Abstract
The relevance of identifying pathological processes in the context of embryonic development is increasingly gaining attention in terms of professionalized prenatal care. To analyze local effects of prenatally administered drugs during embryonic development, the model organism of the chicken embryo can be used in a first exploratory approach. For the examination of local dexamethasone administration—as an exemplary drug—common bead implantation protocols have been adapted to serve as an in vivo technique for local drug testing during embryonic skin regeneration. For this, acrylic beads were soaked in a dexamethasone solution and implanted into skin incisional wounds of 4-day-old chicken embryos. After further incubation, the effects of the applied substance on the process of embryonic skin regeneration were analyzed using histological and molecular biological techniques. This data descriptor contains a detailed microsurgical protocol, a representative video demonstration, and exemplary results of local glucocorticoid-induced changes during embryonic wound healing. To conclude, this method allows for the analysis of the local effects of a particular substance on a cellular level and can be extended to serve as an in vivo technique for numerous other drugs to be tested on embryonic tissue.
Dataset License: CC-BY
1. Summary
The avian embryo is a traditional model organism in developmental biology due to its in ovo accessibility and its genomic similarity to mammalian organisms [1]. Typically, a one-to-one correspondence can be observed between homologous genes in mammals and birds [1,2]. Given the high evolutionary conservation of molecular-level developmental processes in vertebrates, experiments performed on the chicken embryo hold significant relevance for the understanding of human development and pathology.
In recent years, the chicken embryo (Gallus domesticus) has also been employed as an in vivo model for pharmaceutical testing [3]. For this, multiple methods of in ovo drug administration have been established, such as injections into the amniotic cavity or yolk sac and topical application onto the chorioallantoic membrane, leading to a systemic intake of the respective substance and unveiling biocompatibility and drug activity during development [3,4].
To study local effects of a particular substance, in ovo bead implantation into avian embryos is a conventional developmental biological method to investigate, for example, local transcriptional changes [5,6]. For this, acrylic beads are soaked in the respective substance—typically inhibitors or receptor agonists—and implanted into the area of interest, mimicking spatially restricted gain- or loss-of-function approaches.
For the subsequent data descriptor of the original dataset [7], the method of in ovo bead implantation was adapted to serve as a technique for local drug administration during the dynamic process of embryonic wound healing. To our knowledge, this protocol is the first to employ bead implantation in the context of embryonic wound healing. Moreover, the concept of implanting beads into embryos at an older developmental stage allows for a local analysis of the respective drug in a confined tissue such as the skin.
Research on embryonic skin regeneration, which differs from adult skin regeneration in several ways, can contribute to advances in prenatal medicine. Fetal surgery allows for the intrauterine repair of developmental defects such as myelomeningocele, genitourinary obstructions, or cardiac outflow abnormalities and has become an increasingly relevant therapeutic option in prenatal medicine [8,9]. In contrast to adult human wounds, fetal wounds heal in a scarless manner, accompanied by a reduced inflammatory reaction and differences in the composition of the extracellular matrix and cytokines [10,11]. The investigation of these molecular differences as well as their interference with various pharmaceuticals functions as basic research, carrying potential implications for clinical practice.
The following data descriptor elaborates on the method of administering dexamethasone (dex), a synthetic glucocorticoid commonly used in prenatal medicine [12], locally into embryonic skin incisional wounds, using bead implantation and investigating the consequences with respect to the process of skin regeneration.
The wounded areas containing the dex and control beads were analyzed after further incubation using standard histological techniques and immunohistochemistry, allowing for analysis of morphological changes and alterations in protein expression in tissue sections. The present protocol further allows analysis through insitu hybridization, enabling a three-dimensional examination of embryo whole-mounts including transcriptional changes in the expression of relevant genes.
Tissue sections can be further studied with software such as QuPath [13], allowing a more objective comparison using quantitative parameters.
To conclude, this data descriptor aims to present an adapted in vivo technique for local drug testing during avian embryonic wound healing, supported by a detailed microsurgical protocol, a corresponding video demonstration, and exemplary results.
