zahn, R.; Pardini, M.; de Oliveira-Souza, R.; Grafman, J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc. Natl. Acad. Sci. USA 2006, 103, 15623–15628. [Google Scholar]
- Keysers, C.; Kaas, J.H.; Gazzola, V. Somatosensation in social perception. Nat. Rev. Neurosci. 2010, 11, 417–428. [Google Scholar] [CrossRef]
- Lamm, C.; Decety, J.; Singer, T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 2011, 54, 2492–2502. [Google Scholar] [CrossRef]
- Inagaki, T.K.; Eisenberger, N.I. Neural correlates of giving support to a loved one. Psychosom. Med. 2012, 74, 3–7. [Google Scholar] [CrossRef]
- Mickleborough, M.J.; Daniels, J.K.; Coupland, N.J.; Kao, R.; Williamson, P.C.; Lanius, U.F.; Hegadoren, K.; Schore, A.; Densmore, M.; Stevens, T.; Lanius, R.A. Effects of trauma-related cues on pain processing in posttraumatic stress disorder: An fMRI investigation. J. Psychiatry Neurosci. 2011, 36, 6–14. [Google Scholar] [CrossRef]
- Schmahl, C.; Bohus, M.; Esposito, F.; Treede, R.D.; Di Salle, F.; Greffrath, W.; Ludaescher, P.; Jochims, A.; Lieb, K.; Scheffler, K.; et al. Neural correlates of antinociception in borderline personality disorder. Arch. Gen. Psychiatry 2006, 63, 659–667. [Google Scholar] [CrossRef]
- Russ, M.J.; Roth, S.D.; Lerman, A.; Kakuma, T.; Harrison, K.; Shindledecker, R.D.; Hull, J.; Mattis, S. Pain perception in self-injurious patients with borderline personality disorder. Biol. Psychiatry 1992, 32, 501–511. [Google Scholar] [CrossRef]
- Bohus, M.; Limberger, M.; Ebner, U.; Glocker, F.X.; Schwarz, B.; Wernz, M.; Lieb, K. Pain perception during self-reported distress and calmness in patients with borderline personality disorder and self-mutilating behavior. Psychiatry Res. 2000, 95, 251–260. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Berry, A.; Bellisario, V.; Capoccia, S.; Francia, N.; Alleva, E.; Cirulli, F. Long-Term Changes in Pain Sensitivity in an Animal Model of Social Anxiety. Vet. Sci. 2014, 1, 77-95. https://doi.org/10.3390/vetsci1020077
Berry A, Bellisario V, Capoccia S, Francia N, Alleva E, Cirulli F. Long-Term Changes in Pain Sensitivity in an Animal Model of Social Anxiety. Veterinary Sciences. 2014; 1(2):77-95. https://doi.org/10.3390/vetsci1020077
Chicago/Turabian StyleBerry, Alessandra, Veronica Bellisario, Sara Capoccia, Nadia Francia, Enrico Alleva, and Francesca Cirulli. 2014. "Long-Term Changes in Pain Sensitivity in an Animal Model of Social Anxiety" Veterinary Sciences 1, no. 2: 77-95. https://doi.org/10.3390/vetsci1020077
APA StyleBerry, A., Bellisario, V., Capoccia, S., Francia, N., Alleva, E., & Cirulli, F. (2014). Long-Term Changes in Pain Sensitivity in an Animal Model of Social Anxiety. Veterinary Sciences, 1(2), 77-95. https://doi.org/10.3390/vetsci1020077