2. Methods
The following methods section contains a detailed protocol as well as a video demonstration (Supplementary Material) of the procedure of in ovo bead implantation for the purpose of local drug testing during embryonic wound healing. The subsequent methods of further analysis have been described before [5,7,14] and, therefore, are only briefly summarized here for comprehensiveness.
2.1. Preparation of Chicken Eggs
According to German law, experiments on embryonic vertebrates only need to be ethically approved if the animal is in the last third of its embryonic development. In the context of chickens, it implies that experiments conducted on animals before day 14 of its embryonic development are not considered animal experiments. Consequently, such experiments do not necessitate formal approval or governmental authorization.
Fertilized chicken eggs of Gallus domesticus were provided by a local farm, cleaned with 70% ethanol in distilled water in order to avoid contamination, and incubated at 37 °C and 80% humidity. The chicken embryos were staged according to Hamburger and Hamilton (HH) [15].
On the third day of development, 3 mL of albumen was removed from the eggshell using a syringe, allowing the blastoderm to sink within the egg and avoiding accidental injuries during the opening process. Afterwards, scissors were used to cut a window into the eggshell, enabling continuous visualization, as previously demonstrated by Yahya et al. (2020) [5]. The window should be covered with medical tape for further incubation to preserve the embryo and prevent dehydration. It can be reopened again for the surgical procedure on day 4 of development.
2.2. Preparation of the Beads
For the in vivo technique of local drug testing during embryonic wound healing, previous bead implantation methods were adapted [5]. The beads (AG 1-X2 resin, 143–1255, Bio-Rad, Hercules, CA, USA) were placed into a microcentrifuge cap and rinsed with phosphate buffered saline (PBS). For this, PBS was dropped onto the beads in the cap. After sinking of the beads, the PBS was replaced with a pipette. This was repeated several times. After rinsing, the drug-containing solution—in this case a 0.4 mg/mL solution of dexamethasone (Sigma Aldrich, St. Louis, MO, USA) dissolved in PBS containing 1% dimethyl sulfoxide (DMSO)—was dropped onto the beads. The specific solvent of each drug should be used as a control solution for the beads to rule out potential mechanical effects of the bead. The beads were left in the respective solution over night at 4 °C in order to absorb the drug before administration.
2.3. In Ovo Bead Implantation into Skin Incisional Wounds
On day four of incubation (Stages HH23-HH24), in ovo bead implantation into skin incisional wounds was performed according to the following protocol:
- Remove the medical tape and visualize the embryo under a stereo microscope (Leica, M165 FC). Ensure that it has developed free from malformations and has attained the intended developmental stage, as this is essential for further comparison between the experimental and the control group.
- To improve visibility, ink dissolved in PBS may be injected beneath the embryo, creating a dark background and providing more contrast.
- Open the extra embryonic membranes to establish access to the embryo. Use a sterile tungsten needle to tear the vitelline membrane and the amnion.
- Use the tungsten needle to make an incisional wound into the skin of the embryo. Make sure to always use the same anatomic region such as the right interlimb region, as shown in our example (Figure 1A,B), as this is also a key requirement for further comparison.
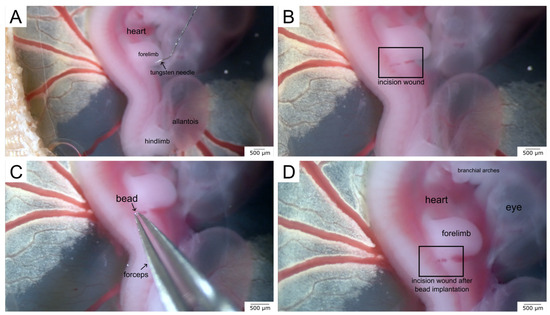 Figure 1. The images display the method of in ovo bead implantation for local drug testing during embryonic wound healing. (A) shows the anatomy of the chicken embryo in ovo and how the incision wound is made using a tungsten needle. (B) shows the incision wound. (C) demonstrates the bead implantation using forceps. (D) displays the final stage after the surgical procedure. A total of 2–3 beads are placed in the incision wound.
Figure 1. The images display the method of in ovo bead implantation for local drug testing during embryonic wound healing. (A) shows the anatomy of the chicken embryo in ovo and how the incision wound is made using a tungsten needle. (B) shows the incision wound. (C) demonstrates the bead implantation using forceps. (D) displays the final stage after the surgical procedure. A total of 2–3 beads are placed in the incision wound. - Use fine forceps to collect several beads from the solution in the microcentrifuge cap. Place the beads near the wound area and push several beads into the wound (Figure 1C,D).
- Close the window of the eggshell with medical tape for further incubation of the wounded embryo. This protects the embryo from dehydration and replaces the eggshell.
A video demonstration of the protocol can be found in the Supplementary Material.
The progress of skin regeneration was analyzed after 1 h and after two days. Potentially, the embryos could be incubated even further, up until 5 days after wounding. However, at that point the beads will be more difficult to localize, as the embryos will have grown substantially.
For fixation, the extra embryonic membranes were cut, the embryos were removed from the egg, and washed in PBS. Afterwards, they were immersed in 4% paraformaldehyde in PBS and stored at 4 °C.
2.4. Histological Processing
The fixed embryos were washed in PBS and dehydrated with an ascending alcohol series. After embedding in paraffin, transverse tissue sections were made using a microtome, seeking the bead-implanted area. Once the beads appeared on a tissue section, up to 15 further tissue sections can be gained from one bead-implanted specimen, depending on the thickness of the sections. During the sectioning process, it is possible for the bead to fall out of the paraffin section. Therefore, occasionally, only the hole of the bead in the paraffin section can be detected on some sections. The paraffin sections were deparaffinized and rehydrated using a descending alcohol series.
For the standard hematoxylin–eosin (HE) staining, the tissue sections were stained with hematoxylin for 15 min and eosin for 2 min (Carl Roth, Karlsruhe, Germany).
After staining and covering the tissue sections, they were photographed with the virtual slide microscope VS120 (Olympus, Tokyo, Japan).
The quantification of wound parameters was performed employing OlyVia software (Version 2.9, Olympus, Tokyo, Japan) and QuPath (Open source software, Version 0.3.2) [13].
2.5. Immunohistochemistry
The paraffin sections were deparaffinized and rehydrated as described above. For antigen unmasking, a heated citrate buffer was used. After several washing steps and tissue permeabilization with 1% Triton X 100 (Sigma Aldrich, St. Louis, MO, USA), the blocking solution containing 7.5% bovine serum in PBS was applied onto the sections.
As an exemplary primary antibody, Vimentin (AMF-17b, DSHB) was dissolved in the blocking solution and applied onto the sections overnight.
For the detection of the primary antibody, the secondary fluorescent antibody Alexa Fluor goat anti-mouse 488 (Thermo Fisher, Waltham, MA, USA) was applied onto the tissue sections after rinsing with PBS followed by 1 h of incubation at room temperature and several further washing steps. Afterward, the nuclei were stained with 4′,6-diamidino-2-phenylindol (DAPI) (Carl Roth, Karlsruhe, Germany).
2.6. Whole-Mount In Situ Hybridization
Whole-mount in-situ hybridization (ISH) was performed as described previously [14]. For the following example, Dermo-1 was used as a riboprobe.
To permeabilize the tissue, proteinase K (10 μg/mL) was applied onto the embryos for 20 min at room temperature. This incubation step must be adapted to the size of the embryo. Following permeabilization, 1 μg/μL of the riboprobe was hybridized for 48 h at 65 °C. An anti-DIG antibody conjugated to alkaline phosphatase (Sigma Aldrich, St. Louis, MO, USA) was employed for the detection of the respective transcripts.
After the ISH, the embryos were photographed using a stereo microscope (M165 FC, Leica, Wetzlar, Germany) with a digital camera (DFC420 C, Leica, Wetzlar, Germany).
InkScape software (Open-source software, Version 1.2.1, 2022) was used for the preparation of all figures. The beads were highlighted by circles to increase visibility.
3. Data Description
3.1. Macroscopic Analysis
The following data descriptor contains exemplary results that can be obtained from the presented in vivo technique for local drug testing during embryonic wound healing. While the following examples are built upon the investigation of dex effects on embryonic wound healing [7], the utilized antibodies or riboprobes can be customized for the respective area of interest. Further interpretation and discussion of the obtained results have been published by Bablok et al. (2022) [7].
Following in ovo bead implantation and further incubation, the process of wound healing can firstly be inspected macroscopically prior to any staining procedures. Due to the transparency of the beads and the embryonic tissue, the beads become increasingly difficult to visualize the longer they stay in the embryo and the more the surrounding tissue adapts to and grows around the implanted beads. Therefore, for visualization purposes, the beads can be highlighted by circles during the digital photographic assembly.
The macroscopic images (Figure 2) show successful bead implantation after 1 h and 2 days, respectively. Note that the beads in the dex wound after 1 h (Figure 2B′) appear more superficial, indicating a prolonged or impaired regeneration process. After 2 days of further incubation, the beads were absorbed by the embryonic tissue even more, and differences between the control and dex group became difficult to discern macroscopically (Figure 2C,D′), requiring deeper analysis using further methods.
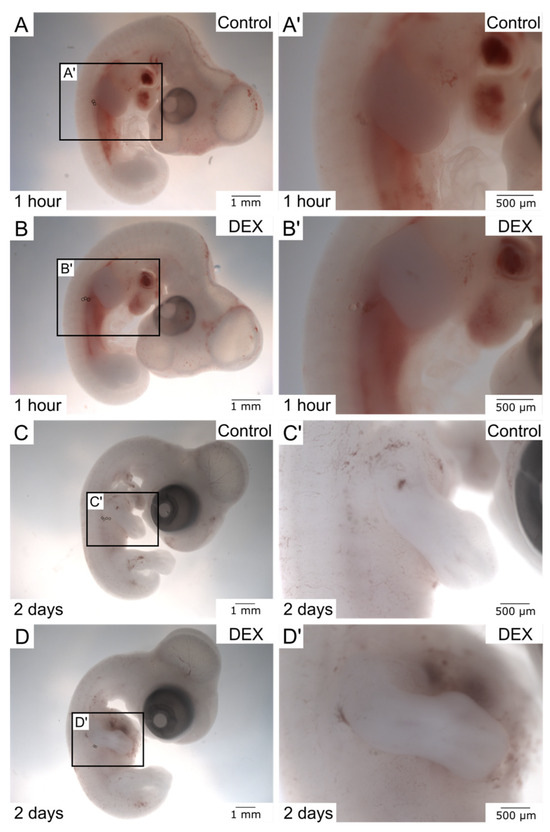
Figure 2.
The images display fixed chicken embryos after in ovo bead implantation. (A) shows an overview of a control embryo after 1 h. (A′) shows the control wound in higher magnification. (B) shows an overview of a dex embryo after 1 h. (B′) shows the dex wound in higher magnification. (C) shows an overview of a control embryo after 2 days. (C′) shows the control wound in higher magnification. (D) shows an overview of a dex embryo after 2 days. (D′) shows the dex wound in higher magnification. The beads are indicated by circles in the overview images.
3.2. Histological Sectioning and Standard Staining
To gain more insights into the process of regeneration of bead-implanted embryonic wounds, transverse HE-stained sections of the respective areas can be compared. This enables visualization of the embryonic wound in its whole depth on a cellular level. The standard HE staining displayed that the dex-treated wound exhibited a larger epithelial defect after 1 h (Figure 3), with erythrocytes covering the open skin wound, but fewer erythrocytes in the granulation tissue in comparison to the control wound.
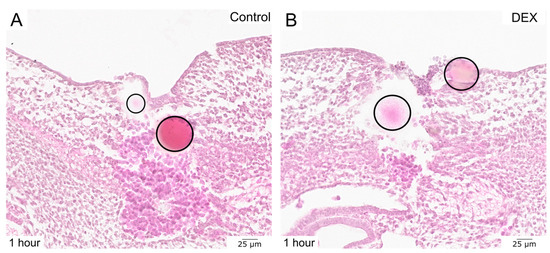
Figure 3.
The images display HE-stained bead-implanted wounds. (A) shows a control wound after 1 h. (B) shows a dex wound after 1 h. Note the larger defect as well as the incomplete re-epithelialization in the dex wound. The beads are indicated by circles.
3.3. Quantification of Histological Wound Parameters
The HE-stained sections can also be used to quantify various wound tissue parameters such as cell density or wound size. Table 1 displays exemplary wound parameters obtained from control and dex wounds, which have been published in [7] with further analysis and interpretation. The distance between the epithelial borders of the incisional wound was measured using the microscopy software OlyVia (Version 2.9, Olympus, Tokyo, Japan). For cell density quantifications, QuPath (Open source software, Version 0.3.2) was utilized to calculate the number of cells in the bead-implanted area following numeric conversion to a set area for comparison (e.g., 1000 μm2). The second and third columns, named control and dex, list the respective data. Each value represents an individual specimen.

Table 1.
Quantitative data obtained from the analysis of histological sections from the bead-implanted embryonic wounds.
3.4. Immunohistochemistry on Histological Sections
To further expand the histological insights, antibody staining can be performed on the paraffin sections, allowing for additional comparison of the experimental groups at the protein level. The exemplary immunohistochemistry showed that there was less Vimentin to be detected in the dex wound after 1 h (Figure 4). Various other antibodies of interest can be used to examine the process of wound healing.
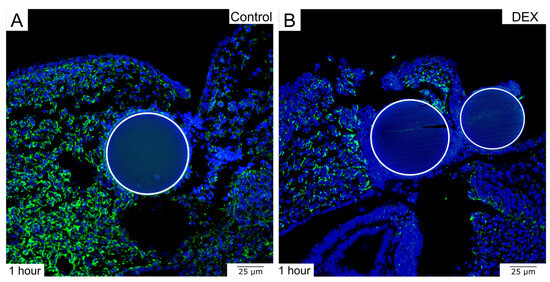
Figure 4.
The images display the immunohistochemical detection of Vimentin in bead-implanted wounds. (A) shows a control wound after 1 h. (B) shows a dex wound after 1 h. Note the decrease in Vimentin expression in the dex wound. The beads are indicated by circles.
3.5. Whole-Mount In Situ Hybridization
Whole-mount in-situ hybridization allows for a three-dimensional analysis of the bead-implanted wounds at the RNA level. The respective transcripts are detected via riboprobes. The presented example of Dermo-1 expression—a gene relevant to skin development—in control and dex wounds shows that, due to the prolonged regeneration in the dex wound, the disruption of the expression pattern in the wound area persisted after 1 h (Figure 5). Various other riboprobes as well as post-hybridization vibratome sectioning can be employed for further analysis of embryonic wound healing.
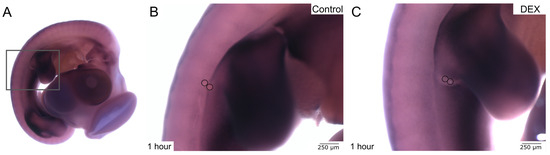
Figure 5.
The images display whole-mounts after in situ hybridization for Dermo-1. (A) serves as an overview. (B) shows a control wound after 1 h. (C) shows a dex wound after 1 h. Note the disruption of the Dermo-1 expression in the dex wound due to the prolonged regeneration. The beads are indicated by circles.
To conclude, the present data descriptor elaborates on a simple and reproducible technique for local drug testing during embryonic wound healing, providing exemplary results and demonstrative video material. The method can be adapted to investigate the interference of various other drugs and their specific molecular mechanisms of action in the context of embryonic development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/data8120178/s1, Video S1.
Author Contributions
Conceptualization, M.B., M.G., B.B.-S. and G.M.-P.; methodology, M.B., M.G., B.B.-S. and G.M.-P.; software, M.B.; validation, M.B., M.G. and G.M.-P.; formal analysis, M.B., M.G. and G.M.-P.; investigation M.B., M.G. and G.M.-P.; resources, B.B.-S. and G.M.-P.; data curation, M.B., M.G. and G.M.-P.; writing—original draft preparation, M.B.; writing—review and editing, M.G., B.B.-S. and G.M.-P.; visualization, M.B.; supervision, M.G., B.B.-S. and G.M.-P.; project administration, B.B.-S. and G.M.-P.; funding acquisition, M.B., M.G., B.B.-S. and G.M.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Ruhr-Universität Bochum, Faculty of Medicine, FoRUM grant (P075R-22), awarded to Martin Bablok. Additionally, this work was funded by the Stem Cell Medicine program scholarship from the Academy of Ruhr University Bochum, awarded to Morris Gellisch.
Institutional Review Board Statement
According to German legislation, the use of embryonic vertebrates in an animal experiment needs approval only if the animal is in the last third of its embryonic development. In the case of chickens, this means that experiments performed on animals before embryonic day 14 (E14) are not regarded as animal experiments by German law and, therefore, do not need approval or governmental permission.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article. The original research article including further results and discussion can be found at: https://doi.org/10.3390/biomedicines10123125 (accessed on 2 October 2023).
Acknowledgments
We acknowledge support by the DFG Open Access Publication Funds of the Ruhr-University Bochum. The AMF-17b antibody developed by Fulton, A.B. was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242, USA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stern, C.D. The Chick—A Great Model System Becomes Even Greater. Dev. Cell 2005, 8, 9–17. [Google Scholar] [CrossRef] [PubMed]
- International Chicken Genome Sequencing Consortium. Sequence and Comparative Analysis of the Chicken Genome Provide Unique Perspectives on Vertebrate Evolution. Nature 2004, 432, 695–716. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.N.M.; Schlemper, A.E.; da Silva, M.V.; Fonseca, B.B. Chicken Embryo: A Useful Animal Model for Drug Testing? Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4828–4839. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Zeisserlaboubebe, M.; Lange, N.; Gurny, R.; Delie, F. The Chick Embryo and Its Chorioallantoic Membrane (CAM) for the in Vivo Evaluation of Drug Delivery Systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Yahya, I.; van Lin, D.J.M.; Böing, M.; Brand-Saberi, B.; Morosan-Puopolo, G. In Ovo Technique for Cell Injection in the CPM Followed by Bead Implantation in the BA2 of Chicken Embryos. MethodsX 2020, 7, 100792. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.H.; Sweetman, D. Grafting of Beads into Developing Chicken Embryo Limbs to Identify Signal Transduction Pathways Affecting Gene Expression. J. Vis. Exp. 2016, 107, e53342. [Google Scholar] [CrossRef]
- Bablok, M.; Gellisch, M.; Brand-Saberi, B.; Morosan-Puopolo, G. Local Glucocorticoid Administration Impairs Embryonic Wound Healing. Biomedicines 2022, 10, 3125. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, H.D.; Flake, A.W. Fetal Surgery. Pediatr. Clin. N. Am. 2019, 66, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Sampat, K.; Losty, P.D. Fetal Surgery. Br. J. Surg. 2021, 108, 632–637. [Google Scholar] [CrossRef]
- Adzick, N.S.; Harrison, M.R. Fetal Surgical Therapy. Lancet 1994, 343, 897–902. [Google Scholar] [CrossRef]
- Moore, A.L.; Marshall, C.D.; Barnes, L.A.; Murphy, M.P.; Ransom, R.C.; Longaker, M.T. Scarless Wound Healing: Transitioning from Fetal Research to Regenerative Healing. WIREs Dev. Biol. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- McGoldrick, E.; Stewart, F.; Parker, R.; Dalziel, S.R. Antenatal Corticosteroids for Accelerating Fetal Lung Maturation for Women at Risk of Preterm Birth. Cochrane Database Syst. Rev. 2020, 2021, CD004454. [Google Scholar]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Yahya, I.; Böing, M.; Brand-Saberi, B.; Morosan-Puopolo, G. How to Distinguish between Different Cell Lineages Sharing Common Markers Using Combinations of Double In-Situ-Hybridization and Immunostaining in Avian Embryos: CXCR4-Positive Mesodermal and Neural Crest-Derived Cells. Histochem. Cell Biol. 2021, 155, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, V.; Hamilton, H.L. A Series of Normal Stages in the Development of the Chick Embryo. Dev. Dyn. 1992, 195, 231–272. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).